Relationship between Short-Range and Homotopic Long-Range Resting State Functional Connectivity in Temporal Lobes in Autism Spectrum Disorder
Abstract
:1. Introduction
2. Materials and Methods
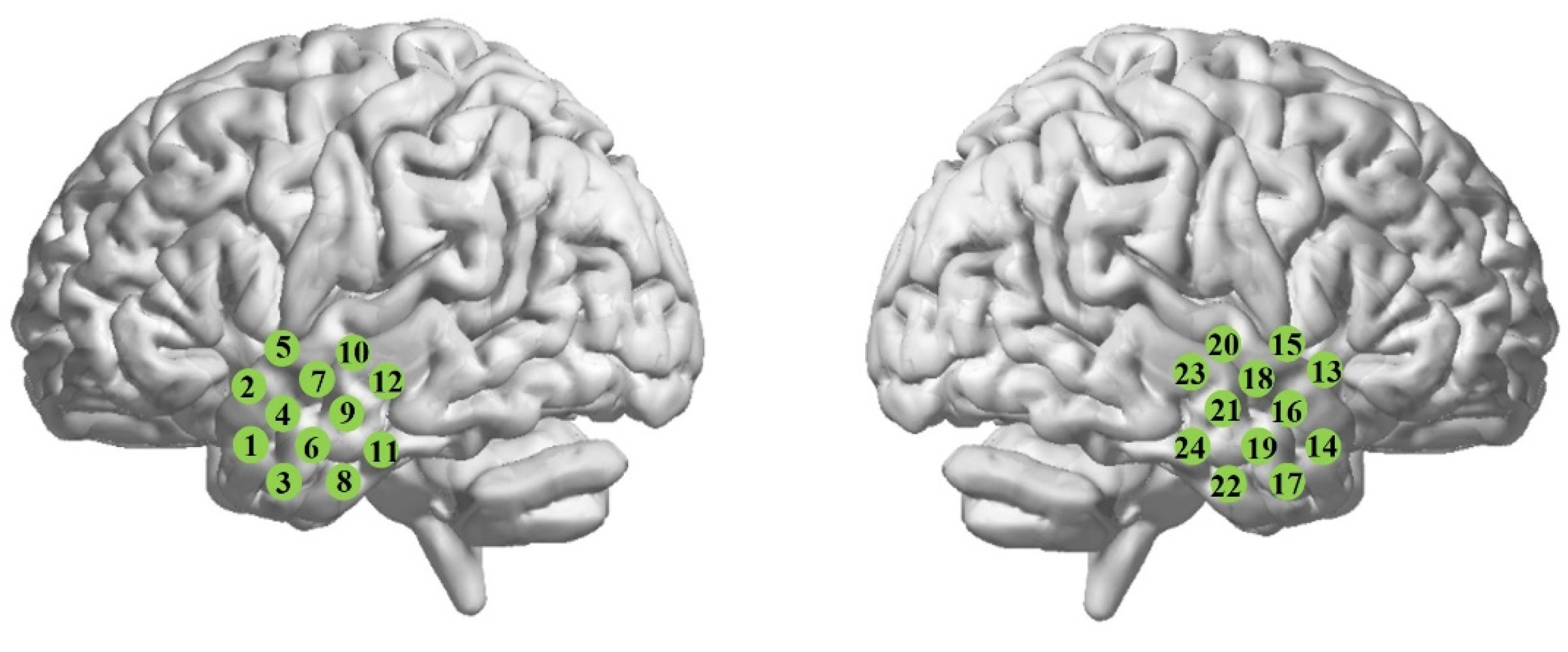
2.1. Experimental Protocol and Participants
2.2. Data Analysis
3. Results
3.1. Short-Range RSFC at the Temporal Lobes
3.2. The Difference between the Short-Range and Homotopic Long-Range RSFC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR; American Psychiatric Press: Washington, DC, USA, 2000. [Google Scholar]
- Maenner, M.J.; Shaw, K.A.; Baio, J. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. JMSS 2020, 69, 1–12. [Google Scholar]
- Hyman, S.L.; Levy, S.E.; Myers, S.M. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics 2020, 145, e20193448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Luyster, R.; Gotham, K.; Guthrie, W.; Coffing, M.; Petrak, R.; Pierce, K.; Bishop, S.; Esler, A.; Hus, V.; Oti, R.; et al. The Autism Diagnostic Observation Schedule-Toddler Module: A new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1305–1320. [Google Scholar]
- Vissers, M.E.; Cohen, M.X.; Geurts, H.M. Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci. Biobehav. Rev. 2012, 36, 604–625. [Google Scholar] [CrossRef]
- Long, Z.; Duan, X.; Mantini, D.; Chen, H. Alteration of functional connectivity in autism spectrum disorder: Effect of age and anatomical distance. Sci. Rep. 2016, 6, 26527. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Liu, X.; Yi, L.; Zhu, C.; Markey, P.S.; Pelowski, M. Assessing autism at its social and developmental roots: A review of Autism Spectrum Disorder studies using functional near-infrared spectroscopy. NeuroImage 2017, 185, 955–967. [Google Scholar]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Dinstein, I.; Pierce, K.; Eyler, L.; Solso, S.; Malach, R.; Behrmann, M.; Courchesne, E. Disrupted neural synchronization in toddlers with autism. Neuron 2011, 70, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Courchesne, E.; Pierce, K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005, 15, 225–230. [Google Scholar]
- Monk, C.S.; Peltier, S.J.; Wiggins, J.L.; Weng, S.-J.; Carrasco, M.; Risi, S.; Lord, C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage 2009, 47, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.S.; Druzgal, T.J.; Froehlich, A.; DuBray, M.B.; Lange, N.; Alexander, A.L.; Abildskov, T.; Nielsen, J.A.; Cariello, A.N.; Cooperrider, J.R.; et al. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex 2011, 21, 1134–1146. [Google Scholar] [CrossRef]
- Allison, T.; Puce, A.; McCarthy, G. Social perception from visual cues: Role of the STS region. Trends Cogn. Sci. 2000, 4, 267–278. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Passamonti, L.; Rowe, J.; Engell, A.D.; Calder, A.J. Connectivity analysis reveals a cortical network for eye gaze perception. Cereb. Cortex 2010, 20, 1780–1787. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qiu, L.; Xu, L.; Pedapati, E.V.; Erickson, C.A.; Sunar, U. Characterization of autism spectrum disorder with spontaneous hemodynamic activity. Biomed. Opt. Express 2016, 7, 3871–3881. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Fan, Y.; Guo, H.; Huang, D.; He, S. Reduced interhemispheric functional connectivity of children with autism spectrum disorder: Evidence from functional near infrared spectroscopy studies. Biomed. Opt. Express 2014, 5, 1262–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, M.; Yoshimura, Y.; Shitamichi, K.; Ueno, S.; Hiraishi, H.; Munesue, T.; Hirosawa, T.; Ono, Y.; Tsubokawa, T.; Inoue, Y. Anterior prefrontal hemodynamic connectivity in conscious 3-to 7-year-old children with typical development and autism spectrum disorder. PLoS ONE 2013, 8, e56087. [Google Scholar] [CrossRef]
- Keown, C.L.; Shih, P.; Nair, A.; Peterson, N.; Mulvey, M.E.; Müller, R.-A. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep. 2013, 5, 567–572. [Google Scholar] [PubMed] [Green Version]
- Maximo, J.O.; Keown, C.L.; Nair, A.; Müller, R.-A. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front. Neurosci. 2013, 7, 605. [Google Scholar]
- Dajani, D.R.; Uddin, L.Q. Local brain connectivity across development in autism spectrum disorder: A cross-sectional investigation. Autism. Res. 2016, 9, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Sepulcre, J.; Liu, H.; Talukdar, T.; Martincorena, I.; Yeo, B.T.; Buckner, R.L. The organization of local and distant functional connectivity in the human brain. PLoS Comput. Biol. 2010, 6, e1000808. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.; Court, J. Raven Manual: Section 3 in Standard Progressive Matrices; Oxford Psychologists Press: Oxford, UK, 1998. [Google Scholar]
- Song, M.; Zhou, Y.; Li, J.; Liu, Y.; Tian, L.; Yu, C.; Jiang, T. Brain spontaneous functional connectivity and intelligence. NeuroImage 2008, 41, 1168–1176. [Google Scholar] [PubMed]
- Pamplona, G.S.; Santos Neto, G.S.; Rosset, S.R.; Rogers, B.P.; Salmon, C.E. Analyzing the association between functional connectivity of the brain and intellectual performance. Front. Neurosci. 2015, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van, D.; Stam, C.J.; Kahn, R.S.; Pol, H.H. Efficiency of functional brain networks and intellectual performance. J. Neurosci. 2009, 29, 7619–7624. [Google Scholar]
- Schubert, A.L.; Hagemann, D.; Lffler, C.; Rummel, J.; Arnau, S.J. A chronometric model of the relationship between frontal midline theta functional connectivity and human intelligence. J. Exp. Psychol. General 2020, 150, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Langeslag, S.J.; Schmidt, M.; Ghassabian, A.; Jaddoe, V.W.; Hofman, A.; van der Lugt, A.; Verhulst, F.C.; Tiemeier, H.; White, T.J. Functional connectivity between parietal and frontal brain regions and intelligence in young children: The Generation R study. Hum. Brain. Mapp. 2013, 34, 3299–3307. [Google Scholar] [CrossRef]
- Molavi, B.; Dumont, G.A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 2012, 33, 259. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, 280–298. [Google Scholar] [CrossRef] [Green Version]
- Kohno, S.; Miyai, I.; Seiyama, A.; Oda, I.; Ishikawa, A.; Tsuneishi, S.; Amita, T.; Shimizu, K. Removal of the skin blood flow artifact in functional near-infrared spectroscopic imaging data through independent component analysis. J. Biomed. Opt. 2007, 12, 062111. [Google Scholar] [CrossRef]
- Garfin, S.R.; Roux, R.; Botte, M.J.; Centeno, R.; Woo, S.L.-Y. Skull osteology as it affects halo pin placement in children. J. Pediatr. Orthoped. 1986, 6, 434–436. [Google Scholar]
- Sharma, S.D.; Park, E.; Purcell, P.L.; Gordon, K.A.; Papsin, B.C.; Cushing, S.L. Age-related variability in pediatric scalp thickness: Implications for auditory prostheses. Int. J. Pediatr. Otorhi. 2020, 130, 109853. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Becker, B.; Jiang, X.; Zhao, Z.; Zhang, Q.; Yao, S.; Kendrick, K.M. Decreased interhemispheric functional connectivity rather than corpus callosum volume as a potential biomarker for autism spectrum disorder. Cortex 2019, 119, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Yan, C.-G.; Li, Q.; Denio, E.; Castellanos, F.X.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Bookheimer, S.Y.; Dapretto, M. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 2014, 19, 659–667. [Google Scholar] [CrossRef] [PubMed]



| Factor | df | Partial η2 | F-Value | p-Value | (1-β) Value |
|---|---|---|---|---|---|
| diagnosis | (1, 45) | 0.329 | 22.074 | <0.001 | 0.996 |
| hemisphere region | (1, 45) | 0.0002 | 0.010 | 0.923 | 0.051 |
| hemisphere region–diagnosis interaction | (1, 45) | 0.004 | 0.199 | 0.658 | 0.072 |
| Factor | df | Partial η2 | F-Value | P-Value | (1-β) Value |
|---|---|---|---|---|---|
| diagnosis | (1, 45) | 0.441 | 35.467 | <0.001 | 0.999 |
| RSFC type | (1, 45) | 0.245 | 14.616 | <0.001 | 0.962 |
| RSFC type–diagnosis interaction | (1, 45) | 0.240 | 14.201 | <0.001 | 0.958 |
| Factor | df | Partial η2 | F-Value | p-Value | (1-β) Value |
|---|---|---|---|---|---|
| diagnosis | (1, 45) | 0.148 | 7.837 | 0.008 | 0.782 |
| functional connectivity type | (1, 45) | 0.300 | 19.299 | <0.001 | 0.990 |
| functional connectivity type-by-diagnosis interaction | (1, 45) | 0.041 | 1.910 | 0.174 | 0.272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Lin, F.; Sun, W.; Zhang, T.; Sun, H.; Li, J. Relationship between Short-Range and Homotopic Long-Range Resting State Functional Connectivity in Temporal Lobes in Autism Spectrum Disorder. Brain Sci. 2021, 11, 1467. https://doi.org/10.3390/brainsci11111467
Wu X, Lin F, Sun W, Zhang T, Sun H, Li J. Relationship between Short-Range and Homotopic Long-Range Resting State Functional Connectivity in Temporal Lobes in Autism Spectrum Disorder. Brain Sciences. 2021; 11(11):1467. https://doi.org/10.3390/brainsci11111467
Chicago/Turabian StyleWu, Xiaoyin, Fang Lin, Weiting Sun, Tingzhen Zhang, Huiwen Sun, and Jun Li. 2021. "Relationship between Short-Range and Homotopic Long-Range Resting State Functional Connectivity in Temporal Lobes in Autism Spectrum Disorder" Brain Sciences 11, no. 11: 1467. https://doi.org/10.3390/brainsci11111467
APA StyleWu, X., Lin, F., Sun, W., Zhang, T., Sun, H., & Li, J. (2021). Relationship between Short-Range and Homotopic Long-Range Resting State Functional Connectivity in Temporal Lobes in Autism Spectrum Disorder. Brain Sciences, 11(11), 1467. https://doi.org/10.3390/brainsci11111467






