The Neural Bases of Egocentric Spatial Representation for Extracorporeal and Corporeal Tasks: An fMRI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
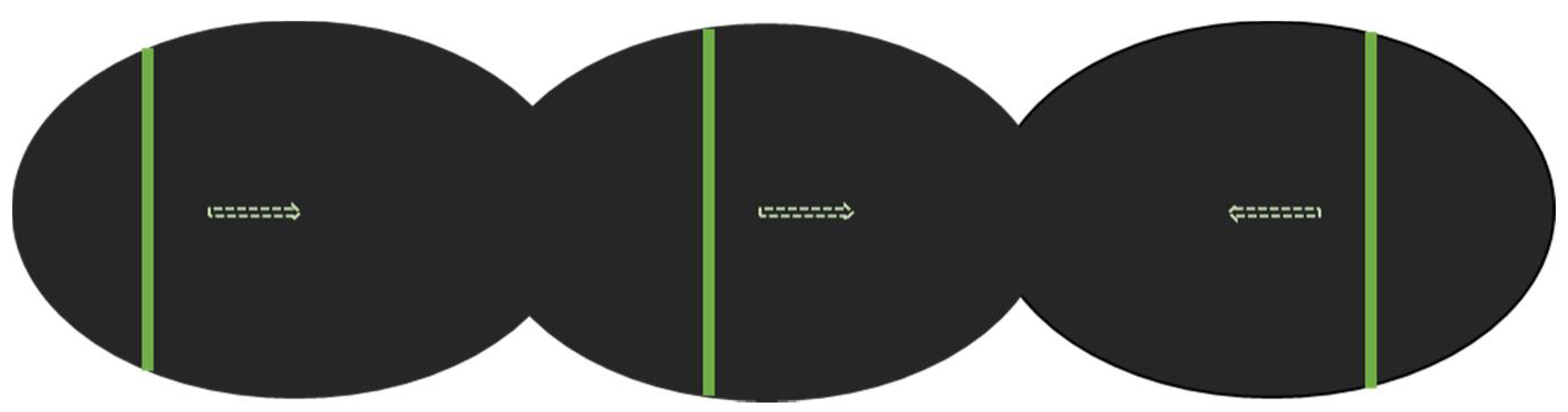
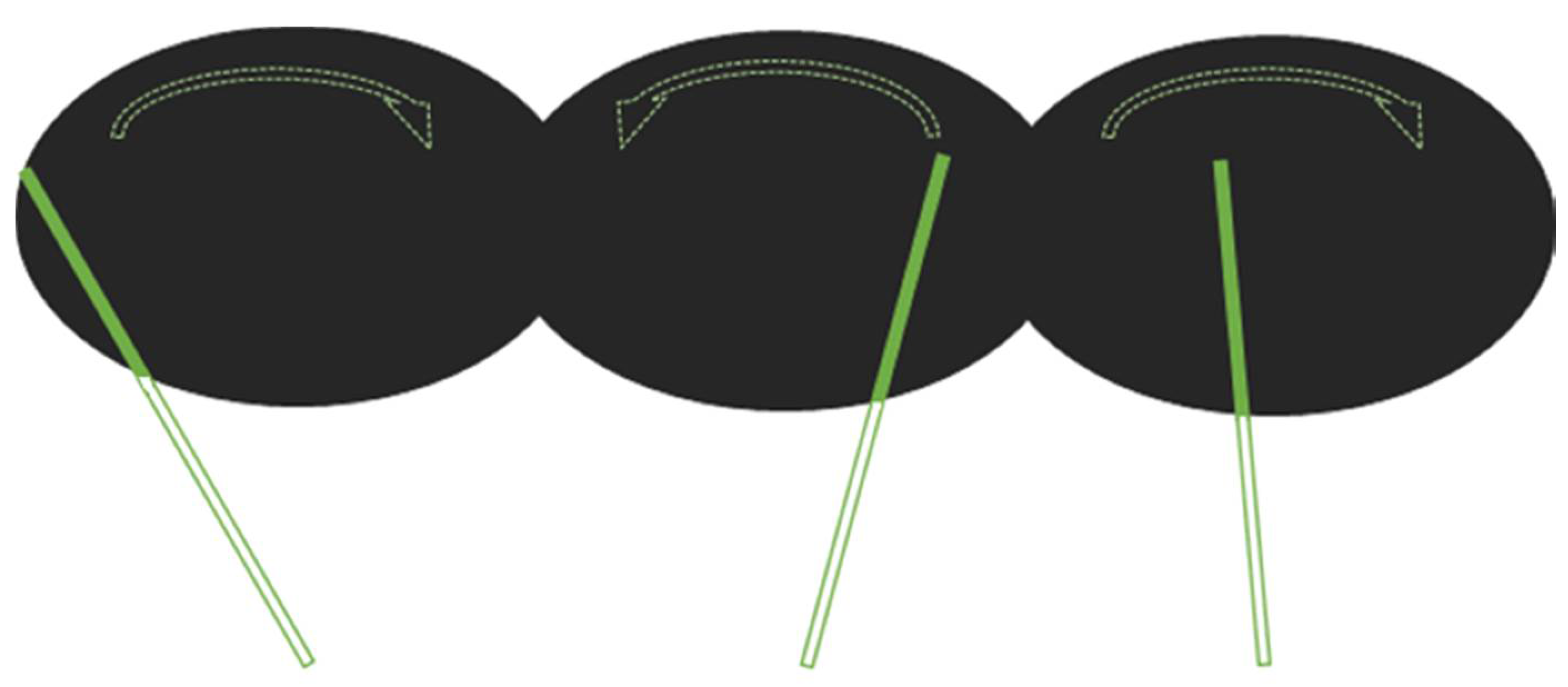
2.2. Experimental Design
2.3. MRI Acquisition
- -
- sagittal morphological isotropic 3D T1 MPRAGE sequence (repetition time (TR)/inversion time (TI)/echo time (TE): 1900/900/2.26 ms, field of view (FOV) 256 × 256 mm2, 160 slices, 1 × 1 × 1 mm3 voxel size);
- -
- field map with two echo times for distortion correction; and
- -
- two blood oxygen level dependent (BOLD) fMRI sequences using a single-shot T2*-weighted EPI sequence (TR/TE: 3000/36 ms, 210 × 210 mm2, FOV, 2 × 2 × 4 mm3, voxel size, 24 slices). Interleaved slices were acquired parallel to the anterior commissure posterior commissure line with no gap.
2.4. Statistical Modeling and Inference Analysis
3. Results
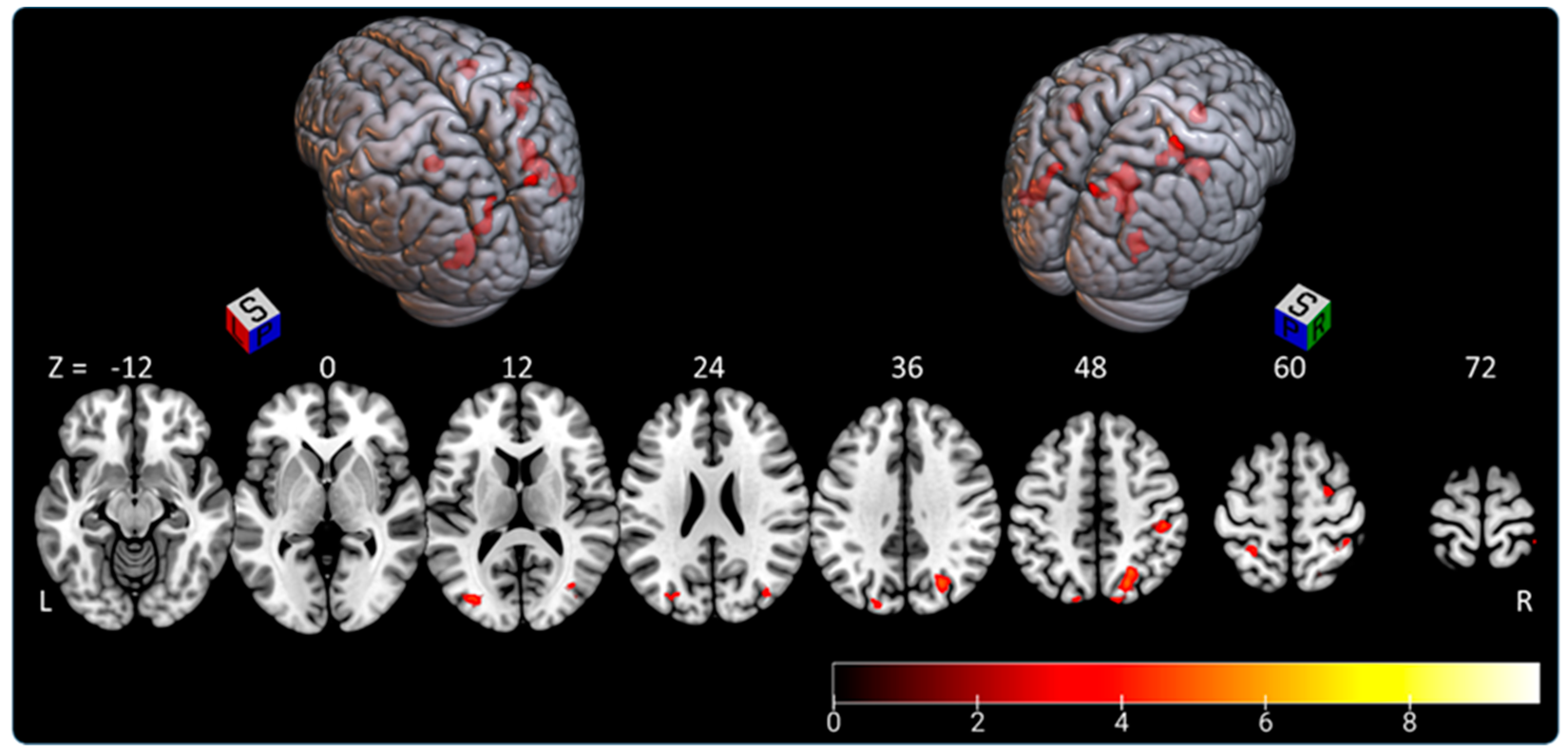
3.1. Subjective Straight-Ahead Task (SSA)
- -
- the right parietal lobe (superior parietal lobule: A7c, A5l, A7pc; inferior parietal lobule: A39c, A40rd, A40rv; precuneus: dmPOS; postcentral gyrus: A2) representing 45.5% of activated areas;
- -
- the right lateral occipital cortex (msOccG, lsOccG, mOccG), representing 21.5% of activated areas;
- -
- the left lateral occipital cortex (V5/MT+, msOccG, lsOccG, mOccG), representing 16% of activated areas;
- -
- the left parietal lobe (superior parietal lobule: A5l, A7pc; and inferior parietal lobule: A39c), representing 9.5% of activated areas; and
- -
- the right premotor cortex (superior frontal gyrus: A6cdl), representing 7.5% of activated areas.
3.2. Subjective Longitudinal Body Plane Task (SLB)
- -
- the right parietal lobe (superior parietal lobule: A7r, A7c, A5l, A7pc, A7ip; inferior parietal lobule: A39c, A40rd, A40rv; precuneus: dmPOS; postcentral gyrus: A1/2/3ulhf, A2, A1/2/3tru), representing 27% of activated areas;
- -
- the right lateral occipital cortex (V5/M+, msOccG, lsOccG, mOccG, iOccG), representing 22.5% of activated areas;
- -
- the right frontal lobe (predominant in the precentral gyrus: A4hf, A6cdl, A4ul, A4tl, A6cvl; superior frontal gyrus: A8m, A6dl, A6m; inferior frontal gyrus: A44d, A44op; paracentral gyrus: A4ll), representing 21% of activated areas;
- -
- the left lateral occipital cortex (V5/MT+, mOccG, iOccG), representing 8% of activated areas;
- -
- the left frontal lobe (supplementary motor area: A6m; precentral gyrus: A4hf, A6cdl, A4ul, A4t; and paracentral lobule: A4ll), representing 7.5% of activated areas; and
- -
- less than 5% in each of the following areas: left parietal lobe (superior parietal lobule: A5l, A7pc; inferior parietal lobule: A39c, A39rv; postcentral gyrus: A1/2/3ulhf, A2), right insular lobe (vIa, dIa, dIg, dId), left temporal lobe (middle temporal gyrus: A37dl; inferior temporal gyrus: A37vl; fusiform gyrus: A37lv), left cingulate gyrus, right temporal lobe (middle temporal gyrus: A37dl; inferior temporal gyrus: A37vl; fusiform gyrus: A37lv), and right cingulate gyrus.
3.3. Subjective Longitudinal Body Plane Task versus Subjective Straight-Ahead Task
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonan, I.V.; Marquer, A.; Eskiizmirliler, S.; Yelnik, A.P.; Vidal, P.-P. Sensory Reweighting in Controls and Stroke Patients. Clin. Neurophysiol. 2013, 124, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Rode, G.; Pérennou, D.; Azouvi, P. Spatial Cognition. Ann. Phys. Rehabil. Med. 2017, 60, 123. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Snyder, H. Coordinate Transformations in the Representation of Spatial Information. Curr. Opin. Neurobiol. 1993, 3, 171–176. [Google Scholar] [CrossRef]
- Galati, G.; Pelle, G.; Berthoz, A.; Committeri, G. Multiple Reference Frames Used by the Human Brain for Spatial Perception and Memory. Exp. Brain Res. 2010, 206, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Jamal, K.; Leplaideur, S.; Rousseau, C.; Chochina, L.; Moulinet-Raillon, A.; Bonan, I. Disturbances of Spatial Reference Frame and Postural Asymmetry after a Chronic Stroke. Exp. Brain Res. 2018, 236, 2377–2385. [Google Scholar] [CrossRef]
- Jeannerod, M.; Biguer, B. Egocentric reference and represented space. Rev. Neurol. 1989, 145, 635–639. [Google Scholar]
- Saj, A.; Cojan, Y.; Musel, B.; Honoré, J.; Borel, L.; Vuilleumier, P. Functional Neuro-Anatomy of Egocentric versus Allocentric Space Representation. Neurophysiol. Clin. Clin. Neurophysiol. 2014, 44, 33–40. [Google Scholar] [CrossRef]
- Ventre, J.; Flandrin, J.M.; Jeannerod, M. In Search for the Egocentric Reference. A Neurophysiological Hypothesis. Neuropsychologia 1984, 22, 797–806. [Google Scholar] [CrossRef]
- Zipser, D.; Andersen, R.A. A Back-Propagation Programmed Network That Simulates Response Properties of a Subset of Posterior Parietal Neurons. Nature 1988, 331, 679–684. [Google Scholar] [CrossRef]
- Chokron, S.; Colliot, P.; Atzeni, T.; Bartolomeo, P.; Ohlmann, T. Active versus Passive Proprioceptive Straight-Ahead Pointing in Normal Subjects. Brain Cogn. 2004, 55, 290–294. [Google Scholar] [CrossRef]
- Bartolomeo, P. Egocentric Frame of Reference: Its Role in Spatial Biasafter Right Hemisphere Lesions. Neuropsychologia 1999, 37, 881–894. [Google Scholar] [CrossRef]
- Rousseaux, M.; Honoré, J.; Saj, A. Body Representations and Brain Damage. Neurophysiol. Clin. Clin. Neurophysiol. 2014, 44, 59–67. [Google Scholar] [CrossRef]
- Barra, J.; Chauvineau, V.; Ohlmann, T.; Gresty, M.; Perennou, D. Perception of Longitudinal Body Axis in Patients with Stroke: A Pilot Study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Barra, J.; Benaim, C.; Chauvineau, V.; Ohlmann, T.; Gresty, M.; Pérennou, D. Are Rotations in Perceived Visual Vertical and Body Axis after Stroke Caused by the Same Mechanism? Stroke 2008, 39, 3099–3101. [Google Scholar] [CrossRef] [Green Version]
- Committeri, G.; Pitzalis, S.; Galati, G.; Patria, F.; Pelle, G.; Sabatini, U.; Castriota-Scanderbeg, A.; Piccardi, L.; Guariglia, C.; Pizzamiglio, L. Neural Bases of Personal and Extrapersonal Neglect in Humans. Brain 2007, 130, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caggiano, P.; Jehkonen, M. The ‘Neglected’ Personal Neglect. Neuropsychol. Rev. 2018, 28, 417–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Brink, A.F.; Biesbroek, J.M.; Oort, Q.; Visser-Meily, J.M.A.; Nijboer, T.C.W. Peripersonal and Extrapersonal Visuospatial Neglect in Different Frames of Reference: A Brain Lesion-Symptom Mapping Study. Behav. Brain Res. 2019, 356, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, F.; Ruggiero, G.; Raemaekers, M.; Iachini, T.; van der Ham, I.J.M.; Fracasso, A.; Postma, A. Neural Correlates of Egocentric and Allocentric Frames of Reference Combined with Metric and Non-Metric Spatial Relations. Neuroscience 2019, 409, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Honore, J.; Bernati, T.; Rousseaux, M. Straight-Ahead Pointing Correlates with Long-Line Bisection in Neglect Patients. Cortex 2004, 40, 75–83. [Google Scholar] [CrossRef]
- Vallar, G.; Lobel, E.; Galati, G.; Berthoz, A.; Pizzamiglio, L.; Le Bihan, D. A Fronto-Parietal System for Computing the Egocentric Spatial Frame of Reference in Humans. Exp. Brain Res. 1999, 124, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Barra, J.; Oujamaa, L.; Chauvineau, V.; Rougier, P.; Perennou, D. Asymmetric Standing Posture after Stroke Is Related to a Biased Egocentric Coordinate System. Neurology 2009, 72, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Penny, W.; Friston, K.; Ashburner, J.; Kiebel, S.; Nichols, T. Statistical Parametric Mapping: The Analysis of Functional Brain Images, 1st ed.; Academic Press: Cambridge, MA, USA, 2006; Available online: https://www.elsevier.com/books/statistical-parametric-mapping-the-analysis-of-functional-brain-images/penny/978-0-12-372560-8 (accessed on 13 January 2020).
- Boxerman, J.L.; Bandettini, P.A.; Kwong, K.K.; Baker, J.R.; Davis, T.L.; Rosen, B.R.; Weisskoff, R.M. The Intravascular Contribution to Fmri Signal Change: Monte Carlo Modeling and Diffusion-Weighted Studies in Vivo. Magn. Reson. Med. 1995, 34, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Slotnick, S.D. Resting-State FMRI Data Reflects Default Network Activity Rather than Null Data: A Defense of Commonly Employed Methods to Correct for Multiple Comparisons. Cogn. Neurosci. 2017, 8, 141–143. [Google Scholar] [CrossRef]
- Slotnick, S.D. Cluster Success: FMRI Inferences for Spatial Extent Have Acceptable False-Positive Rates. Cogn. Neurosci. 2017, 8, 150–155. [Google Scholar] [CrossRef]
- Galati, G.; Committeri, G.; Sanes, J.N.; Pizzamiglio, L. Spatial Coding of Visual and Somatic Sensory Information in Body-Centred Coordinates: Body-Centred Spatial Coding of Multimodal Stimuli. Eur. J. Neurosci. 2001, 14, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Galati, G.; Lobel, E.; Vallar, G.; Berthoz, A.; Pizzamiglio, L.; Le Bihan, D. The Neural Basis of Egocentric and Allocentric Coding of Space in Humans: A Functional Magnetic Resonance Study. Exp. Brain Res. 2000, 133, 156–164. [Google Scholar] [CrossRef]
- Neggers, S.F.W.; Van der Lubbe, R.H.J.; Ramsey, N.F.; Postma, A. Interactions between Ego- and Allocentric Neuronal Representations of Space. NeuroImage 2006, 31, 320–331. [Google Scholar] [CrossRef]
- Zaehle, T.; Jordan, K.; Wüstenberg, T.; Baudewig, J.; Dechent, P.; Mast, F.W. The Neural Basis of the Egocentric and Allocentric Spatial Frame of Reference. Brain Res. 2007, 1137, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Lacadie, C.M.; Fulbright, R.K.; Constable, R.T.; Papademetris, X. More Accurate Talairach Coordinates for NeuroImaging Using Nonlinear Registration. NeuroImage 2008, 42, 717–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain; Thieme: New York, NY, USA, 1988; Available online: https://www.thieme.com/books-main/neurosurgery/product/414-co-planar-stereotaxic-atlas-of-the-human-brain (accessed on 10 January 2020).
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Schleicher, A.; Zilles, K. Cytoarchitecture of the Cerebral Cortex—More than Localization. NeuroImage 2007, 37, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Zilles, K. Architectonic Mapping of the Human Brain beyond Brodmann. Neuron 2015, 88, 1086–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K.; Amunts, K. Centenary of Brodmann’s Map—Conception and Fate. Nat. Rev. Neurosci. 2010, 11, 139–145. [Google Scholar] [CrossRef]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef]
- Andersen, R.A. Multimodal Integration for the Representation of Space in the Posterior Parietal Cortex. Philos. Trans. R. Soc. B Lond. Biol. Sci. 1997, 352, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Duhamel, J.-R.; Bremmer, F.; Ben Hamed, S.; Graf, W. Spatial Invariance of Visual Receptive Fields in Parietal Cortex Neurons. Nature 1997, 389, 845–848. [Google Scholar] [CrossRef]
- Genovesio, A.; Ferraina, S. Integration of Retinal Disparity and Fixation-Distance Related Signals toward an Egocentric Coding of Distance in the Posterior Parietal Cortex of Primates. J. Neurophysiol. 2004, 91, 2670–2684. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; DeAngelis, G.C.; Angelaki, D.E. Flexible Egocentric and Allocentric Representations of Heading Signals in Parietal Cortex. Proc. Natl. Acad. Sci. USA 2018, 115, E3305–E3312. [Google Scholar] [CrossRef] [Green Version]
- Evrard, H.C. The Organization of the Primate Insular Cortex. Front. Neuroanat. 2019, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galletti, C.; Battaglini, P.P.; Fattori, P. Parietal Neurons Encoding Spatial Locations in Craniotopic Coordinates. Exp. Brain Res. 1993, 96, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Grüsser, O.J.; Pause, M.; Schreiter, U. Localization and Responses of Neurones in the Parieto-Insular Vestibular Cortex of Awake Monkeys (Macaca Fascicularis). J. Physiol. 1990, 430, 537–557. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, J. Place Units in the Hippocampus of the Freely Moving Rat. Exp. Neurol. 1976, 51, 78–109. [Google Scholar] [CrossRef]
- Schneider, R.J.; Friedman, D.P.; Mishkin, M. A Modality-Specific Somatosensory Area within the Insula of the Rhesus Monkey. Brain Res. 1993, 621, 116–120. [Google Scholar] [CrossRef]
- Zacks, J.; Rypma, B.; Gabrieli, J.D.E.; Tversky, B.; Glover, G.H. Imagined Transformations of Bodies: An FMRI Investigation. Neuropsychologia 1999, 37, 1029–1040. [Google Scholar] [CrossRef]
- Seghier, M.L. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Rushworth, M.F.S.; Johansen-Berg, H.; Göbel, S.M.; Devlin, J.T. The Left Parietal and Premotor Cortices: Motor Attention and Selection. NeuroImage 2003, 20, S89–S100. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.-P.; Eugène, F.; Michon, P.-E.; Jackson, P.L. The Neural Network of Motor Imagery: An ALE Meta-Analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Nakata, H.; Hayashi, T.; Sakamoto, M.; Muraoka, T.; Uchida, Y.; Kanosue, K. Brain Activity during Motor Imagery of an Action with an Object: A Functional Magnetic Resonance Imaging Study. Neurosci. Res. 2013, 76, 150–155. [Google Scholar] [CrossRef]
- Lorey, B.; Pilgramm, S.; Bischoff, M.; Stark, R.; Vaitl, D.; Kindermann, S.; Munzert, J.; Zentgraf, K. Activation of the Parieto-Premotor Network Is Associated with Vivid Motor Imagery—A Parametric FMRI Study. PLoS ONE 2011, 6, e20368. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Weidner, R.; Vossel, S.; Weiss, P.H.; Fink, G.R. Neural Mechanisms of Attentional Reorienting in Three-Dimensional Space. J. Neurosci. 2012, 32, 13352–13362. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, G.; D’Errico, O.; Iachini, T. Development of Egocentric and Allocentric Spatial Representations from Childhood to Elderly Age. Psychol. Res. 2016, 80, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Serino, S.; Tuena, C.; Pedroli, E.; Dakanalis, A.; Cipresso, P.; Riva, G. Egocentric and Allocentric Spatial Reference Frames in Aging: A Systematic Review. Neurosci. Biobehav. Rev. 2017, 80, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Moffat, S.D.; Elkins, W.; Resnick, S.M. Age Differences in the Neural Systems Supporting Human Allocentric Spatial Navigation. Neurobiol. Aging 2006, 27, 965–972. [Google Scholar] [CrossRef] [PubMed]


| Cluster Voxels/Side | AAL3 % in the Area | Peak MNI Coordinates (x y z)/T Value | Brainnetome % in the Area |
|---|---|---|---|
| 226/right | 42.5 occipital sup 33.6 parietal sup | 24 −62 46/5.630 | 31.9 lsOccg 28.8 A7c |
| 92/left | 96.7 occipital mid | −30 −84 14/5.023 | 44.6 mOccG |
| 35.9 A39c | |||
| 81/right | 61.7 postcentral | 46 −28 42/5.440 | 69.1 A40rd |
| 29.6 supramarginal | 19.8 A2 | ||
| 66/right | 87.9 occipital mind | 42 −80 18/5.471 | 54.5 A39c 31.8 mOccG |
| 58/right | 55.2 postcentral 25.9 parietal inf 19.0 parietal sup | 38 −44 66/5.216 | 12.1 A7pc 60.3 A5l 19.0 A7ip |
| 40/right | 72.5 frontal sup 17.5 precentral | 26 −4 58/5.087 | 85.0 A6cdl 10.0 A6dl |
| 39/left | 76.9 occipital sup 23.1 parietal sup | −18 −86 38/4.506 | 10.3 msOccG 79.5 lsOccG |
| 31/left | 51.6 parietal sup | −26 −46 58/6.259 | 19.4 A7pc |
| 25.8 parietal inf 22.6 postcentral | 38.7 A5l |
| Cluster Voxels/Side | AAL3 % in the Area | Peak MNI Coordinates (x y z)/T Value | Brainnetome % in the Area |
|---|---|---|---|
| 565/right | 25.3 occipital mid 25.1 temporal mid 18.9 occipital sup 13.3 temporal inf | 44 −66 −6/10.448 | 16.6 mOccG 9.7 msOccG 8.5 lsOccG 38.1 V5/MT+ |
| 512/right | 59.4 postcentral 16.8 supramarginal 15.0 parietal sup | 50 −22 42/6.4996 | 18.8 A2 24.2 A40rd 15.4 A5l 11.1 A40rv |
| 240/right | 64.6 frontal sup | 36 −6 58/6.921 | 56.3 A6cdl |
| 15.0 precentral 11.3 frontal mid | 22.5 A6dl | ||
| 218/left | 59.2 occipital mid 20.2 temporal mid 18.4 occipital inf | −34 −78 10/6.0758 | 19.7 mOccG 45.4 V5/MT+ 11.0 A39c |
| 152/right-left | 59.9 supp motor area L 31.6 sup motor area R | −4 −12 58/6.0816 | 51.3 lA6m 26.3 rA6m |
| 107/right | 60.8 frontal inf oper 39.3 precentral | 58 12 30/6.2155 | 92.5 A6cvl |
| 78/right | 87.2 insula | 44 20 −6/5.5758 | 34.6 dla 44.9 dld 11.5 A44op |
| 55/left | 98.2 precentral | −40 −10 66/5.086 | 45.5 A6cdl 29.1 A4hf 18.2 A4ul |
| 52/left | 73.1 postcentral 26.9 parietal sup | −40 −44 62/4.699 | 36.5 A7pc 38.5 A5l 19.2 A2 |
| 45/right-left | 53.3 cingulate mid L 31.1 cingulate mid R 11.1 sup motor area L | −2 6 42/6.001 | 57.8 lA24cd 35.6 rA24cd |
| 31/left | 51.6 postcentral | −38 −18 46/5.1726 | 38.7 A1/2/3ulhf |
| 48.4 precentral | 41.9 A4hf |
| Cluster Voxels/Side | AAL3 % in the Area | PeakMNI Coordinates (x y z)/T Value | Brainnetome % in the Area |
|---|---|---|---|
| 28/left | 71.0 angular 7.1 parietal inf | −44 −66 50/4.9301 | 57.1 A39rd 7.1 A39rv |
| 25/left | 92.0 precentral | −30 −14 70/6.430 | 52.0 A6cdl |
| 40.0 A4ul |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leplaideur, S.; Moulinet-Raillon, A.; Duché, Q.; Chochina, L.; Jamal, K.; Ferré, J.-C.; Bannier, E.; Bonan, I. The Neural Bases of Egocentric Spatial Representation for Extracorporeal and Corporeal Tasks: An fMRI Study. Brain Sci. 2021, 11, 963. https://doi.org/10.3390/brainsci11080963
Leplaideur S, Moulinet-Raillon A, Duché Q, Chochina L, Jamal K, Ferré J-C, Bannier E, Bonan I. The Neural Bases of Egocentric Spatial Representation for Extracorporeal and Corporeal Tasks: An fMRI Study. Brain Sciences. 2021; 11(8):963. https://doi.org/10.3390/brainsci11080963
Chicago/Turabian StyleLeplaideur, Stephanie, Annelise Moulinet-Raillon, Quentin Duché, Lucie Chochina, Karim Jamal, Jean-Christophe Ferré, Elise Bannier, and Isabelle Bonan. 2021. "The Neural Bases of Egocentric Spatial Representation for Extracorporeal and Corporeal Tasks: An fMRI Study" Brain Sciences 11, no. 8: 963. https://doi.org/10.3390/brainsci11080963
APA StyleLeplaideur, S., Moulinet-Raillon, A., Duché, Q., Chochina, L., Jamal, K., Ferré, J.-C., Bannier, E., & Bonan, I. (2021). The Neural Bases of Egocentric Spatial Representation for Extracorporeal and Corporeal Tasks: An fMRI Study. Brain Sciences, 11(8), 963. https://doi.org/10.3390/brainsci11080963







