Neural Mechanisms of Prism Adaptation in Healthy Adults and Individuals with Spatial Neglect after Unilateral Stroke: A Review of fMRI Studies
Abstract
:1. Introduction
1.1. A Brief History
1.2. Neural Mechanisms of Spatial Neglect
1.3. Neural Mechanisms of Prism Adaptation (PA)
1.4. Present Study
2. Materials and Methods
3. Results
3.1. Task-Specific fMRI Studies
3.1.1. Meta-Analysis 1: Brain Activity before vs. after PA in HC
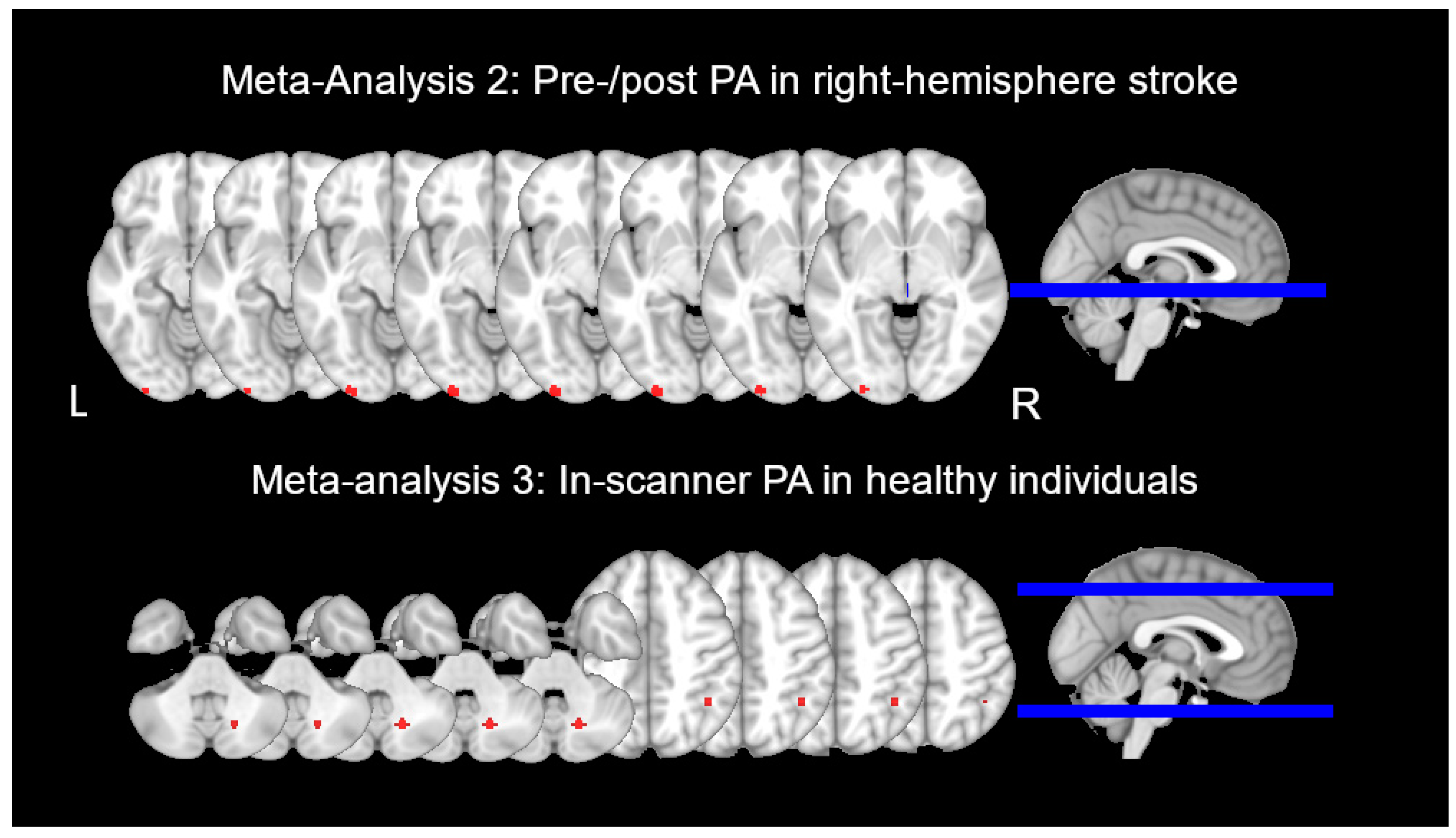
3.1.2. Meta-Analysis 2: Brain Activity before vs. after PA in RBD Patients
3.1.3. Meta-Analysis 3: Progressive Change in Brain Activity during PA in HC
3.1.4. PA Effects in LBD Patients
3.2. Resting State Functional Connectivity (rsFC) fMRI Studies
4. Discussion
4.1. Neural Mechanisms of Prism Adaptation in Healthy Adults
4.1.1. Brain Activity before vs. after Prism Adaptation
4.1.2. Brain Activity during Prism Adaptation
4.2. Neural Mechanisms of Prism Adaptation in Patients with Unilateral Stroke
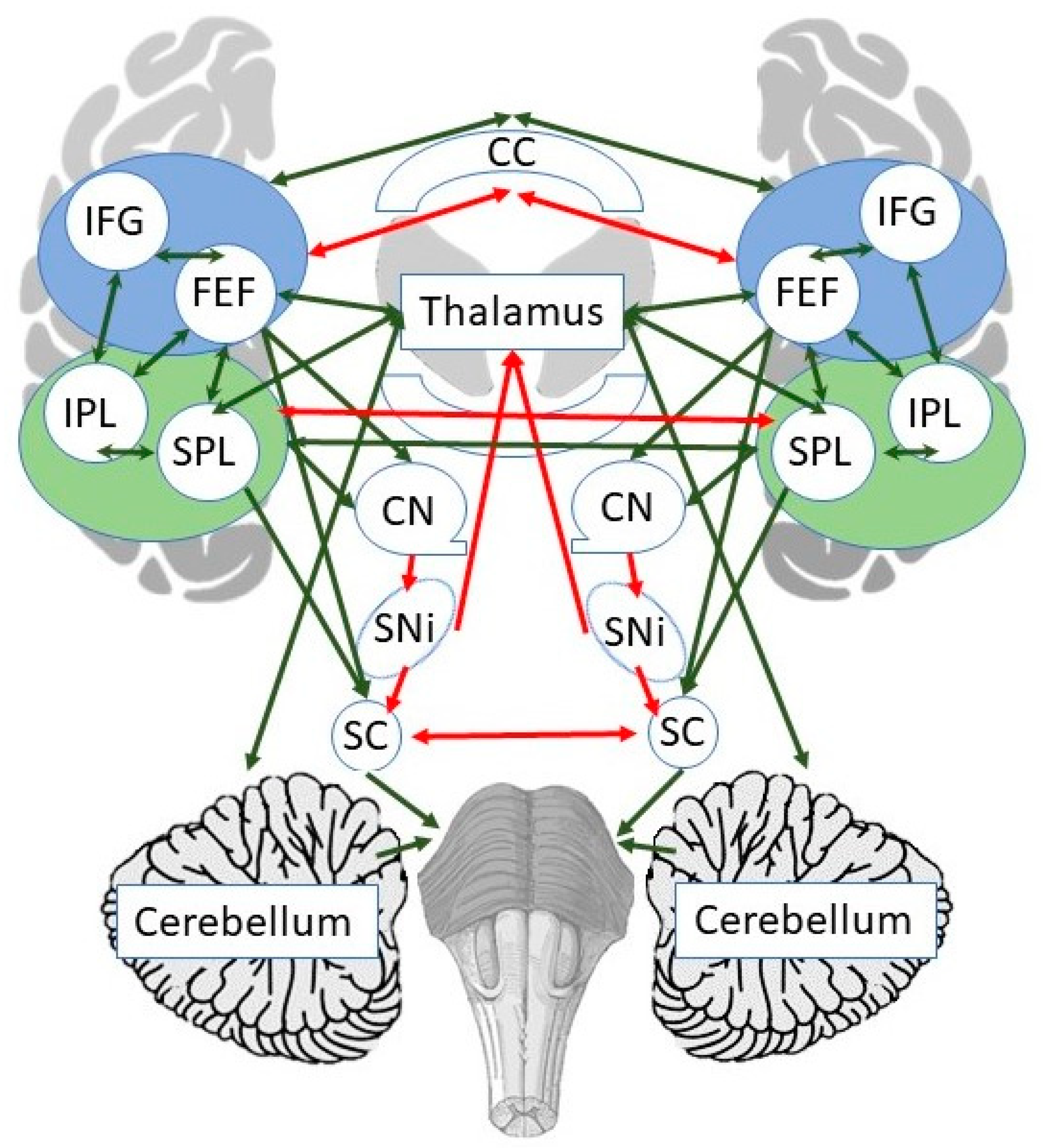
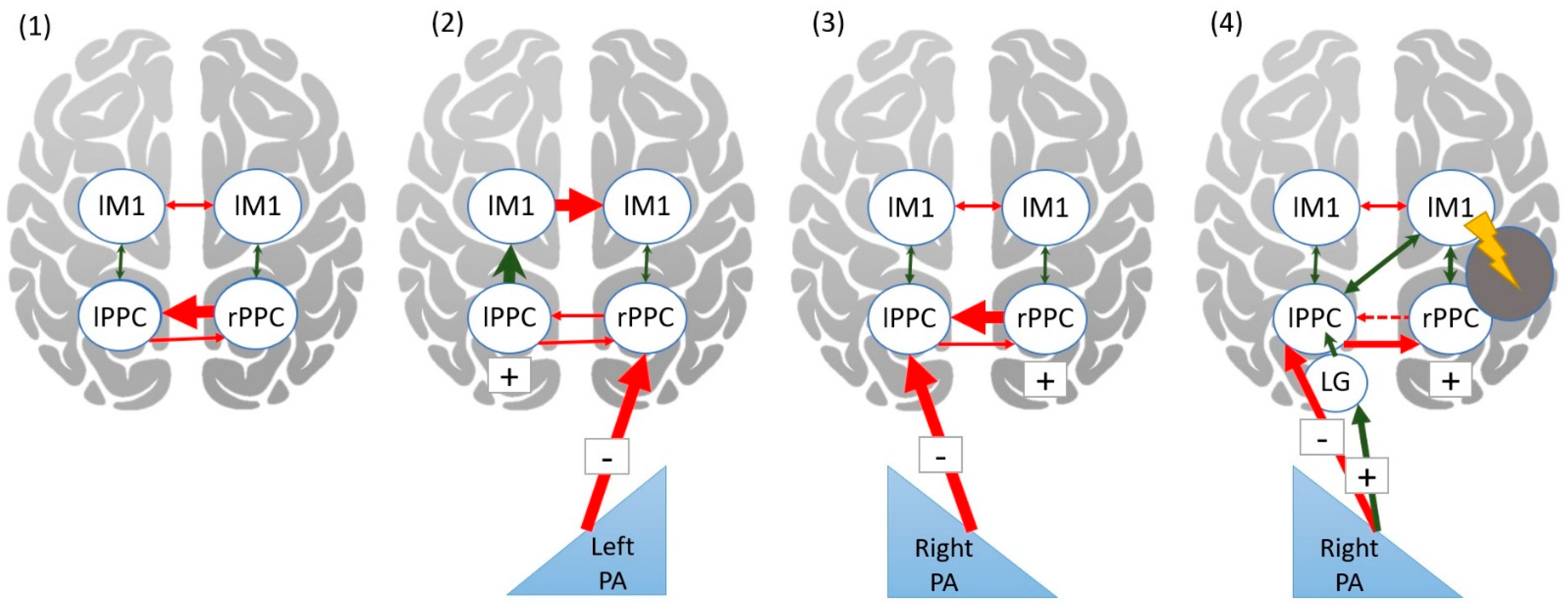
4.3. Theoretical Framework for Prism Adaptation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, E.; Shekhtman, G.; Chen, P. Prevalence of spatial neglect post stroke: A systematic review. Ann. Phys. Rehabil. Med. 2021, 64, 101459. [Google Scholar] [CrossRef]
- Chen, P.; Ward, I.; Khan, U.; Liu, Y.; Hreha, K. Spatial neglect hinders success of inpatient rehabilitation in individuals with traumatic brain injury: A retrospective study. Neurorehabil. Neural. Repair. 2016, 30, 451–460. [Google Scholar] [CrossRef]
- Gomes, D.; Fonseca, M.; Garrotes, M.; Lima, M.R.; Mendonca, M.; Pereira, M.; Lourenco, M.; Oliveira, E.; Lavrador, J.P. Corpus callosum and neglect syndrome: Clinical findings after meningioma removal and anatomical review. J. Neurosci. Rural. Pract. 2017, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Brain, W.R. Visual disorientation with special reference to lesions of the right cerebral hemisphere. Brain 1941, 64, 244–272. [Google Scholar] [CrossRef]
- Mesulam, M.M. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1999, 354, 1325–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbetta, M.; Shulman, G.L. Spatial neglect and attention networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef] [Green Version]
- Heilman, K.M.; Valenstein, E. Mechanisms underlying hemispatial neglect. Ann. Neurol. 1979, 5, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Heilman, K.M.; Watson, R.T.; Valenstein, E. Neglect and related disorders. In Clinical Neuropsychology, 5th ed.; Heilman, K.M., Valenstein, E., Eds.; Oxford University: New York, NY, USA, 2012; pp. 296–348. [Google Scholar]
- Adair, J.C.; Barrett, A.M. Spatial neglect: Clinical and neuroscience review: A wealth of information on the poverty of spatial attention. Ann. N. Y. Acad. Sci. 2008, 1142, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.; Rossit, S. Visuospatial neglect in action. Neuropsychologia 2012, 50, 1018–1028. [Google Scholar] [CrossRef]
- Ogourtsova, T.; Archambault, P.; Lamontagne, A. Impact of post-stroke unilateral spatial neglect on goal-directed arm movements: Systematic literature review. Top. Stroke Rehabil. 2015, 22, 397–428. [Google Scholar] [CrossRef]
- Rode, G.; Pagliari, C.; Huchon, L.; Rossetti, Y.; Pisella, L. Semiology of neglect: An update. Ann. Phys. Rehabil. Med. 2017, 60, 177–185. [Google Scholar] [CrossRef]
- Katz, N.; Hartman-Maeir, A.; Ring, H.; Soroker, N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch. Phys. Med. Rehabil. 1999, 80, 379–384. [Google Scholar] [CrossRef]
- Wee, J.Y.; Hopman, W.M. Comparing consequences of right and left unilateral neglect in a stroke rehabilitation population. Am. J. Phys. Med. Rehabil. 2008, 87, 910–920. [Google Scholar] [CrossRef]
- Chen, P.; Hreha, K.; Kong, Y.; Barrett, A.M. Impact of spatial neglect in stroke rehabilitation: Evidence from the setting of an inpatient rehabilitation facility. Arch. Phys. Med. Rehabil. 2015, 96, 1458–1466. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Mizuno, K.; Miyamoto, A.; Kondo, K.; Liu, M. Influence of right versus left unilateral spatial neglect on the functional recovery after rehabilitation in sub-acute stroke patients. Neuropsychol. Rehabil. 2020, 1–22. [Google Scholar] [CrossRef]
- Champod, A.S.; Frank, R.C.; Taylor, K.; Eskes, G.A. The effects of prism adaptation on daily life activities in patients with visuospatial neglect: A systematic review. Neuropsychol. Rehabil. 2018, 28, 491–514. [Google Scholar] [CrossRef]
- Chen, P.; Diaz-Segarra, N.; Hreha, K.; Kaplan, E.; Barrett, A.M. Prism adaptation treatment improves inpatient rehabilitation outcome in individuals with spatial neglect: A retrospective matched control study. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100130. [Google Scholar] [CrossRef]
- Mizuno, K.; Tsuji, T.; Takebayashi, T.; Fujiwara, T.; Hase, K.; Liu, M. Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: A randomized, controlled trial. Neurorehabil. Neural. Repair. 2011, 25, 711–720. [Google Scholar] [CrossRef]
- Ten Brink, A.F.; Visser-Meily, J.M.A.; Schut, M.J.; Kouwenhoven, M.; Eijsackers, A.L.H.; Nijboer, T.C.W. Prism adaptation in rehabilitation? No additional effects of prism adaptation on neglect recovery in the subacute phase poststroke: A randomized controlled trial. Neurorehabil. Neural. Repair. 2017, 31, 1017–1028. [Google Scholar] [CrossRef] [Green Version]
- Vilimovsky, T.; Chen, P.; Hoidekrova, K.; Petioky, J.; Harsa, P. Prism adaptation treatment to address spatial neglect in an intensive rehabilitation program: A randomized pilot and feasibility trial. PLoS ONE 2021, 16, e0245425. [Google Scholar] [CrossRef]
- Serino, A.; Barbiani, M.; Rinaldesi, M.L.; Ladavas, E. Effectiveness of prism adaptation in neglect rehabilitation: A controlled trial study. Stroke 2009, 40, 1392–1398. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, L.; Yang, Y.; Chen, S. Effects of prism adaptation for unilateral spatial neglect after stroke: A systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 2021, 100, 584–591. [Google Scholar] [CrossRef]
- Harris, C.S. Adaptation to displaced vision: Visual, motor, or proprioceptive change? Science 1963, 140, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Taub, E.; Goldberg, L.A. Prism adaptation: Control of intermanual transfer by distribution of practice. Science 1973, 180, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Redding, G.M.; Wallace, B. Sources of “overadditivity” in prism adaptation. Percept. Psychophys. 1978, 24, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Redding, G.M.; Wallace, B. Intermanual transfer of prism adaptation. J. Mot. Behav. 2008, 40, 246–262. [Google Scholar] [CrossRef]
- Rossetti, Y.; Rode, G.; Pisella, L.; Farne, A.; Li, L.; Boisson, D.; Perenin, M.T. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 1998, 395, 166–169. [Google Scholar] [CrossRef]
- Ladavas, E.; Bonifazi, S.; Catena, L.; Serino, A. Neglect rehabilitation by prism adaptation: Different procedures have different impacts. Neuropsychologia 2011, 49, 1136–1145. [Google Scholar] [CrossRef]
- Facchin, A.; Bultitude, J.H.; Mornati, G.; Peverelli, M.; Daini, R. A comparison of prism adaptation with terminal versus concurrent exposure on sensorimotor changes and spatial neglect. Neuropsychol. Rehabil. 2020, 30, 613–640. [Google Scholar] [CrossRef]
- Facchin, A.; Beschin, N.; Toraldo, A.; Cisari, C.; Daini, R. Aftereffect induced by prisms of different power in the rehabilitation of neglect: A multiple single case report. Neurorehabilitation 2013, 32, 839–853. [Google Scholar] [CrossRef]
- Sarri, M.; Greenwood, R.; Kalra, L.; Papps, B.; Husain, M.; Driver, J. Prism adaptation aftereffects in stroke patients with spatial neglect: Pathological effects on subjective straight ahead but not visual open-loop pointing. Neuropsychologia 2008, 46, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Gossmann, A.; Kastrup, A.; Kerkhoff, G.; Lopez-Herrero, C.; Hildebrandt, H. Prism adaptation improves ego-centered but not allocentric neglect in early rehabilitation patients. Neurorehabil. Neural. Repair. 2013, 27, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Damora, A.; Abbruzzese, L.; Zoccolotti, P. Prism adaptation improves egocentric but not allocentric unilateral neglect: A case study. Eur. J. Phys. Rehabil. Med. 2018, 54, 85–89. [Google Scholar] [CrossRef]
- Jacquin-Courtois, S.; O’Shea, J.; Luaute, J.; Pisella, L.; Revol, P.; Mizuno, K.; Rode, G.; Rossetti, Y. Rehabilitation of spatial neglect by prism adaptation: A peculiar expansion of sensorimotor after-effects to spatial cognition. Neurosci. Biobehav. Rev. 2013, 37, 594–609. [Google Scholar] [CrossRef]
- Rode, G.; Rossetti, Y.; Boisson, D. Prism adaptation improves representational neglect. Neuropsychologia 2001, 39, 1250–1254. [Google Scholar] [CrossRef]
- Crottaz-Herbette, S.; Fornari, E.; Notter, M.P.; Bindschaedler, C.; Manzoni, L.; Clarke, S. Reshaping the brain after stroke: The effect of prismatic adaptation in patients with right brain damage. Neuropsychologia 2017, 104, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lunven, M.; Rode, G.; Bourlon, C.; Duret, C.; Migliaccio, R.; Chevrillon, E.; Thiebaut de Schotten, M.; Bartolomeo, P. Anatomical predictors of successful prism adaptation in chronic visual neglect. Cortex 2019, 120, 629–641. [Google Scholar] [CrossRef]
- Saj, A.; Cojan, Y.; Assal, F.; Vuilleumier, P. Prism adaptation effect on neural activity and spatial neglect depend on brain lesion site. Cortex 2019, 119, 301–311. [Google Scholar] [CrossRef]
- Payne, B.R.; Rushmore, R.J. Functional circuitry underlying natural and interventional cancellation of visual neglect. Exp. Brain Res. 2004, 154, 127–153. [Google Scholar] [CrossRef]
- Payne, B.R.; Rushmore, R.J. Animal models of cerebral neglect and its cancellation. Neuroscientist 2003, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Burcham, K.J.; Corwin, J.V.; Stoll, M.L.; Reep, R.L. Disconnection of medial agranular and posterior parietal cortex produces multimodal neglect in rats. Behav. Brain Res. 1997, 86, 41–47. [Google Scholar] [CrossRef]
- Sprague, J.M. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 1966, 153, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cabre, A.; Toba, M.N.; Hilgetag, C.C.; Rushmore, R.J. Perturbation-driven paradoxical facilitation of visuo-spatial function: Revisiting the ’Sprague effect’. Cortex 2020, 122, 10–39. [Google Scholar] [CrossRef]
- Wiesen, D.; Karnath, H.O.; Sperber, C. Disconnection somewhere down the line: Multivariate lesion-symptom mapping of the line bisection error. Cortex 2020, 133, 120–132. [Google Scholar] [CrossRef]
- Baldassarre, A.; Ramsey, L.; Hacker, C.L.; Callejas, A.; Astafiev, S.V.; Metcalf, N.V.; Zinn, K.; Rengachary, J.; Snyder, A.Z.; Carter, A.R.; et al. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain 2014, 137, 3267–3283. [Google Scholar] [CrossRef] [Green Version]
- He, B.J.; Snyder, A.Z.; Vincent, J.L.; Epstein, A.; Shulman, G.L.; Corbetta, M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007, 53, 905–918. [Google Scholar] [CrossRef] [Green Version]
- Toba, M.N.; Migliaccio, R.; Batrancourt, B.; Bourlon, C.; Duret, C.; Pradat-Diehl, P.; Dubois, B.; Bartolomeo, P. Common brain networks for distinct deficits in visual neglect. A combined structural and tractography MRI approach. Neuropsychologia 2018, 115, 167–178. [Google Scholar] [CrossRef]
- Prablanc, C.; Panico, F.; Fleury, L.; Pisella, L.; Nijboer, T.; Kitazawa, S.; Rossetti, Y. Adapting terminology: Clarifying prism adaptation vocabulary, concepts, and methods. Neurosci. Res. 2020, 153, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Luaute, J.; Schwartz, S.; Rossetti, Y.; Spiridon, M.; Rode, G.; Boisson, D.; Vuilleumier, P. Dynamic changes in brain activity during prism adaptation. J. Neurosci. 2009, 29, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Chapman, H.L.; Eramudugolla, R.; Gavrilescu, M.; Strudwick, M.W.; Loftus, A.; Cunnington, R.; Mattingley, J.B. Neural mechanisms underlying spatial realignment during adaptation to optical wedge prisms. Neuropsychologia 2010, 48, 2595–2601. [Google Scholar] [CrossRef]
- Panico, F.; Rossetti, Y.; Trojano, L. On the mechanisms underlying prism adaptation: A review of neuro-imaging and neuro-stimulation studies. Cortex 2020, 123, 57–71. [Google Scholar] [CrossRef]
- Kuper, M.; Wunnemann, M.J.; Thurling, M.; Stefanescu, R.M.; Maderwald, S.; Elles, H.G.; Goricke, S.; Ladd, M.E.; Timmann, D. Activation of the cerebellar cortex and the dentate nucleus in a prism adaptation fMRI study. Hum. Brain Mapp. 2014, 35, 1574–1586. [Google Scholar] [CrossRef]
- Crottaz-Herbette, S.; Fornari, E.; Tissieres, I.; Clarke, S. A brief exposure to leftward prismatic adaptation enhances the representation of the ipsilateral, right visual field in the right inferior parietal lobule. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Crottaz-Herbette, S.; Fornari, E.; Clarke, S. Prismatic adaptation changes visuospatial representation in the inferior parietal lobule. J. Neurosci. 2014, 34, 11803–11811. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Mizuno, K.; Nishida, D.; Tahara, M.; Yamada, E.; Shindo, S.; Kasuga, S.; Liu, M. Prism adaptation changes resting-state functional connectivity in the dorsal stream of visual attention networks in healthy adults: A fMRI study. Cortex 2019, 119, 594–605. [Google Scholar] [CrossRef]
- Clarke, S.; Crottaz-Herbette, S. Modulation of visual attention by prismatic adaptation. Neuropsychologia 2016, 92, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redding, G.M.; Wallace, B. Prism adaptation and unilateral neglect: Review and analysis. Neuropsychologia 2006, 44, 1–20. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crottaz-Herbette, S.; Tissieres, I.; Fornari, E.; Rapin, P.A.; Clarke, S. Remodelling the attentional system after left hemispheric stroke: Effect of leftward prismatic adaptation. Cortex 2019, 115, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Tissieres, I.; Fornari, E.; Clarke, S.; Crottaz-Herbette, S. Supramodal effect of rightward prismatic adaptation on spatial representations within the ventral attentional system. Brain Struct. Funct. 2018, 223, 1459–1471. [Google Scholar] [CrossRef]
- Saj, A.; Cojan, Y.; Vocat, R.; Luaute, J.; Vuilleumier, P. Prism adaptation enhances activity of intact fronto-parietal areas in both hemispheres in neglect patients. Cortex 2013, 49, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Danckert, J.; Ferber, S.; Goodale, M.A. Direct effects of prismatic lenses on visuomotor control: An event-related functional MRI study. Eur. J. Neurosci. 2008, 28, 1696–1704. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Mizuno, K.; Nishida, D.; Tahara, M.; Yamada, E.; Shindo, S.; Watanabe, Y.; Kasuga, S.; Liu, M. Correlation between changes in functional connectivity in the dorsal attention network and the after-effects induced by prism adaptation in healthy humans: A dataset of resting-state fMRI and pointing after prism adaptation. Data Brief 2019, 22, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Wilf, M.; Serino, A.; Clarke, S.; Crottaz-Herbette, S. Prism adaptation enhances decoupling between the default mode network and the attentional networks. Neuroimage 2019, 200, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Schintu, S.; Freedberg, M.; Gotts, S.J.; Cunningham, C.A.; Alam, Z.M.; Shomstein, S.; Wassermann, E.M. Prism adaptation modulates connectivity of the intraparietal sulcus with multiple brain networks. Cereb. Cortex 2020, 30, 4747–4758. [Google Scholar] [CrossRef]
- Yang, N.Y.; Zhou, D.; Chung, R.C.; Li-Tsang, C.W.; Fong, K.N. Rehabilitation interventions for unilateral neglect after stroke: A systematic review from 1997 through 2012. Front. Hum. Neurosci. 2013, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Harris, C.S. Perceptual adaptation to inverted, reversed, and displaced vision. Psychol. Rev. 1965, 72, 419–444. [Google Scholar] [CrossRef]
- Redding, G.M.; Wallace, B. Generalization of prism adaptation. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 1006–1022. [Google Scholar] [CrossRef] [Green Version]
- Held, R.; Schlank, M. Adaptation to disarranged eye-hand coordination in the distance-dimension. Am. J. Psychol. 1959, 72, 603–605. [Google Scholar] [CrossRef]
- Barrett, A.M.; Boukrina, O.; Saleh, S. Ventral attention and motor network connectivity is relevant to functional impairment in spatial neglect after right brain stroke. Brain Cogn. 2019, 129, 16–24. [Google Scholar] [CrossRef]
- Corbetta, M.; Kincade, M.J.; Lewis, C.; Snyder, A.Z.; Sapir, A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat. Neurosci. 2005, 8, 1603–1610. [Google Scholar] [CrossRef]
- Thimm, M.; Fink, G.R.; Sturm, W. Neural correlates of recovery from acute hemispatial neglect. Restor. Neurol. Neurosci. 2008, 26, 481–492. [Google Scholar]
- Goedert, K.M.; Chen, P.; Foundas, A.L.; Barrett, A.M. Frontal lesions predict response to prism adaptation treatment in spatial neglect: A randomised controlled study. Neuropsychol. Rehabil. 2020, 30, 32–53. [Google Scholar] [CrossRef]
- Priftis, K.; Passarini, L.; Pilosio, C.; Meneghello, F.; Pitteri, M. Visual scanning training, limb activation treatment, and prism adaptation for rehabilitating left neglect: Who is the winner? Front. Hum. Neurosci. 2013, 7, 360. [Google Scholar] [CrossRef] [Green Version]
- Nys, G.M.S.; de Haan, E.H.F.; Kunneman, A.; de Kort, P.L.M.; Dijkerman, H.C. Acute neglect rehabilitation using repetitive prism adaptation: A randomized placebo-controlled trial. Restor. Neurol. Neurosci. 2008, 26, 1–12. [Google Scholar]
- Vaes, N.; Nys, G.; Lafosse, C.; Dereymaeker, L.; Oostra, K.; Hemelsoet, D.; Vingerhoets, G. Rehabilitation of visuospatial neglect by prism adaptation: Effects of a mild treatment regime. A randomised controlled trial. Neuropsychol. Rehabil. 2018, 28, 899–918. [Google Scholar] [CrossRef]
- Ladavas, E.; Giulietti, S.; Avenanti, A.; Bertini, C.; Lorenzini, E.; Quinquinio, C.; Serino, A. a-tDCS on the ipsilesional parietal cortex boosts the effects of prism adaptation treatment in neglect. Restor. Neurol. Neurosci. 2015, 33, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, H.; Yamakawa, Y.; Itou, A.; Muraki, T.; Asada, T. Long-term effects of prism adaptation on chronic neglect after stroke. Neurorehabilitation 2008, 23, 137–151. [Google Scholar] [CrossRef]
- Nyffeler, T.; Vanbellingen, T.; Kaufmann, B.C.; Pflugshaupt, T.; Bauer, D.; Frey, J.; Chechlacz, M.; Bohlhalter, S.; Muri, R.M.; Nef, T.; et al. Theta burst stimulation in neglect after stroke: Functional outcome and response variability origins. Brain 2019, 142, 992–1008. [Google Scholar] [CrossRef]
- Bartolomeo, P. Visual neglect: Getting the hemispheres to talk to each other. Brain 2019, 142, 840–842. [Google Scholar] [CrossRef]
- Baumann, O.; Borra, R.J.; Bower, J.M.; Cullen, K.E.; Habas, C.; Ivry, R.B.; Leggio, M.; Mattingley, J.B.; Molinari, M.; Moulton, E.A.; et al. Consensus paper: The role of the cerebellum in perceptual processes. Cerebellum 2015, 14, 197–220. [Google Scholar] [CrossRef]
- Chao, C.C.; Karabanov, A.N.; Paine, R.; Carolina de Campos, A.; Kukke, S.N.; Wu, T.; Wang, H.; Hallett, M. Induction of motor associative plasticity in the posterior parietal cortex-primary motor network. Cereb. Cortex 2015, 25, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Karabanov, A.N.; Chao, C.C.; Paine, R.; Hallett, M. Mapping different intra-hemispheric parietal-motor networks using twin Coil TMS. Brain Stimul. 2013, 6, 384–389. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, J.; Revol, P.; Cousijn, H.; Near, J.; Petitet, P.; Jacquin-Courtois, S.; Johansen-Berg, H.; Rode, G.; Rossetti, Y. Induced sensorimotor cortex plasticity remediates chronic treatment-resistant visual neglect. eLife 2017, 6, e26602. [Google Scholar] [CrossRef]
- Martin-Arevalo, E.; Schintu, S.; Farne, A.; Pisella, L.; Reilly, K.T. Adaptation to leftward shifting prisms alters motor interhemispheric inhibition. Cereb. Cortex 2018, 28, 528–537. [Google Scholar] [CrossRef]
- Koch, G.; Cercignani, M.; Bonni, S.; Giacobbe, V.; Bucchi, G.; Versace, V.; Caltagirone, C.; Bozzali, M. Asymmetry of parietal interhemispheric connections in humans. J. Neurosci. 2011, 31, 8967–8975. [Google Scholar] [CrossRef] [Green Version]
- Jewell, G.; McCourt, M.E. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000, 38, 93–110. [Google Scholar] [CrossRef]
- Goedert, K.M.; Leblanc, A.; Tsai, S.W.; Barrett, A.M. Asymmetrical effects of adaptation to left- and right-shifting prisms depends on pre-existing attentional biases. J. Int. Neuropsychol. Soc. 2010, 16, 795–804. [Google Scholar] [CrossRef] [Green Version]
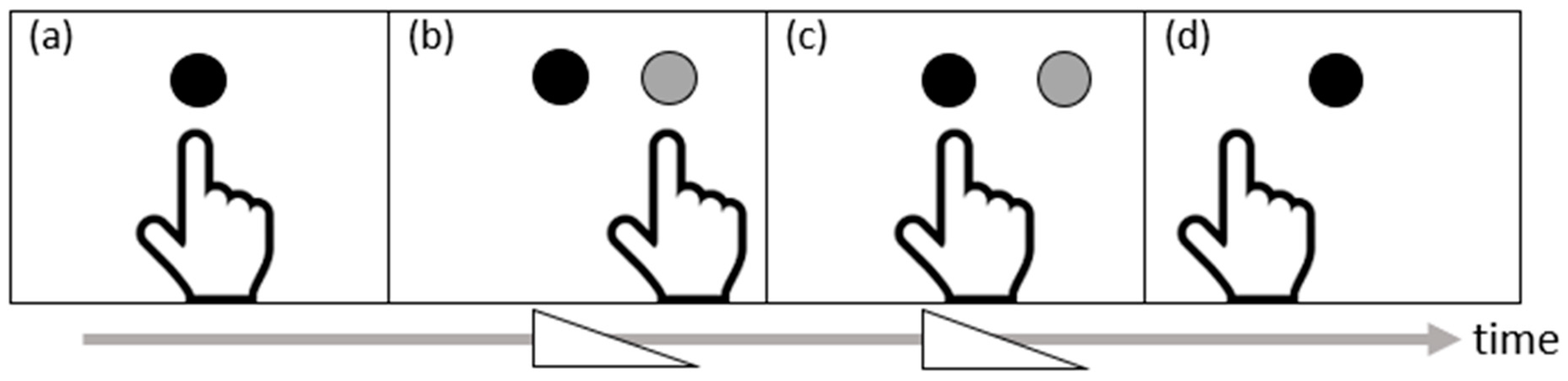
| Meta-Analysis 1: Before and after PA in Healthy Individuals | |||||||
| Study | Participant Population | N | PA Procedure | Task in Scanner | Scanned PA Phase | Findings | Quality Assessment |
| Crottaz-Herbette et al., 2014 [55] | HC | 28 |
| Visual detection (pressing a button when a white stimulus appeared on black background), visuospatial short-term memory, verbal short-term memory | ☒Before ☐Early ☐Late ☒After | Visual detection--
| Strength:
|
| Crottaz-Herbette et al., 2017 [37] | Age-matched HC (only HC results noted here) | 11 |
| Visual detection (same as above) | ☒Before ☐Early ☐Late ☒After | Stimulus location (left, right, center) x session (after vs. before PA)
| Strength:
|
| Crottaz-Herbette et al., 2017 [54] | HC | 42 |
| Visual detection (same as above) | ☒Before ☐Early ☐Late ☒After |
| Strength:
Weakness:
|
| Tissieres, et al., 2018 [62] | HC | 30 |
| Auditory detection and visual detection | ☒Before ☐Early ☐Late ☒After |
Stimulus location (left, right, center) x session (after vs. before PA)
| Strength:
Weakness:
|
| Crottaz-Herbette et al., 2019 [61] | HC | 14 |
| Visual detection (same as that used in their 2014 study) | ☒Before ☐Early ☐Late ☒After | After vs. Before PA:
| Strength:
Weakness:
|
| Meta-Analysis 2: Before and after PA in individuals with RBD | |||||||
| Saj et al., 2013 [63] | RBD | 7 |
| “bisection” (conceptually similar to a landmark task), visual search, and spatial memory | ☒Before ☐Early ☐Late ☒After | “bisection”--
Visual search--
Summary: Reduction of left neglect after PA associated with bilateral increases in SPL, MFG, occipital lobe | Strength:
|
| Saj et al., 2019 [39] | RBD | 10 |
| Imaginary finger pointing and encouraged rapid saccades to visual target | ☒Before ☒Early ☒Late ☒After | Frontal group
Parietal group
Summary: Increased activity in bilateral fronto-parietal networks and the occipital lobe following PA treatment, larger increases in patient group with frontal than parietal lesions | Strength:
Weakness:
|
| Crottaz-Herbette et al., 2017 [37] | RBD | 15 |
| Visual detection (same as that used in their 2014 study above) | ☒Before ☐Early ☐Late ☒After | Stimulus location (left, right, center) x session (after vs. before PA)
Summary: PA in patients affected IPL, prefrontal, and temporal cortex. Anterior STG/MTG activity correlated with neglect severity, with greater increases after PA for more severe spatial neglect. | Strength:
|
| Meta-Analysis 3: During PA in healthy individuals | |||||||
| Chapman et al., 2010 [51] | HC | 12 |
| Laser pointing to visual target while viewing it through a monocular lens | ☒Before ☒Early ☒Late ☒After |
Summary: Anterior IPL and cerebellum activated during early and late phase of PA | Weakness:
|
| Danckert et al., 2008 [64] | HC | 8 |
| Finger pointing to visual target while viewing through a monocular prism lens during the ON condition | ☐Before ☒Early ☒Late ☐After |
Summary: Anterior cingulate, anterior intraparietal sulcus and right medial cerebellum activated early during PA | Weakness:
|
| Küper et al., 2014 [53] | HC | 19 |
| Finger pointing to visual target while viewing through a monocular lens | ☒Before ☒Early ☒Late ☒After |
Summary: Strategic learning in PA associated with ventro-caudal dentate and posterior cerebellum activity; spatial realignment associated with superior posterior cerebellum | Strength:
Weakness:
|
| Luauté et al., 2009 [50] | HC | 11 |
| Finger pointing to visual target while viewing through a monocular lens | ☒Before ☒Early ☒Late ☒After |
Summary: PA activated STG/STS; Early PA activated right cerebellum, SPL, IPS, left IPS. Activity in anterior IPS modulated by error size; de-adaptation activated left IPL | Strength:
|
| Study | Participant Population | N | PA Procedure | Task in Scanner | Findings | Quality Assessment |
|---|---|---|---|---|---|---|
| Schintu et al., 2020 [67] | HC | 38 (18 used rightward prisms; 20 used leftward prisms) |
| Looking at a white central cross appearing on a black screen for an unspecified period of time | R
ight vs. left PA in decreasing rsFC between IPS seeds and …
After vs. before PA in rsFC between IPS seeds and …
| Strength:
Weakness:
|
| Tsujimoto et al., 2019 [56] | HC | 19 |
| 10 min with unspecified instructions |
| Strength:
Weakness:
|
| Tsujimoto et al., 2019 [65] | HC | 19 |
| 10 min with unspecified instructions |
| Strength:
Weakness:
|
| Wilf et al., 2019 [66] | HC | 26 (14 in the PA group; 12 in the control group) |
| Looking at a red fixation cross for 8 min | After vs. before PA in the PA group (n = 14)
| Strength:
Weakness:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukrina, O.; Chen, P. Neural Mechanisms of Prism Adaptation in Healthy Adults and Individuals with Spatial Neglect after Unilateral Stroke: A Review of fMRI Studies. Brain Sci. 2021, 11, 1468. https://doi.org/10.3390/brainsci11111468
Boukrina O, Chen P. Neural Mechanisms of Prism Adaptation in Healthy Adults and Individuals with Spatial Neglect after Unilateral Stroke: A Review of fMRI Studies. Brain Sciences. 2021; 11(11):1468. https://doi.org/10.3390/brainsci11111468
Chicago/Turabian StyleBoukrina, Olga, and Peii Chen. 2021. "Neural Mechanisms of Prism Adaptation in Healthy Adults and Individuals with Spatial Neglect after Unilateral Stroke: A Review of fMRI Studies" Brain Sciences 11, no. 11: 1468. https://doi.org/10.3390/brainsci11111468
APA StyleBoukrina, O., & Chen, P. (2021). Neural Mechanisms of Prism Adaptation in Healthy Adults and Individuals with Spatial Neglect after Unilateral Stroke: A Review of fMRI Studies. Brain Sciences, 11(11), 1468. https://doi.org/10.3390/brainsci11111468








