Resting-State Networks Associated with Behavioral and Self-Reported Measures of Persecutory Ideation in Psychosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Methods
2.2.1. Behavioral Measure of Persecution: Minnesota Trust Game (MTG)
2.2.2. Self-Reported Measures of Persecution: Green et al. Paranoid Thought Scales (GPTS)
2.3. Resting-State Functional Neuroimaging Data
2.4. Data Analysis Plan
3. Results
3.1. Performance and Demographic Characteristics
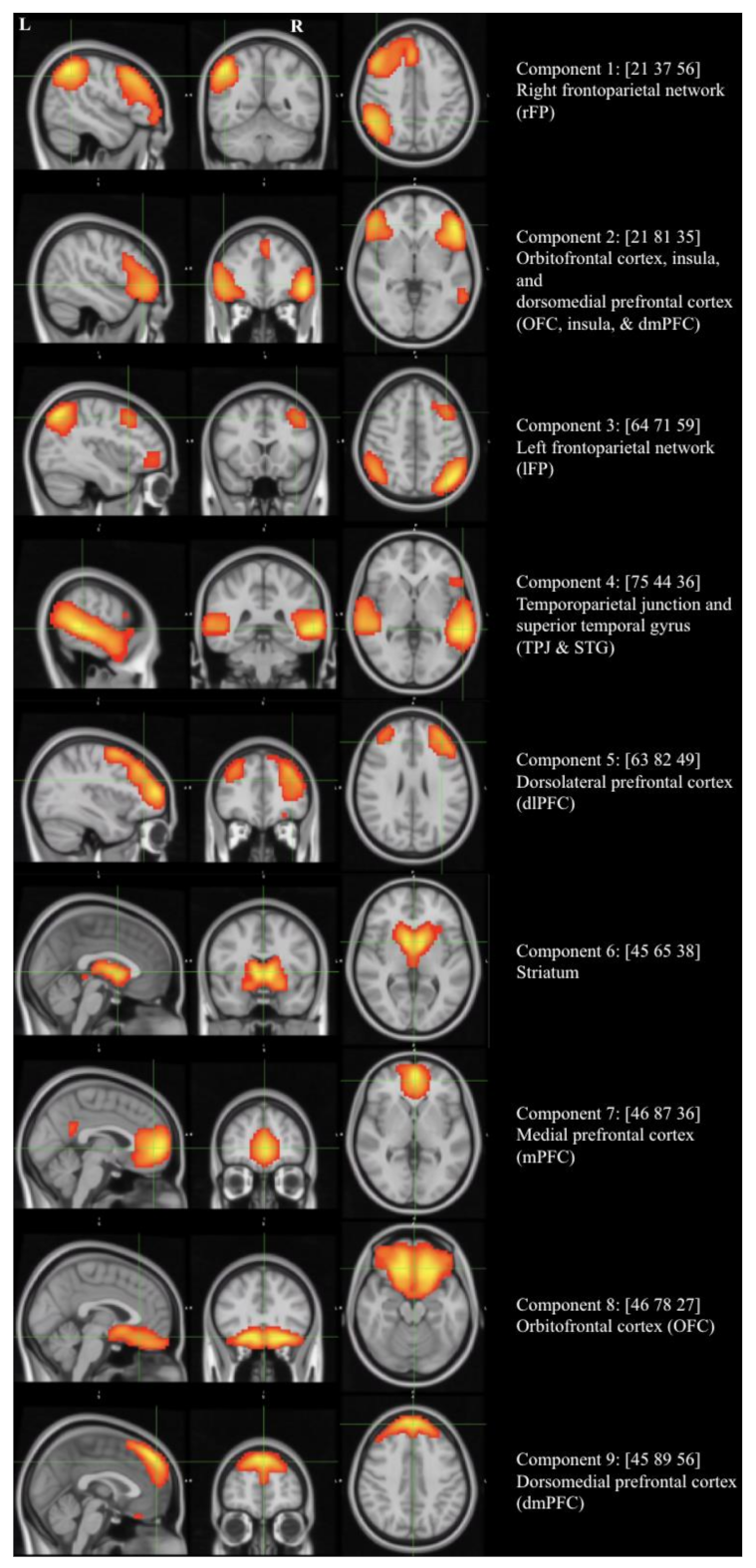
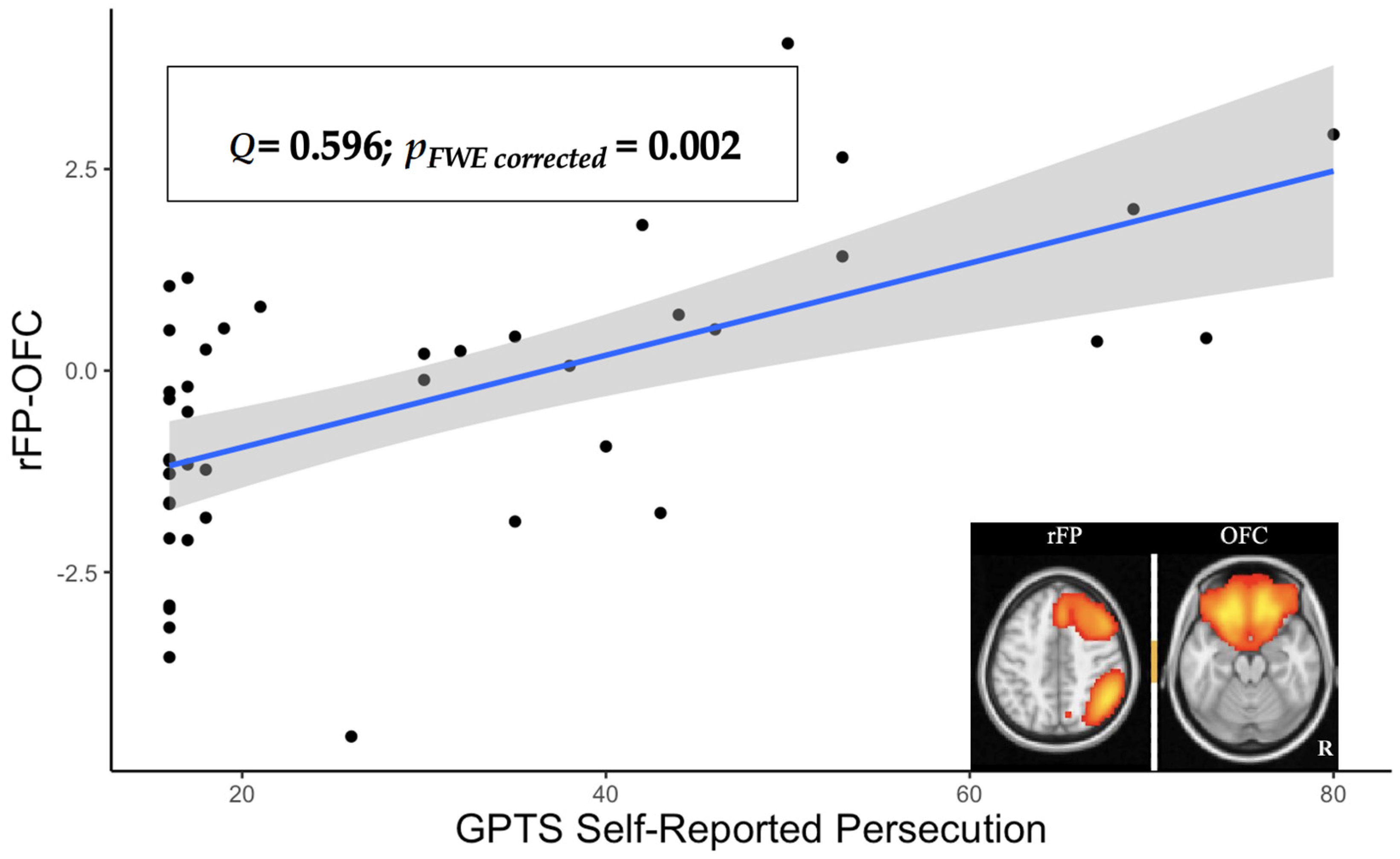
3.2. Brain Network Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
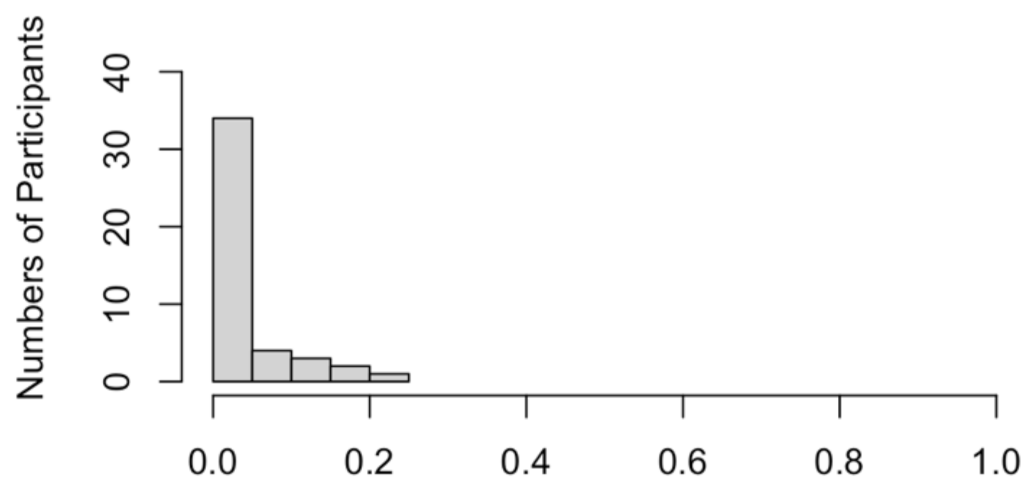
Appendix B
References
- Appelbaum, P.S.; Robbins, P.C.; Roth, L.H. Dimensional approach to delusions: Comparison across types and diagnoses. Am. J. Psychiatry 1999, 156, 1938–1943. [Google Scholar]
- Van Os, J.; Reininghaus, U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry 2016, 15, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Cheung, P.; Schweitzer, I.; Crowley, K.; Tuckwell, V. Violence in schizophrenia: Role of hallucinations and delusions. Schizophr. Res. 1997, 26, 181–190. [Google Scholar] [CrossRef]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Beckmann, C.F. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deco, G.; Corbetta, M. The dynamical balance of the brain at rest. Neuroscientist 2011, 17, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.D.; Huang, J.; Chun, M.M.; Constable, R.T. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Schatzberg, A.F. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liang, M.; Zhou, Y.; He, Y.; Hao, Y.; Song, M.; Jiang, T. Disrupted small-world networks in schizophrenia. Brain 2008, 131, 945–961. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, S.A.; Damoiseaux, J.S.; Goekoop, R.; Barkhof, F.; Scheltens, P.; Smith, S.M.; Beckmann, C.F. Model-free group analysis shows altered BOLD FMRI networks in dementia. Hum. Brain Mapp. 2009, 30, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Garrity, A.G.; Pearlson, G.D.; McKiernan, K.; Lloyd, D.; Kiehl, K.A.; Calhoun, V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry 2007, 164, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Littow, H.; Huossa, V.; Karjalainen, S.; Jääskeläinen, E.; Haapea, M.; Miettunen, J.; Kiviniemi, V.J. Aberrant functional connectivity in the default mode and central executive networks in subjects with schizophrenia–a whole-brain resting-state ICA study. Front. Psychiatry 2015, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotarska-Jagiela, A.; van de Ven, V.; Oertel-Knöchel, V.; Uhlhaas, P.J.; Vogeley, K.; Linden, D.E. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010, 117, 21–30. [Google Scholar] [CrossRef]
- Orliac, F.; Naveau, M.; Joliot, M.; Delcroix, N.; Razafimandimby, A.; Brazo, P.; Delamillieure, P. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr. Res. 2013, 148, 74–80. [Google Scholar] [CrossRef]
- Manoliu, A.; Riedl, V.; Zherdin, A.; Mühlau, M.; Schwerthöffer, D.; Scherr, M.; Sorg, C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2014, 40, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Nekovarova, T.; Fajnerova, I.; Horacek, J.; Spaniel, F. Bridging disparate symptoms of schizophrenia: A triple network dysfunction theory. Front. Behav. Neurosci. 2014, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Wisner, K.M.; Johnson, M.K.; Porter, J.N.; Krueger, R.F.; MacDonald, A.W., III. Task-related neural mechanisms of persecutory ideation in schizophrenia and community monozygotic twin-pairs. Hum. Brain Mapp. 2021, 46, 5244–5263. [Google Scholar] [CrossRef]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef] [Green Version]
- Sanislow, C.A.; Pine, D.S.; Quinn, K.J.; Kozak, M.J.; Garvey, M.A.; Heinssen, R.K.; Cuthbert, B.N. Developing constructs for psychopathology research: Research domain criteria. J. Abnorm. Psychol. 2010, 119, 631. [Google Scholar] [CrossRef] [Green Version]
- Kotov, R.; Krueger, R.F.; Watson, D.; Achenbach, T.M.; Althoff, R.R.; Bagby, R.M.; Zimmerman, M. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J. Abnorm. Psychol. 2017, 126, 454–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wechsler, D. Wechsler Test of Adult Reading: WTAR; Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Jeste, D.V.; Palmer, B.W.; Appelbaum, P.S.; Golshan, S.; Glorioso, D.; Dunn, L.B.; Kraemer, H.C. A new brief instrument for assessing decisional capacity for clinical research. Arch. Gen. Psychiatry 2007, 64, 966–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecrubier, Y.; Sheehan, D.V.; Weiller, E.; Amorim, P.; Bonora, I.; Sheehan, K.H.; Dunbar, G.C. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur. Psychiatry 1997, 12, 224–231. [Google Scholar] [CrossRef]
- Johnson, M.K.; Rustichini, A.; MacDonald, A.W., III. Suspicious personality predicts behavior on a social decision-making task. Personal. Individ. Differ. 2009, 47, 30–35. [Google Scholar] [CrossRef]
- Green, C.E.L.; Freeman, D.; Kuipers, E.; Bebbington, P.; Fowler, D.; Dunn, G.; Garety, P.A. Measuring ideas of persecution and social reference: The Green et al. Paranoid Thought Scales (GPTS). Psychol. Med. 2008, 38, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.S.; Power, J.D.; Dubis, J.W.; Vogel, A.C.; Church, J.A.; Schlaggar, B.L.; Petersen, S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014, 35, 1981–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueter, A.R.; Abram, S.V.; MacDonald, A.W., III; Rustichini, A.; DeYoung, C.G. The goal priority network as a neural substrate of Conscientiousness. Hum. Brain Mapp. 2018, 39, 3574–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FSLNets. Available online: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets (accessed on 15 January 2020).
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Rudebeck, P.H.; Murray, E.A. The orbitofrontal oracle: Cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron 2014, 84, 1143–1156. [Google Scholar] [CrossRef] [Green Version]
- Schoenbaum, G.; Takahashi, Y.; Liu, T.L.; McDannald, M.A. Does the orbitofrontal cortex signal value? Ann. N. Y. Acad. Sci. 2011, 1239, 87. [Google Scholar] [CrossRef] [Green Version]
- Wallis, J.D. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007, 30, 31–56. [Google Scholar] [CrossRef] [Green Version]
- Barbas, H. Flow of information for emotions through temporal and orbitofrontal pathways. J. Anat. 2007, 211, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.D.; Straccia, M.A.; Meyer, M.L.; Du, M.; Tan, K.M. Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): Causal, multivariate, and reverse inference evidence. Neurosci. Biobehav. Rev. 2019, 99, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Dodell-Feder, D.; Tully, L.M.; Dudek, E.; Hooker, C.I. The representation of mental state information in schizophrenia and first-degree relatives: A multivariate pattern analysis of fMRI data. Soc. Cogn. Affect. Neurosci. 2021, 16, 608–620. [Google Scholar] [CrossRef]
- Vargas, T.; Damme, K.S.; Hooker, C.I.; Gupta, T.; Cowan, H.R.; Mittal, V.A. Differentiating implicit and explicit theory of mind and associated neural networks in youth at Clinical High Risk (CHR) for psychosis. Schizophr. Res. 2019, 208, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Evans, N.; Lister, R. Gut feelings, deliberative thought, and paranoid ideation: A study of experiential and rational reasoning. Psychiatry Res. 2012, 197, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Bentall, R.P.; Rowse, G.; Shryane, N.; Kinderman, P.; Howard, R.; Blackwood, N.; Corcoran, R. The cognitive and affective structure of paranoid delusions: A transdiagnostic investigation of patients with schizophrenia spectrum disorders and depression. Arch. Gen. Psychiatry 2009, 66, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Krkovic, K.; Krink, S.; Lincoln, T.M. Emotion regulation as a moderator of the interplay between self-reported and physiological stress and paranoia. Eur. Psychiatry 2018, 49, 43–49. [Google Scholar] [CrossRef]
- Shaikh, M.; Ellett, L.; Dutt, A.; Day, F.; Laing, J.; Kroll, J.; Valmaggia, L.R. Perceived ethnic discrimination and persecutory paranoia in individuals at ultra-high risk for psychosis. Psychiatry Res. 2016, 241, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Chin, A.L.; Negash, S.; Xie, S.; Arnold, S.E.; Hamilton, R. Quality, and not just quantity, of education accounts for differences in psychometric performance between african americans and white non-hispanics with Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2012, 18, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.S.; Lesh, T.A.; Barch, D.M. Thresholds, power, and sample sizes in clinical neuroimaging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Marek, S.; Tervo-Clemmens, B.; Calabro, F.J.; Montez, D.F.; Kay, B.P.; Hatoum, A.S.; Dosenbach, N.U. Towards reproducible brain-wide association studies. BioRxiv 2020. [Google Scholar] [CrossRef]
- Lemmers-Jansen, I.L.; Krabbendam, L.; Veltman, D.J.; Fett, A.K.J. Boys vs. girls: Gender differences in the neural development of trust and reciprocity depend on social context. Dev. Cogn. Neurosci. 2017, 25, 235–245. [Google Scholar] [CrossRef] [PubMed]

| Mean (SD) | Relationship with MTG 1 Percentage of Suspicious Mistrust | Relationship with MTG Suspicion Threshold | Relationship with GPTS 2 Persecution | |
|---|---|---|---|---|
| N | 44 | / | / | / |
| Age | 29.7 (7.9) | ρ = −0.037, p = 0.814 | ρ = 0.029, p = 0.850 | ρ = 0.169, p = 0.273 |
| Sex (% Male) | 63.6% | W = 187, p = 0.372 | W = 187.5, p = 0.361 | W = 160, p = 0.114 |
| % Racial Minority | 25% | W = 288.5, p = 0.004 | W = 289.5, p = 0.002 | W = 137, p = 0.223 |
| Estimated Intelligence (WTAR 3 Raw Score) | 38.6 (8.7) | ρ = −0.392, p = 0.009 | ρ = −0.403, p = 0.007 | ρ = −0.111, p = 0.475 |
| Education (yrs) | 15.5 (2.3) | ρ = −0.249, p = 0.108 | ρ = −0.229, p = 0.139 | ρ = −0.023, p = 0.886 |
| Parental Education (average yrs) | 16.0 (3.5) | ρ = −0.134, p = 0.393 | ρ = −0.154, p = 0.324 | ρ = −0.092, p = 0.555 |
| Handedness (1 = left; 5 = right) | 4.2 (0.9) | ρ = 0.105, p = 0.498 | ρ = 0.214, p = 0.164 | ρ = −0.151, p = 0.329 |
| MTG Percentage of Suspicious Mistrust | 25.8% (26.6%) | - | - | - |
| MTG Suspicion Threshold | −5.7 (11.3) | ρ = 0.882, p < 0.001 | - | - |
| GPTS Persecution | 29.6 (18.1) | ρ = 0.161, p = 0.296 | ρ = 0.087, p = 0.577 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Kazinka, R.; Pratt, D.; Kwashie, A.; MacDonald, A.W., III. Resting-State Networks Associated with Behavioral and Self-Reported Measures of Persecutory Ideation in Psychosis. Brain Sci. 2021, 11, 1490. https://doi.org/10.3390/brainsci11111490
Yu L, Kazinka R, Pratt D, Kwashie A, MacDonald AW III. Resting-State Networks Associated with Behavioral and Self-Reported Measures of Persecutory Ideation in Psychosis. Brain Sciences. 2021; 11(11):1490. https://doi.org/10.3390/brainsci11111490
Chicago/Turabian StyleYu, Lingyan, Rebecca Kazinka, Danielle Pratt, Anita Kwashie, and Angus W. MacDonald, III. 2021. "Resting-State Networks Associated with Behavioral and Self-Reported Measures of Persecutory Ideation in Psychosis" Brain Sciences 11, no. 11: 1490. https://doi.org/10.3390/brainsci11111490
APA StyleYu, L., Kazinka, R., Pratt, D., Kwashie, A., & MacDonald, A. W., III. (2021). Resting-State Networks Associated with Behavioral and Self-Reported Measures of Persecutory Ideation in Psychosis. Brain Sciences, 11(11), 1490. https://doi.org/10.3390/brainsci11111490






