Brain Substrates for Distinct Spatial Processing Components Contributing to Hemineglect in Humans
Abstract
:1. Introduction
2. Experiment 1
2.1. Methods
2.1.1. Participants
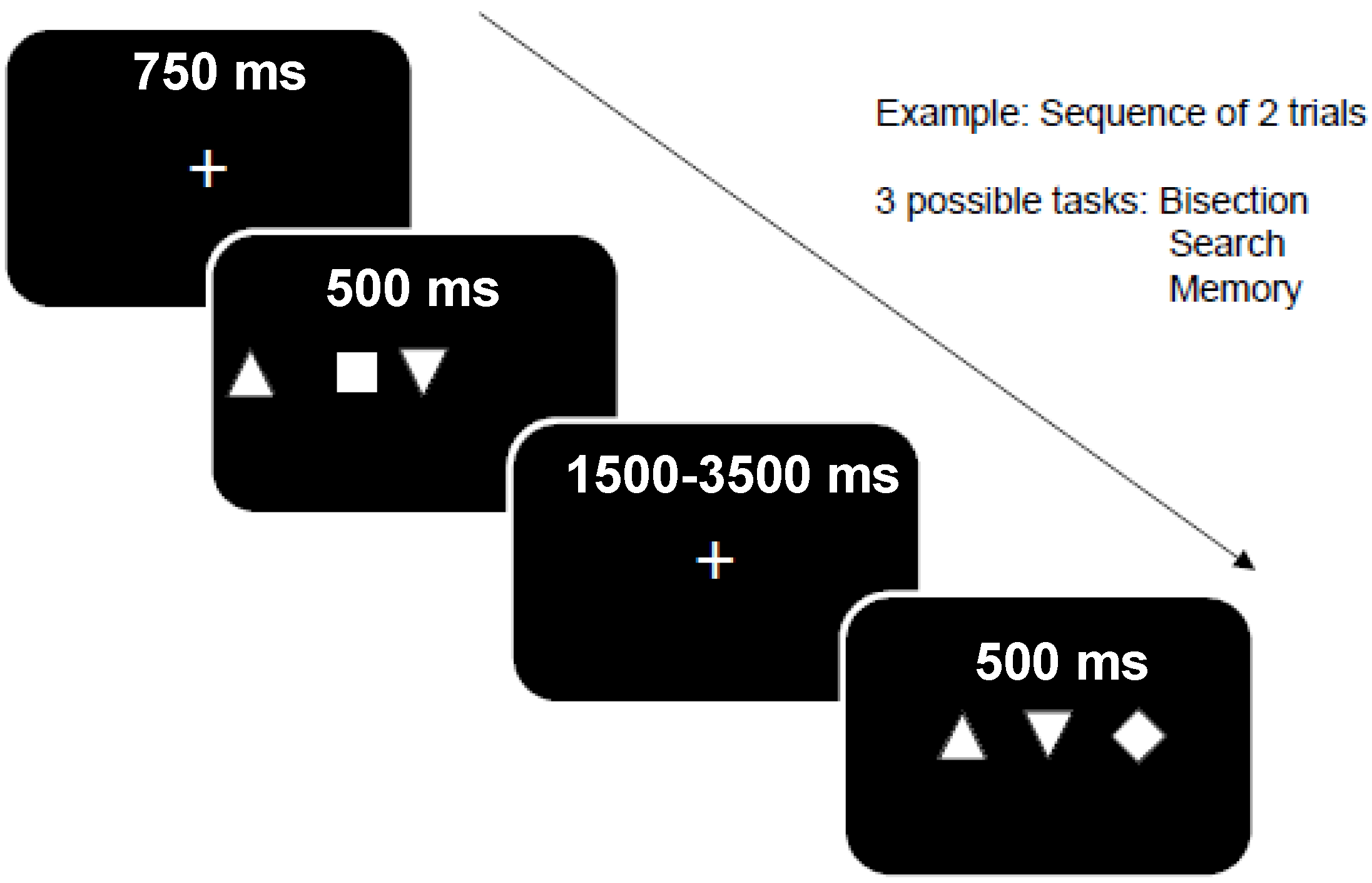
2.1.2. Behavioral “Triplet Task” Design during fMRI
- (i)
- Bisection task: participants must indicate whether the central item is located at the midpoint between the two others, or not (response: yes or no).
- (ii)
- Visual search task: participants must indicate whether the single-odd item in triplets is a square or a diamond (response: square or diamond).
- (iii)
- Memory task: participants must indicate whether the three items appeared at the same location as in the preceding trial, or not (response: same vs. different). Successive trials were constrained such that the positions of items on trial n corresponded to positions on trial n − 1 on 50% of all trials (one-back task), irrespective of the actual shape (square, diamond, triangle) of items.
2.1.3. Acquisition of fMRI Data
2.1.4. Analysis of fMRI Data
2.2. Results
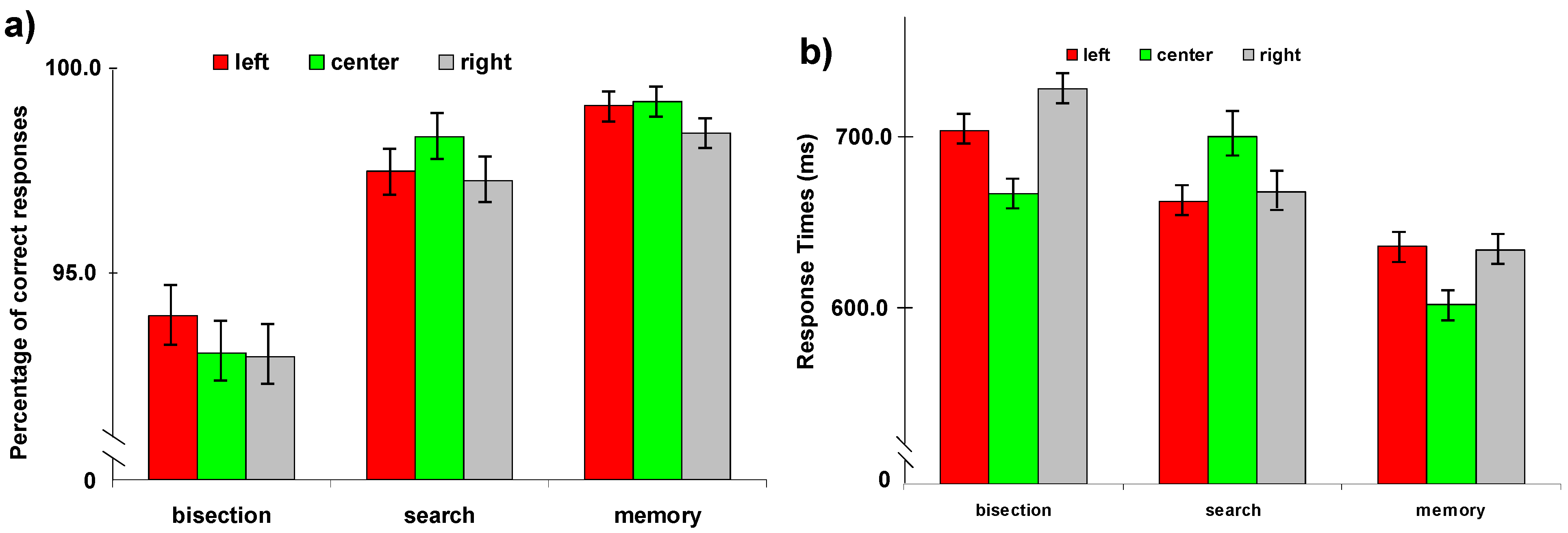
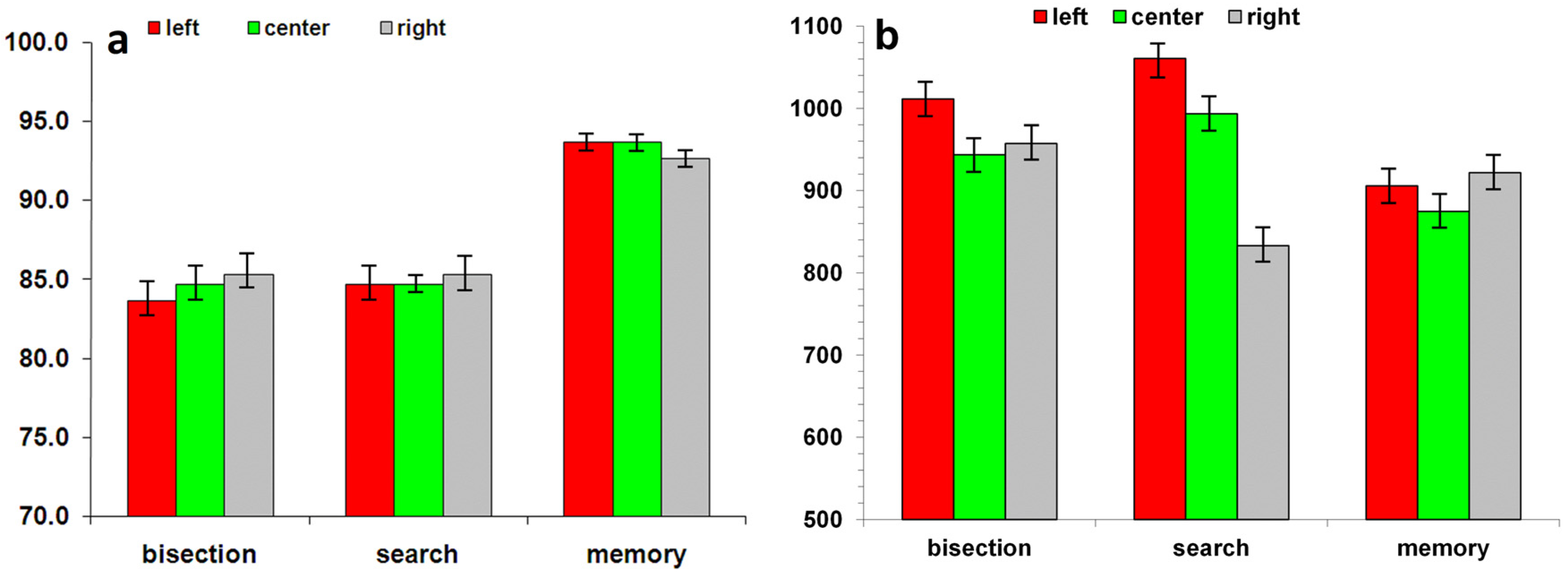
2.2.1. Behavioral Performance during fMRI Scanning
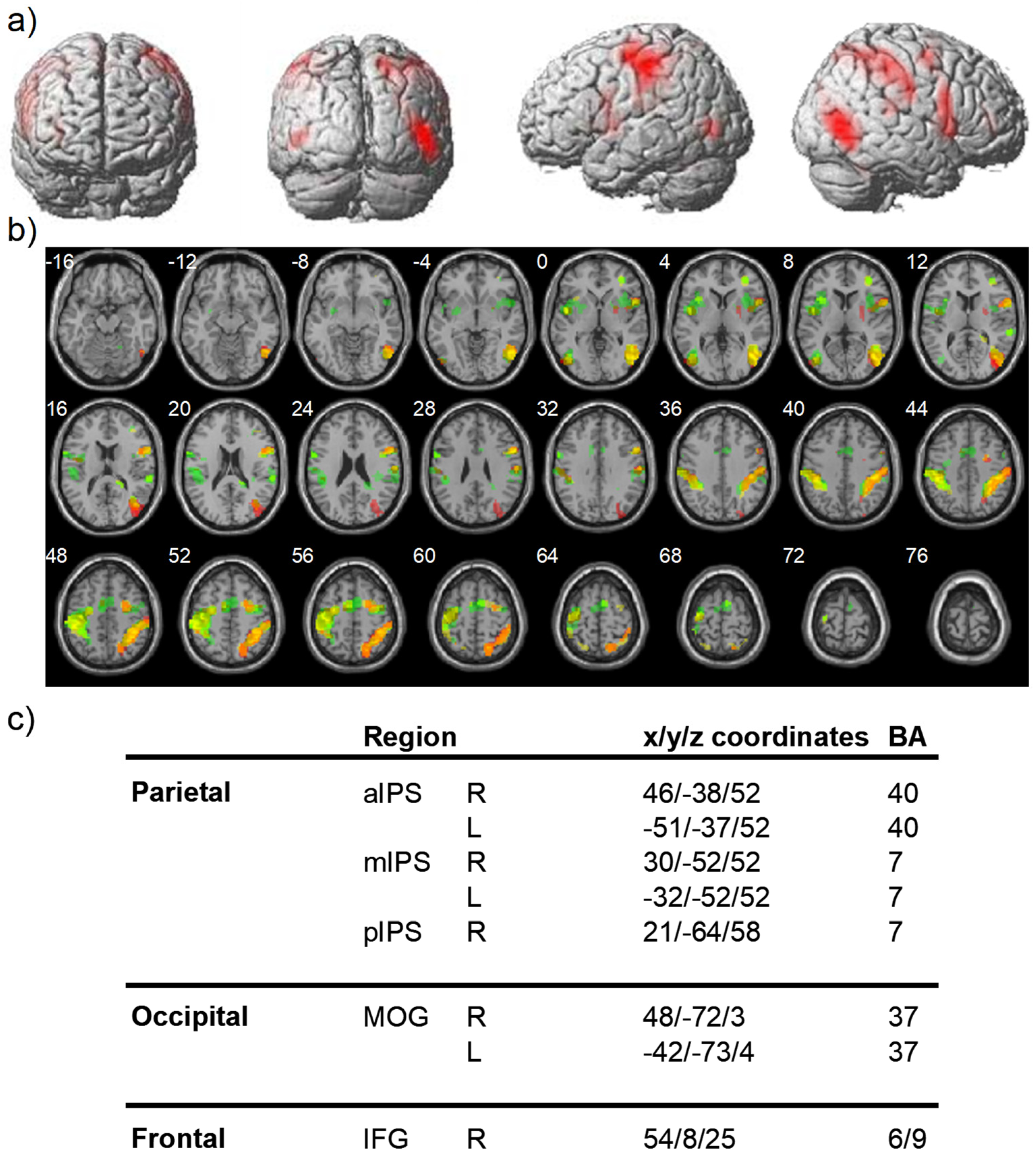
2.2.2. Event-Related fMRI Results
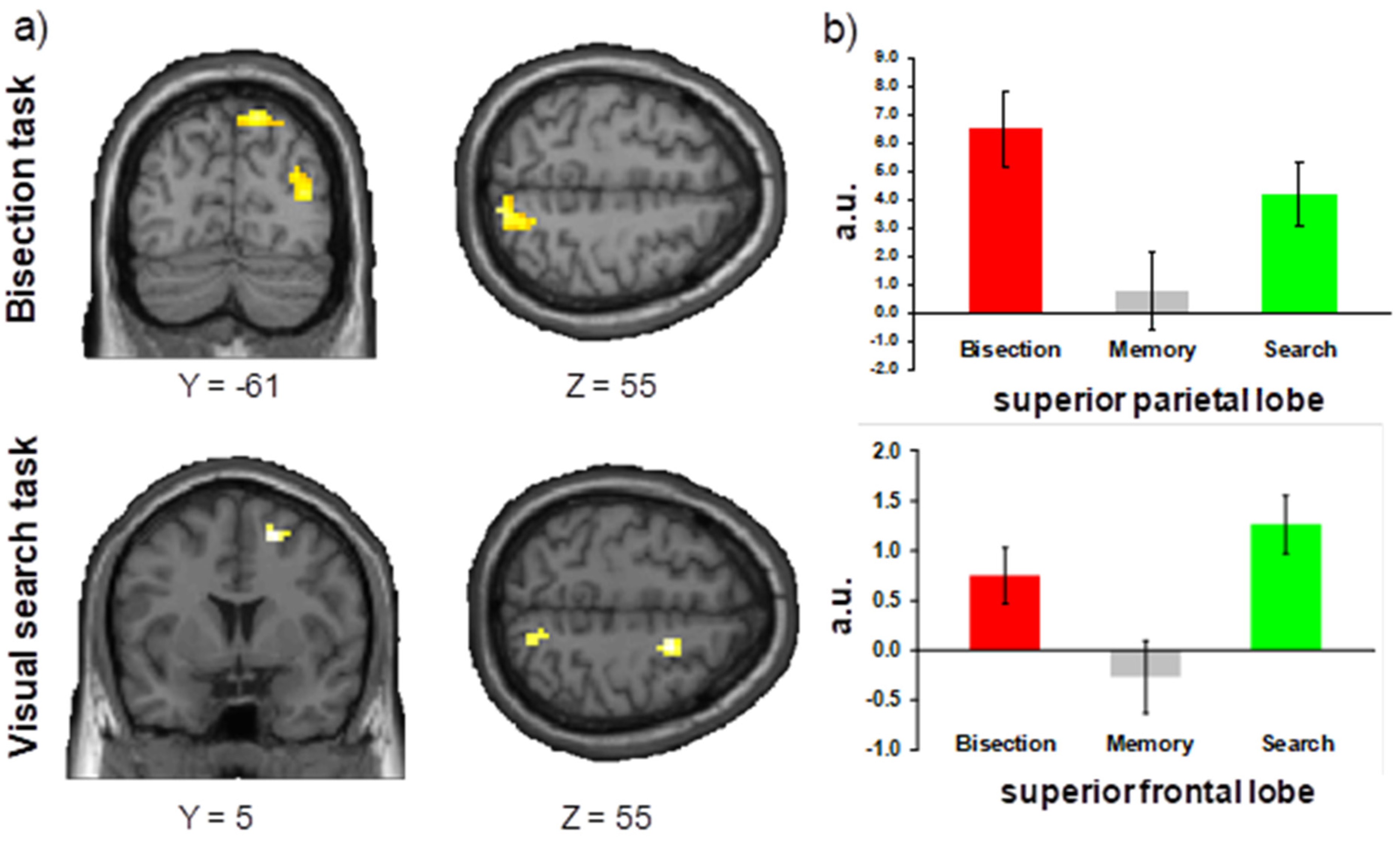
Bisection Task
Visual Search Task
Spatial Memory Task
3. Experiment 2
3.1. Methods
3.1.1. Participants
3.1.2. Brain Imaging and Lesion Analysis
3.1.3. Behavioral “Triplet Task”
3.2. Results
3.2.1. Behavioral Performance
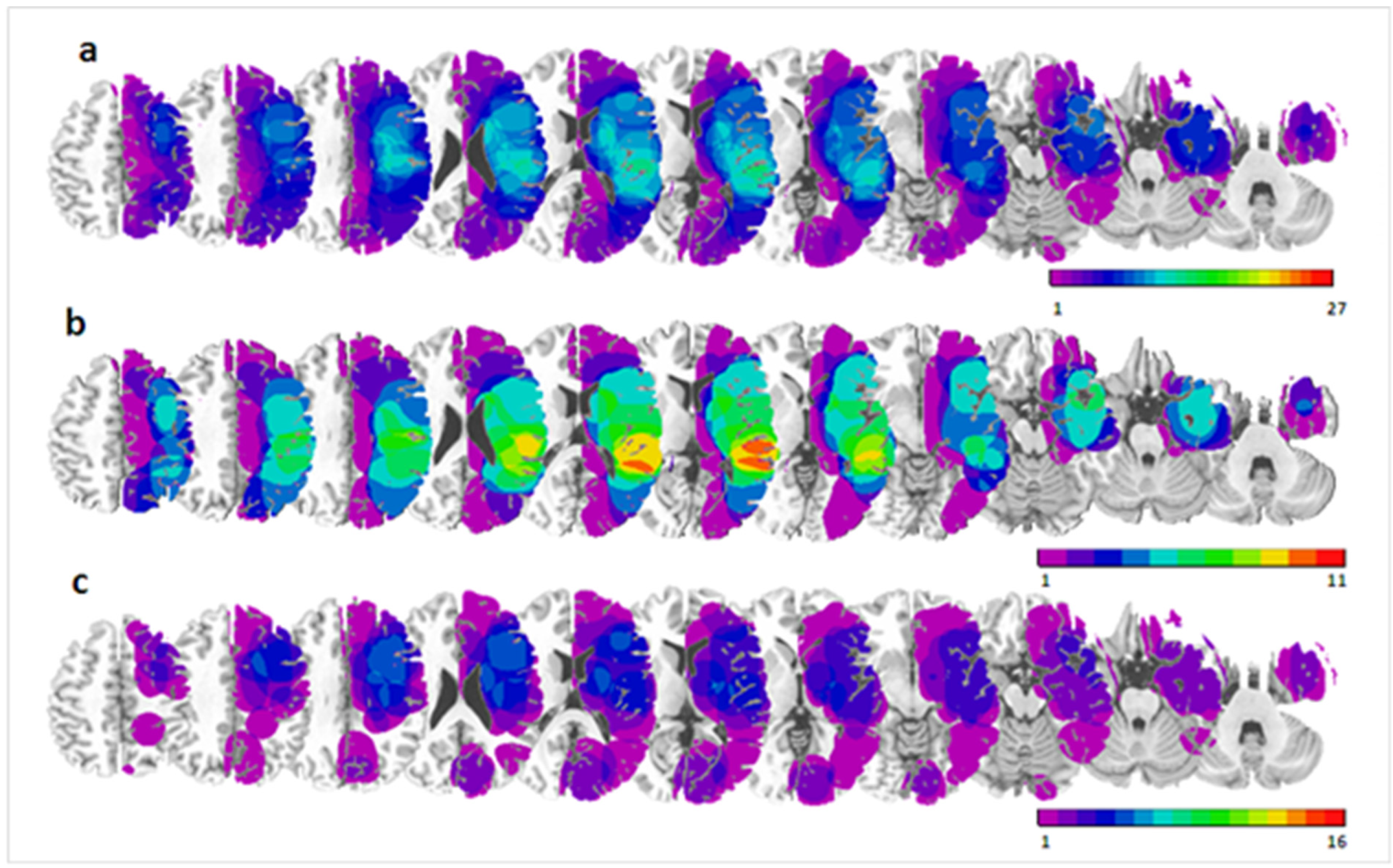
3.2.2. VLSM Analysis
Bisection Task
Visual Search Task
Spatial Memory Task
4. Discussion
4.1. Bisection Task
4.2. Visual Search Task
4.3. Attentional Network
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mesulam, M.-M. Large-Scale Neurocognitive Networks and Distributed Processing for Attention, Language, and Memory. Ann. Neurol. 1990, 28, 597–613. [Google Scholar] [CrossRef]
- Posner, M.I.; Petersen, S.E. The Attention System of the Human Brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- LaBar, K.S.; Gitelman, D.R.; Parrish, T.B.; Mesulam, M.-M. Neuroanatomic Overlap of Working Memory and Spatial Attention Networks: A Functional MRI Comparison within Subjects. NeuroImage 1999, 10, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbetta, M.; Akbudak, E.; Conturo, T.E.; Snyder, A.Z.; Ollinger, J.M.; Drury, H.A.; Linenweber, M.R.; Petersen, S.E.; Raichle, M.E.; Van Essen, D.C.; et al. A Common Network of Functional Areas for Attention and Eye Movements. Neuron 1998, 21, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Shulman, G.L. Spatial Neglect and Attention Networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef] [Green Version]
- Verdon, V.; Schwartz, S.; Lovblad, K.-O.; Hauert, C.-A.; Vuilleumier, P. Neuroanatomy of Hemispatial Neglect and Its Functional Components: A Study Using Voxel-Based Lesion-Symptom Mapping. Brain 2010, 133, 880–894. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Kincade, J.M.; Shulman, G.L. Neural Systems for Visual Orienting and Their Relationships to Spatial Working Memory. J. Cogn. Neurosci. 2002, 14, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Gitelman, D.R.; Nobre, A.C.; Parrish, T.B.; LaBar, K.S.; Kim, Y.-H.; Meyer, J.R.; Mesulam, M.-M. A Large-Scale Distributed Network for Covert Spatial Attention: Further Anatomical Delineation Based on Stringent Behavioural and Cognitive Controls. Brain 1999, 122, 1093–1106. [Google Scholar] [CrossRef] [Green Version]
- Donner, T.H.; Kettermann, A.; Diesch, E.; Ostendorf, F.; Villringer, A.; Brandt, S.A. Visual Feature and Conjunction Searches of Equal Difficulty Engage Only Partially Overlapping Frontoparietal Networks. NeuroImage 2002, 15, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Donner, T.H.; Kettermann, A.; Diesch, E.; Villringer, A.; Brandt, S.A. Parietal Activation during Visual Search in the Absence of Multiple Distractors. NeuroReport 2003, 14, 2257–2261. [Google Scholar] [CrossRef]
- Nobre, A.C.; Coull, J.T.; Walsh, V.; Frith, C.D. Brain Activations during Visual Search: Contributions of Search Efficiency versus Feature Binding. NeuroImage 2003, 18, 91–103. [Google Scholar] [CrossRef]
- Fink, G.R.; Marshall, J.C.; Shah, N.J.; Weiss, P.H.; Halligan, P.W.; Grosse-Ruyken, M.; Ziemons, K.; Zilles, K.; Freund, H.-J. Line Bisection Judgments Implicate Right Parietal Cortex and Cerebellum as Assessed by FMRI. Neurology 2000, 54, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.E. Prefrontal and Parietal Contributions to Spatial Working Memory. Neuroscience 2006, 139, 173–180. [Google Scholar] [CrossRef]
- Mechelli, A.; Henson, R.N.A.; Price, C.J.; Friston, K.J. Comparing Event-Related and Epoch Analysis in Blocked Design FMRI. NeuroImage 2003, 18, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Vandenberghe, R.; Gillebert, C.R. Parcellation of Parietal Cortex: Convergence between Lesion-Symptom Mapping and Mapping of the Intact Functioning Brain. Behav. Brain Res. 2009, 199, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Mort, D.J.; Malhotra, P.; Mannan, S.K.; Rorden, C.; Pambakian, A.; Kennard, C.; Husain, M. The Anatomy of Visual Neglect. Brain 2003, 126, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Saj, A.; Cojan, Y.; Vocat, R.; Luauté, J.; Vuilleumier, P. Prism Adaptation Enhances Activity of Intact Fronto-Parietal Areas in Both Hemispheres in Neglect Patients. Cortex 2013, 49, 107–119. [Google Scholar] [CrossRef]
- Chechlacz, M.; Rotshtein, P.; Bickerton, W.-L.; Hansen, P.C.; Deb, S.; Humphreys, G.W. Separating Neural Correlates of Allocentric and Egocentric Neglect: Distinct Cortical Sites and Common White Matter Disconnections. Cogn. Neuropsychol. 2010, 27, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, M.; Beis, J.M.; Pradat-Diehl, P.; Martin, Y.; Bartolomeo, P.; Bernati, T.; Chokron, S.; Leclercq, M.; Louis-Dreyfus, A.; Marchal, F.; et al. Presenting a battery for assessing spatial neglect. Norms and effects of age, educational level, sex, hand and laterality. Rev. Neurol. (Paris) 2001, 157, 1385–1400. [Google Scholar] [PubMed]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Deidda, C.; Malhotra, P.; Crinion, J.T.; Merola, S.; Husain, M. A Deficit of Spatial Remapping in Constructional Apraxia after Right-Hemisphere Stroke. Brain 2010, 133, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Saj, A.; Verdon, V.; Vocat, R.; Vuilleumier, P. ‘The Anatomy Underlying Acute versus Chronic Spatial Neglect’ Also Depends on Clinical Tests. Brain 2012, 135, e207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuilleumier, P.; Schwartz, S.; Verdon, V.; Maravita, A.; Hutton, C.; Husain, M.; Driver, J. Abnormal Attentional Modulation of Retinotopic Cortex in Parietal Patients with Spatial Neglect. Curr. Biol. 2008, 18, 1525–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicek, M.; Deouell, L.; Knight, R. Brain Activity during Landmark and Line Bisection Tasks. Front. Hum. Neurosci. 2009, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Weiss, P.H.; Marshall, J.C.; Zilles, K.; Fink, G.R. Are Action and Perception in near and Far Space Additive or Interactive Factors? NeuroImage 2003, 18, 837–846. [Google Scholar] [CrossRef]
- Waberski, T.D.; Gobbelé, R.; Lamberty, K.; Buchner, H.; Marshall, J.C.; Fink, G.R. Timing of Visuo-Spatial Information Processing: Electrical Source Imaging Related to Line Bisection Judgements. Neuropsychologia 2008, 46, 1201–1210. [Google Scholar] [CrossRef]
- Vallar, G.; Lobel, E.; Galati, G.; Berthoz, A.; Pizzamiglio, L.; Le Bihan, D. A Fronto-Parietal System for Computing the Egocentric Spatial Frame of Reference in Humans. Exp. Brain Res. 1999, 124. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Lobel, E.; Vallar, G.; Berthoz, A.; Pizzamiglio, L.; Le Bihan, D. The Neural Basis of Egocentric and Allocentric Coding of Space in Humans: A Functional Magnetic Resonance Study. Exp. Brain Res. 2000, 133, 156–164. [Google Scholar] [CrossRef]
- Saj, A.; Cojan, Y.; Assal, F.; Vuilleumier, P. Prism Adaptation Effect on Neural Activity and Spatial Neglect Depend on Brain Lesion Site. Cortex J. Devoted Study Nerv. Syst. Behav. 2019, 119, 301–311. [Google Scholar] [CrossRef]
- Anderson, E.J.; Mannan, S.K.; Husain, M.; Rees, G.; Sumner, P.; Mort, D.J.; McRobbie, D.; Kennard, C. Involvement of Prefrontal Cortex in Visual Search. Exp. Brain Res. 2007, 180, 289–302. [Google Scholar] [CrossRef]
- Anderson, E.J.; Mannan, S.K.; Rees, G.; Sumner, P.; Kennard, C. Overlapping Functional Anatomy for Working Memory and Visual Search. Exp. Brain Res. 2010, 200, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Dassonville, P. Activation in a Frontoparietal Cortical Network Underlies Individual Differences in the Performance of an Embedded Figures Task. PLoS ONE 2011, 6, e20742. [Google Scholar] [CrossRef] [Green Version]
- Husain, M.; Kennard, C. Distractor-Dependent Frontal Neglect. Neuropsychologia 1997, 35, 829–841. [Google Scholar] [CrossRef]
- de Schotten, M.T.; Urbanski, M.; Duffau, H.; Volle, E.; Lévy, R.; Dubois, B.; Bartolomeo, P. Direct Evidence for a Parietal-Frontal Pathway Subserving Spatial Awareness in Humans. Science 2005, 309, 2226–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxbaum, L.J.; Ferraro, M.K.; Veramonti, T.; Farne, A.; Whyte, J.; Ladavas, E.; Frassinetti, F.; Coslett, H.B. Hemispatial Neglect: Subtypes, Neuroanatomy, and Disability. Neurology 2004, 62, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Ogden, J.A. MRI Brain Scan Analyses and Neuropsychological Profiles of Nine Patients with Persisting Unilateral Neglect. Neuropsychologia 2002, 40, 879–887. [Google Scholar] [CrossRef]
- Samuelsson, H.; Jensen, C.; Ekholm, S.; Naver, H.; Blomstrand, C. Anatomical and Neurological Correlates of Acute and Chronic Visuospatial Neglect Following Right Hemisphere Stroke* *Some of the Results of This Study Were Presented at: The 5th Nordic Meeting in Neuropsychology, Uppsala, Sweden, August 17-19, 1995. Cortex 1997, 33, 271–285. [Google Scholar] [CrossRef]
- Doricchi, F.; Tomaiuolo, F. The Anatomy of Neglect without Hemianopia: A Key Role for Parietal–Frontal Disconnection? NeuroReport 2003, 14, 2239–2243. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Thiebaut de Schotten, M.; Doricchi, F. Left Unilateral Neglect as a Disconnection Syndrome. Cereb. Cortex 2007, 17, 2479–2490. [Google Scholar] [CrossRef] [Green Version]
- Ptak, R.; Bourgeois, A.; Cavelti, S.; Doganci, N.; Schnider, A.; Iannotti, G.R. Discrete Patterns of Cross-Hemispheric Functional Connectivity Underlie Impairments of Spatial Cognition after Stroke. J. Neurosci. 2020, 40, 6638–6648. [Google Scholar] [CrossRef] [PubMed]
- Vaessen, M.; Saj, A.; Lovblad, K.; Gschwind, M.; Vuilleumier, P. Structural White-Matter Connections Mediating Distinct Behavioral Components of Spatial Neglect in Right Brain-Damaged Patients. Cortex 2016, 77, 54–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Spatial Neglect | No Neglect | |
|---|---|---|
| Age | 73.1 (12.1) | 64 (6.1) |
| Sex (F/M) | 7/4 | 6/10 |
| Etiology (isch/hem) | 9/2 | 14/2 |
| Mini Mental State (/27) | 24.9 (1.9) | 25.2 (1.0) |
| Bell cancellation (total omission) | 17.5 (4.6) | 3.0 (2.3) |
| Line bisection (% deviation) | 47.7 (20.9) | 4.7 (4.2) |
| Copy of scene (omission) | 1.8 (1.5) | 0.1 (0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojan, Y.; Saj, A.; Vuilleumier, P. Brain Substrates for Distinct Spatial Processing Components Contributing to Hemineglect in Humans. Brain Sci. 2021, 11, 1584. https://doi.org/10.3390/brainsci11121584
Cojan Y, Saj A, Vuilleumier P. Brain Substrates for Distinct Spatial Processing Components Contributing to Hemineglect in Humans. Brain Sciences. 2021; 11(12):1584. https://doi.org/10.3390/brainsci11121584
Chicago/Turabian StyleCojan, Yann, Arnaud Saj, and Patrik Vuilleumier. 2021. "Brain Substrates for Distinct Spatial Processing Components Contributing to Hemineglect in Humans" Brain Sciences 11, no. 12: 1584. https://doi.org/10.3390/brainsci11121584
APA StyleCojan, Y., Saj, A., & Vuilleumier, P. (2021). Brain Substrates for Distinct Spatial Processing Components Contributing to Hemineglect in Humans. Brain Sciences, 11(12), 1584. https://doi.org/10.3390/brainsci11121584





