Measuring the Effects of Social Isolation and Dissatisfaction on Depressive Symptoms during the COVID-19 Pandemic: The Moderating Role of Sleep and Physical Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Measures
2.2.1. Social Isolation and Satisfaction
2.2.2. Sleep
2.2.3. Physical Activity
2.2.4. Depressive Symptoms
2.3. Statistical Analyses
3. Results
3.1. Descriptive Statistics
3.2. Regression Analyses
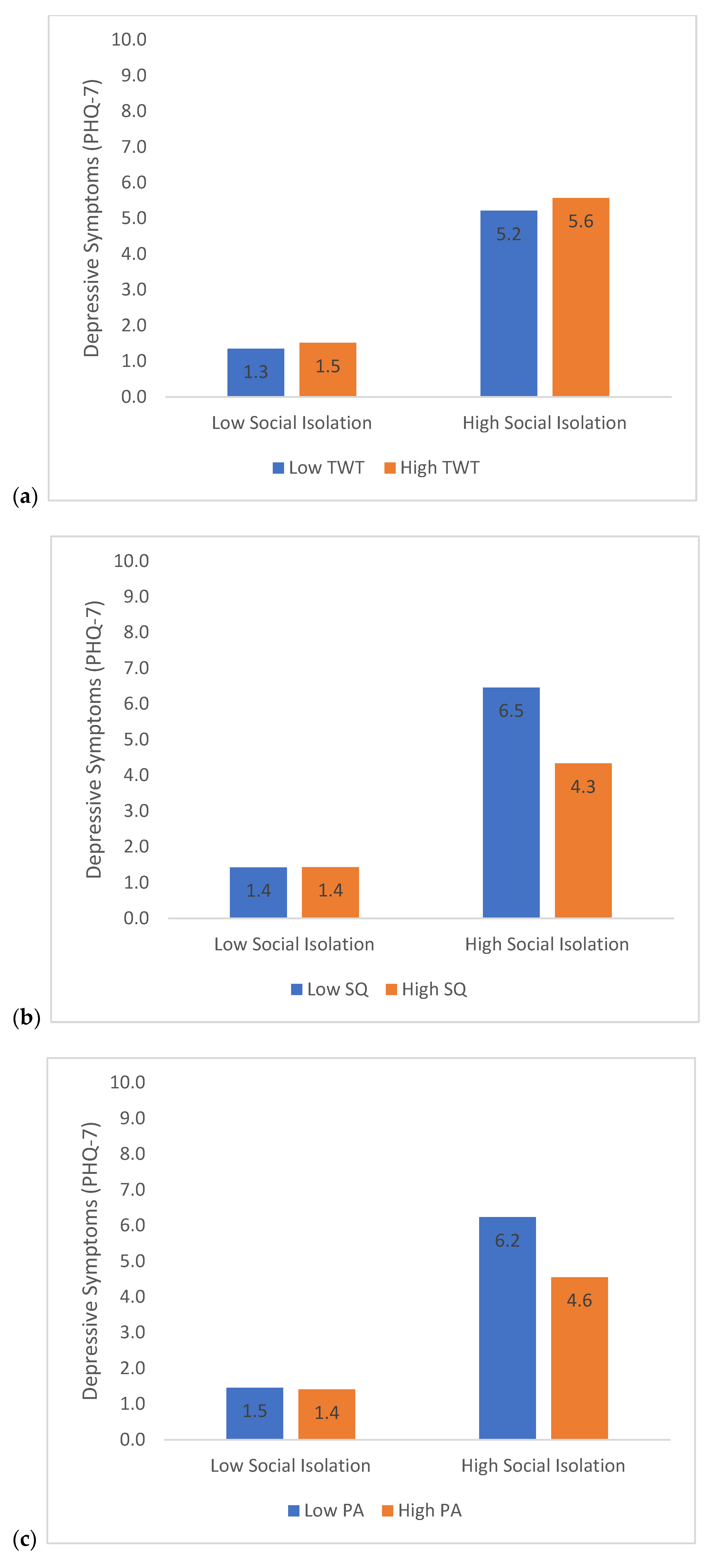
3.3. Moderation Analyses—Social Isolation
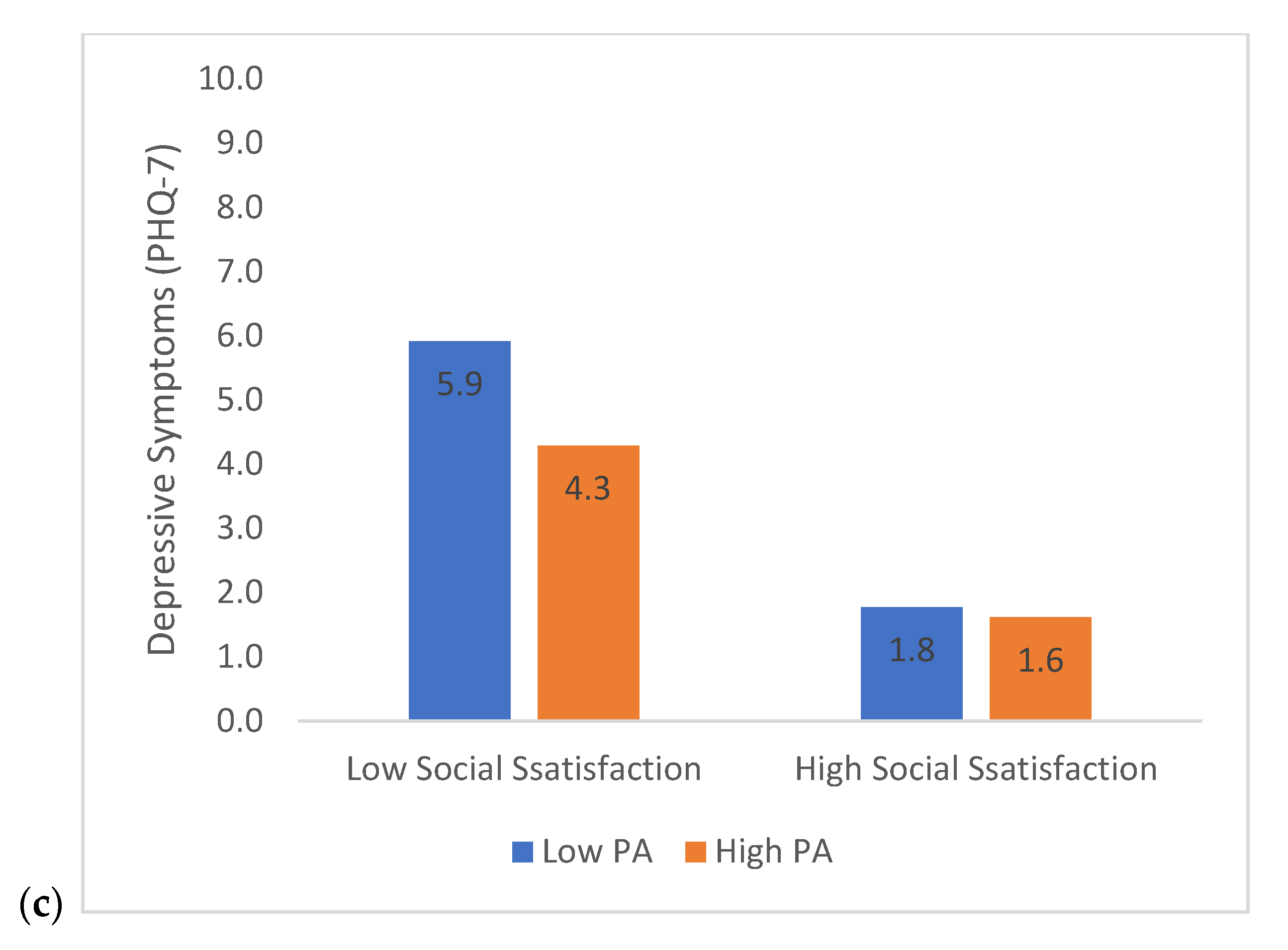
3.4. Moderation Analyses—Social Satisfaction
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huremović, D. Social Distancing, Quarantine, and Isolation. In Psychiatry Pandemics; Springer: Cham, Switzeland, 2019; pp. 85–94. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Lo, N.C. Scientific and ethical basis for social-distancing interventions against COVID-19. Lancet Infect. Dis. 2020, 20, 631–633. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M. Early Evidence on Social Distancing in Response to COVID-19 in the United States. 2020. Available online: https://ssrn.com/abstract=3569368 (accessed on 25 October 2021).
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mental Health and Psychosocial Considerations during the COVID-19 Outbreak 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/331490/WHO-2019-nCoV-MentalHealth-2020.1-eng.pdf (accessed on 17 June 2020).
- Hossain, M.; Tasnim, S.; Sultana, A.; Faizah, F.; Mazumder, H.; Zou, L.; McKyer, E.L.J.; Ahmed, H.U.; Ma, P. Epidemiology of mental health problems in COVID-19: A review. F1000Research 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Palsson, O.S.; Ballou, S.; Gray, S. The US National Pandemic Emotional Impact Report Page Index 2020. Available online: https://pandemicimpactreport.com/index.html (accessed on 25 October 2021).
- Pappa, S.; Ntella, V.; Giannakas, T.; Giannakoulis, V.G.; Papoutsi, E.; Katsaounou, P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 901–907. [Google Scholar] [CrossRef]
- Muller, A.E.; Hafstad, E.V.; Himmels, J.P.W.; Smedslund, G.; Flottorp, S.; Stensland, S.Ø.; Stroobants, S.; Van de Velde, S.; Vist, G.E. The mental health impact of the COVID-19 pandemic on healthcare workers, and interventions to help them: A rapid systematic review. Psychiatry Res. 2020, 293, 113441. [Google Scholar] [CrossRef]
- Hummel, S.; Oetjen, N.; Du, J.; Posenato, E.; de Almeida, R.M.R.; Losada, R.; Ribeiro, O.; Frisardi, V.; Hopper, L.; Rashid, A.; et al. Mental health among medical professionals during the COVID-19 pandemic in eight european countries: Cross-sectional survey study. J. Med. Internet Res. 2021, 23, e24983. [Google Scholar] [CrossRef] [PubMed]
- Santini, Z.I.; Koyanagi, A.; Tyrovolas, S.; Mason, C.; Haro, J.M. The association between social relationships and depression: A systematic review. J. Affect. Disord. 2015, 175, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.L.; Galliher, R.V. Predicting depression and self-esteem from social connectedness, support, and competence. J. Soc. Clin. Psychol. 2006, 25, 855–874. [Google Scholar] [CrossRef]
- Hom, M.A.; Chu, C.; Rogers, M.L.; Joiner, T.E. A Meta-Analysis of the Relationship between Sleep Problems and Loneliness. Clin. Psychol. Sci. 2020, 8, 799–824. [Google Scholar] [CrossRef]
- Grippo, A.J.; Gerena, D.; Huang, J.; Kumar, N.; Shah, M.; Ughreja, R.; Carter, C.S. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 2007, 32, 966–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkesman, O.; Maayan, R.; Weizman, A.; Weller, A. Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav. Brain Res. 2006, 175, 408–414. [Google Scholar] [CrossRef]
- Arrigo, B.A.; Bullock, J.L. The psychological effects of solitary confinement on prisoners in supermax units: Reviewing what we know and recommending what should change. Int. J. Offender. Ther. Comp. Criminol. 2008, 52, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.H.; Callagan, J.E.; Newman, A.F. Effect of solitary confinement on prisoners. Am. J. Psychiatry 1963, 119, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Buchman-Schmitt, J.M.; Stanley, I.H.; Hom, M.A.; Tucker, R.P.; Hagan, C.R.; Rogers, M.L.; Podlogar, M.C.; Chiurliza, B.; Ringer-Moberg, F.B.; et al. The interpersonal theory of suicide: A systematic review and meta-analysis of a decade of cross-national research. Psychol. Bull. 2017, 143, 1313–1345. [Google Scholar] [CrossRef]
- Opperman, K.; Czyz, E.K.; Gipson, P.Y.; King, C.A. Connectedness and Perceived Burdensomeness among Adolescents at Elevated Suicide Risk: An Examination of the Interpersonal Theory of Suicidal Behavior. Arch. Suicide Res. 2015, 19, 385–400. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; ASME Press: New York, NY, USA, 2018. [CrossRef]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Difrancesco, S.; Lamers, F.; Riese, H.; Merikangas, K.R.; Beekman, A.T.F.; van Hemert, A.M.; Schoevers, R.A.; Penninx, B.W.J.H. Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: A 2-week ambulatory assessment study. Depress. Anxiety 2019, 36, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Gan, Y.; Qu, X.; Lu, Z. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry 2016, 16, 375. [Google Scholar] [CrossRef] [Green Version]
- Adamson, B.C.; Yang, Y.; Motl, R.W. Association between compliance with physical activity guidelines, sedentary behavior and depressive symptoms. Prev. Med. 2016, 91, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeon, S.W.; Shin, D.W.; Oh, K.S.; Shin, Y.C.; Lim, S.W. Association between physical activity and depressive symptoms in general adult populations: An analysis of the dose-response relationship. Psychiatry Res. 2018, 269, 258–263. [Google Scholar] [CrossRef]
- Garfield, V.; Llewellyn, C.H.; Kumari, M. The relationship between physical activity, sleep duration and depressive symptoms in older adults: The English Longitudinal Study of Ageing (ELSA). Prev. Med. Rep. 2016, 4, 512–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebar, A.L.; Stanton, R.; Geard, D.; Short, C.; Duncan, M.J.; Vandelanotte, C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev. 2015, 9, 366–378. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.E.A.; Shapiro, C.M. Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: A systematic review. J. Psychosom. Res. 2018, 106, 1–12. [Google Scholar] [CrossRef]
- Kokou-Kpolou, C.K.; Megalakaki, O.; Laimou, D.; Kousouri, M. Insomnia during COVID-19 pandemic and lockdown: Prevalence, severity, and associated risk factors in French population. Psychiatry Res. 2020, 290, 113128. [Google Scholar] [CrossRef] [PubMed]
- Voitsidis, P.; Gliatas, I.; Bairachtari, V.; Papadopoulou, K.; Papageorgiou, G.; Parlapani, E.; Syngelakis, M.; Holeva, V.; Diakogiannis, I. Insomnia during the COVID-19 pandemic in a Greek population. Psychiatry Res. 2020, 289, 113076. [Google Scholar] [CrossRef] [PubMed]
- Cénat, J.M.; Blais-Rochette, C.; Kokou-Kpolou, C.K.; Noorishad, P.-G.; Mukunzi, J.N.; McIntee, S.-E.; Dalexis, R.D.; Goulet, M.-A.; Labelle, P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2021, 295, 113599. [Google Scholar] [CrossRef]
- Marelli, S.; Castelnuovo, A.; Somma, A.; Castronovo, V.; Mombelli, S.; Bottoni, D.; Leitner, C.; Fossati, A.; Ferini-Strambi, L. Impact of COVID-19 lockdown on sleep quality in university students and administration staff. J. Neurol. 2021, 268, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: Results of the ECLB-COVID19 international online survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Lesser, I.A.; Nienhuis, C.P. The impact of COVID-19 on physical activity behavior and well-being of canadians. Int. J. Environ. Res. Public Health 2020, 17, 3899. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Babarro, A.; Coca, A.; Arbillaga-Etxarri, A.; Gutiérrez-Santamaría, B. Physical activity change during COVID-19 confinement. Int. J. Environ. Res. Public Health 2020, 17, 6878. [Google Scholar] [CrossRef] [PubMed]
- Ben Simon, E.; Walker, M.P. Sleep loss causes social withdrawal and loneliness. Nat. Commun. 2018, 9, 3146. [Google Scholar] [CrossRef]
- Schrempft, S.; Jackowska, M.; Hamer, M.; Steptoe, A. Associations between social isolation, loneliness, and objective physical activity in older men and women. BMC Public Health 2019, 19, 74. [Google Scholar] [CrossRef] [Green Version]
- Werneck, A.O.; Collings, P.J.; Barboza, L.L.; Stubbs, B.; Silva, D.R. Associations of sedentary behaviors and physical activity with social isolation in 100,839 school students: The Brazilian Scholar Health Survey. Gen. Hosp. Psychiatry 2019, 59, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- IPAQ. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms. 2005. Available online: https://sites.google.com/site/theipaq/home (accessed on 25 October 2021).
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.W. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 605–613. [Google Scholar] [CrossRef]
- Williams, L.S.; Brizendine, E.J.; Plue, L.; Bakas, T.; Tu, W.; Hendrie, H.; Kroenke, K. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005, 36, 635–638. [Google Scholar] [CrossRef] [Green Version]
- Coiro, M.J.; Asraf, K.; Tzischinsky, O.; Hadar-Shoval, D.; Tannous-Haddad, L.; Wolfson, A.R. Sleep quality and COVID-19-related stress in relation to mental health symptoms among Israeli and U.S. adults. Sleep Health 2021, 7, 127–133. [Google Scholar] [CrossRef]
- Gu, S.; He, Z.; Sun, L.; Jiang, Y.; Xu, M.; Feng, G.; Ma, X.; Wang, F.; Huang, J.H. Effects of Coronavirus-19 Induced Loneliness on Mental Health: Sleep Quality and Intolerance for Uncertainty as Mediators. Front. Psychiatry 2021, 12, 1606. [Google Scholar] [CrossRef]
- Crew, E.C.; Baron, K.G.; Grandner, M.A.; Ievers-Landis, C.E.; McCrae, C.S.; Nadorff, M.R.; Nowakowski, S.; Margolies, S.O.; Hansen, K. The Society of Behavioral Sleep Medicine (SBSM) COVID-19 Task Force: Objectives and Summary Recommendations for Managing Sleep during a Pandemic. Behav. Sleep Med. 2020, 18, 570–572. [Google Scholar] [CrossRef]
- Altena, E.; Baglioni, C.; Espie, C.A.; Ellis, J.; Gavriloff, D.; Holzinger, B.; Schlarb, A.; Frase, L.; Jernelöv, S.; Riemann, D. Dealing with sleep problems during home confinement due to the COVID-19 outbreak: Practical recommendations from a task force of the European CBT-I Academy. J. Sleep Res. 2020, 29, e13052. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Storfer-Isser, A.; Taylor, H.G.; Rosen, C.L.; Redline, S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics 2009, 123, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Gruber, R.; Cassoff, J. The Interplay Between Sleep and Emotion Regulation: Conceptual Framework Empirical Evidence and Future Directions. Curr. Psychiatry Rep. 2014, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; Oosterhoff, B.; Bower, J.L.; Kaplow, J.B.; Alfano, C.A. Associations among adolescent sleep problems, emotion regulation, and affective disorders: Findings from a nationally representative sample. J. Psychiatr. Res. 2018, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wilckens, K.A.; Woo, S.G.; Kirk, A.R.; Erickson, K.I.; Wheeler, M.E. Role of sleep continuity and total sleep time in executive function across the adult lifespan. Psychol. Aging 2014, 29, 658–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernhart, S.; Weihe, E.; Rassaf, T. Reduced physical activity and weight gain are associated with an increase of depressive symptoms during the COVID-19 pandemic. A general practitioners’ prospective observational study. JRSM Cardiovasc. Dis. 2021, 10, 204800402110477. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Puyat, J.H.; Ranote, H.; Vila-Rodriguez, F.; Kazanjian, A. A cross-sectional survey of activities to support mental wellness during the COVID-19 pandemic. J Affect. Disord. Rep. 2021, 5, 100167. [Google Scholar] [CrossRef]
- Zalewska, A.; Gałczyk, M.; Sobolewski, M.; Białokoz-Kalinowska, I. Depression as compared to level of physical activity and internet addiction among polish physiotherapy students during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2021, 18, 10072. [Google Scholar] [CrossRef]
- Wipfli, B.; Landers, D.; Nagoshi, C.; Ringenbach, S. An examination of serotonin and psychological variables in the relationship between exercise and mental health. Scand. J. Med. Sci. Sport 2011, 21, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Singh, R.H.; Dey, P.K. Exercise training: Significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol. Behav. 1992, 52, 1095–1099. [Google Scholar] [CrossRef]
- Harmer, C.J. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology 2008, 55, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Droste, S.; Gesing, A.; Ulbricht, S.; Müller, M.B.; Linthorst, A.C.E.; Reul, J.M.H.M. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology 2003, 144, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Salmon, P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar] [CrossRef]
- Anderson, E.; Shivakumar, G. Effects of exercise and physical activity on anxiety. Front. Psychiatry 2013, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Juchnowicz, D.; Baj, J.; Forma, A.; Karakuła, K.; Sitarz, E.; Bogucki, J.; Karakula-Juchnowicz, H. The outbreak of sars-cov-2 pandemic and the well-being of polish students: The risk factors of the emotional distress during COVID-19 lockdown. J. Clin. Med. 2021, 10, 944. [Google Scholar] [CrossRef]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.W.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef]

| n | % | |
|---|---|---|
| Gender | ||
| Female | 2885 | 78.9 |
| Male | 691 | 18.9 |
| Transgender Female | 4 | 0.1 |
| Transgender Male | 14 | 0.4 |
| Gender Variant/Non-Conforming | 42 | 1.1 |
| Other | 19 | 0.5 |
| Race/Ethnicity | ||
| American Indian, Native American, or Alaska Native | 18 | 0.5 |
| Asian or Asian American | 150 | 4.1 |
| Black, African American, or African | 175 | 4.8 |
| Latino or Latina | 117 | 3.2 |
| Middle Eastern or Arab | 12 | 0.3 |
| Native Hawaiian or Other Pacific Islander | 5 | 0.1 |
| White or Caucasian | 3042 | 83.2 |
| Multi-racial | 102 | 2.8 |
| Other | 33 | 0.9 |
| Marital Status | ||
| Married | 1639 | 44.8 |
| Widowed | 136 | 3.7 |
| Divorced | 506 | 13.8 |
| Separated | 52 | 1.4 |
| Never Married | 1317 | 36.0 |
| Educational History | ||
| Less than high school | 11 | 0.3 |
| High school graduate | 169 | 4.6 |
| Some college | 494 | 13.5 |
| 2-year degree | 276 | 7.5 |
| 4-year degree | 1313 | 35.9 |
| Professional degree | 1108 | 30.3 |
| Doctorate | 272 | 7.4 |
| Employment | ||
| Unemployed | 1314 | 35.9 |
| Employed 1–20 h | 348 | 9.5 |
| Employed 20–30 h | 231 | 6.3 |
| Employed full time (40+ h) | 1744 | 47.7 |
| Work Shift | ||
| First (9 a.m.–5 p.m.) | 1964 | 53.7 |
| Second (4 p.m.–12 a.m.) | 128 | 3.5 |
| Third (12 a.m.–8 a.m.) | 45 | 1.2 |
| PTE (less than 3 day per week) | 145 | 4.0 |
| Work at home | 949 | 25.9 |
| b | t | p-Value | |
|---|---|---|---|
| Model 1 | |||
| Social Isolation | 0.034 | 43.04 | <0.001 |
| Total Wake Time (TWT) | 0.001 | 3.65 | <0.001 |
| Sleep Quality (SQ) | −0.272 | −13.25 | <0.001 |
| Physical Activity (PA) | −0.001 | −10.35 | <0.001 |
| Social Isolation × TWT | 0.00001 | 0.79 | 0.43 |
| Social Isolation × SQ | −0.005 | −7.39 | <0.001 |
| Social Isolation × PA | −0.00001 | −5.59 | <0.001 |
| Model 2 | |||
| Social Satisfaction | −0.591 | −41.00 | <0.001 |
| Total Wake Time (TWT) | 0.001 | 3.97 | <0.001 |
| Sleep Quality (SQ) | −0.249 | −12.22 | <0.001 |
| Physical Activity (PA) | −0.001 | −10.84 | <0.001 |
| Social Satisfaction × TWT | −0.001 | −3.76 | <0.001 |
| Social Satisfaction × SQ | −0.068 | 5.54 | <0.001 |
| Social Satisfaction × PA | −0.0002 | 5.35 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, I.; Howie, E.K.; Muench, A.; Perlis, M.L. Measuring the Effects of Social Isolation and Dissatisfaction on Depressive Symptoms during the COVID-19 Pandemic: The Moderating Role of Sleep and Physical Activity. Brain Sci. 2021, 11, 1449. https://doi.org/10.3390/brainsci11111449
Vargas I, Howie EK, Muench A, Perlis ML. Measuring the Effects of Social Isolation and Dissatisfaction on Depressive Symptoms during the COVID-19 Pandemic: The Moderating Role of Sleep and Physical Activity. Brain Sciences. 2021; 11(11):1449. https://doi.org/10.3390/brainsci11111449
Chicago/Turabian StyleVargas, Ivan, Erin Kaye Howie, Alexandria Muench, and Michael L. Perlis. 2021. "Measuring the Effects of Social Isolation and Dissatisfaction on Depressive Symptoms during the COVID-19 Pandemic: The Moderating Role of Sleep and Physical Activity" Brain Sciences 11, no. 11: 1449. https://doi.org/10.3390/brainsci11111449
APA StyleVargas, I., Howie, E. K., Muench, A., & Perlis, M. L. (2021). Measuring the Effects of Social Isolation and Dissatisfaction on Depressive Symptoms during the COVID-19 Pandemic: The Moderating Role of Sleep and Physical Activity. Brain Sciences, 11(11), 1449. https://doi.org/10.3390/brainsci11111449






