The Directionality of Fronto-Posterior Brain Connectivity Is Associated with the Degree of Individual Autistic Traits
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. EEG Acquisition and Preprocessing
2.3. Cortical Source Reconstruction and ROI Definition
2.4. Granger Causality Analysis
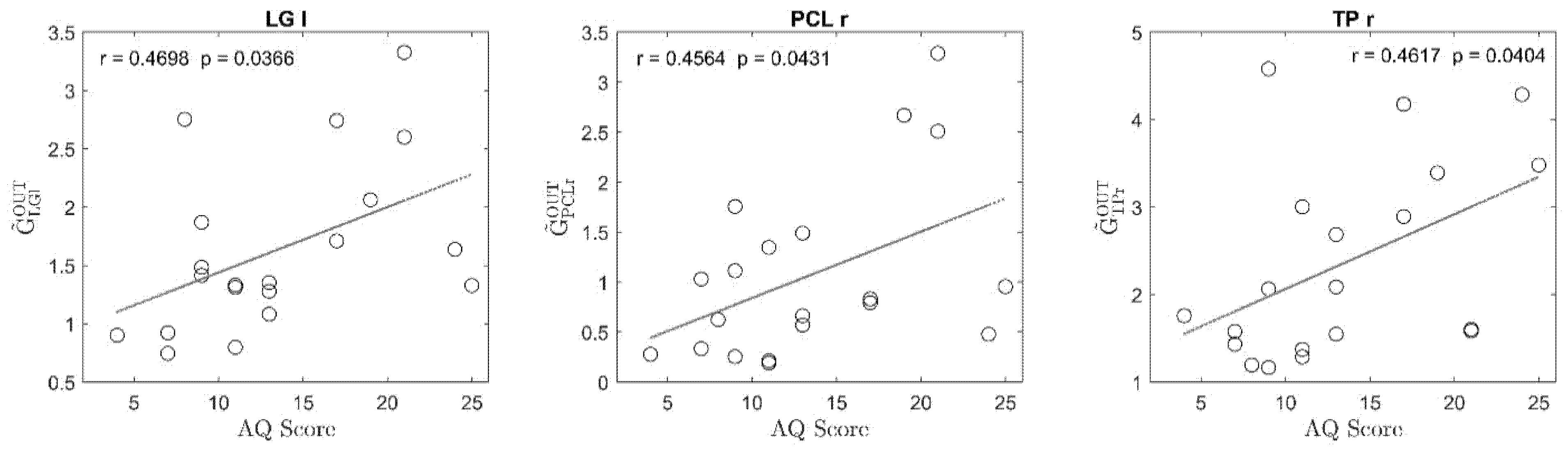
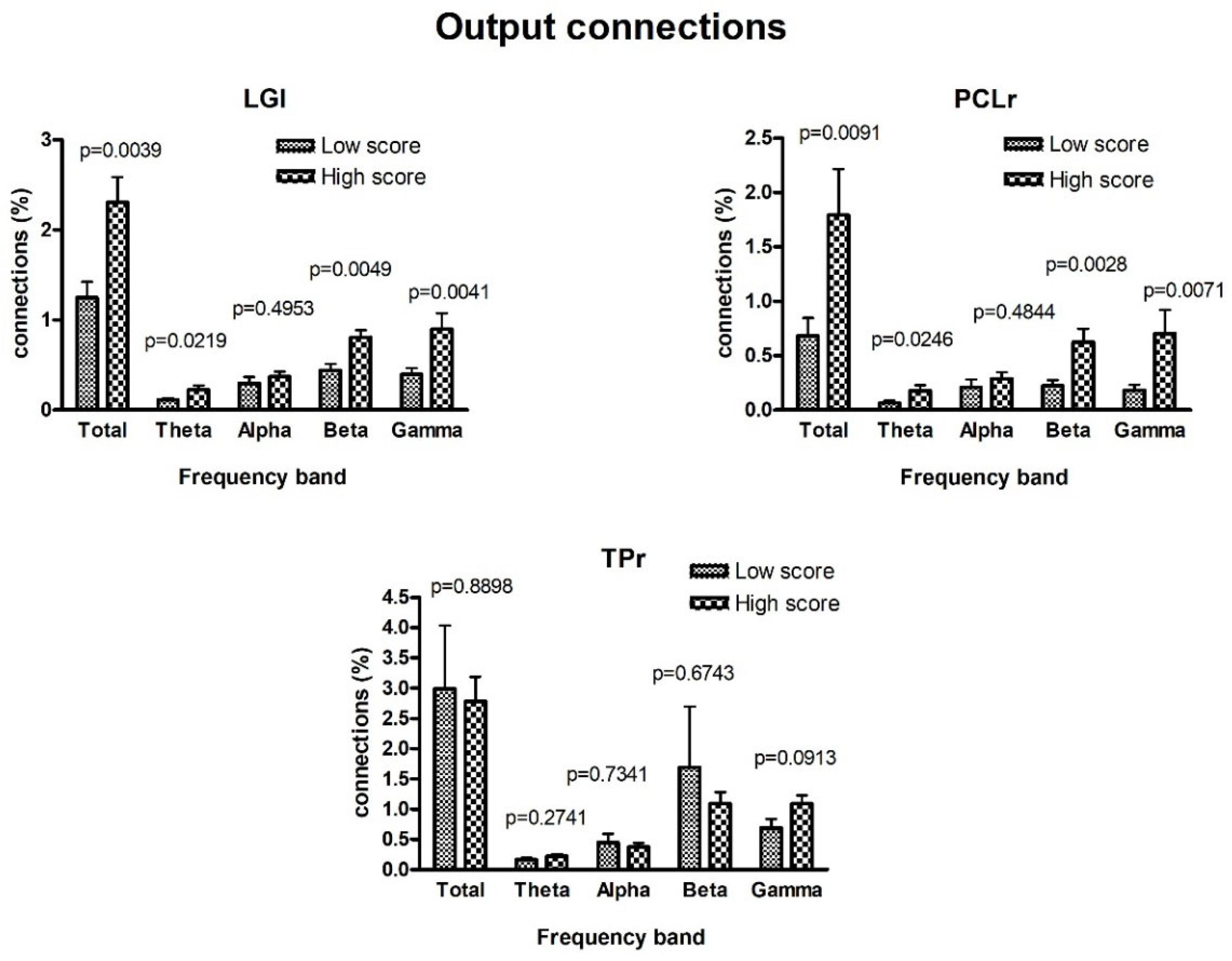
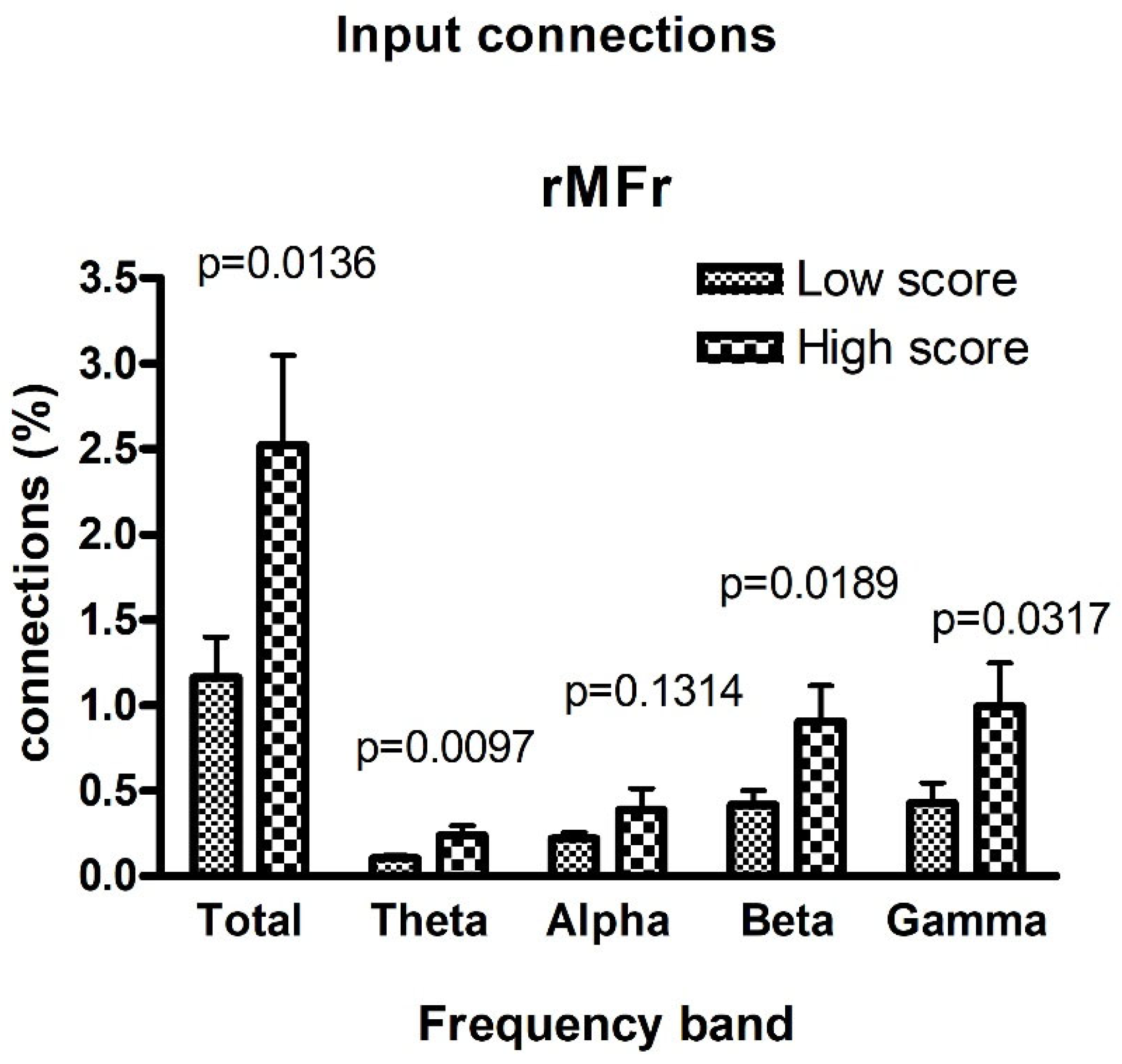
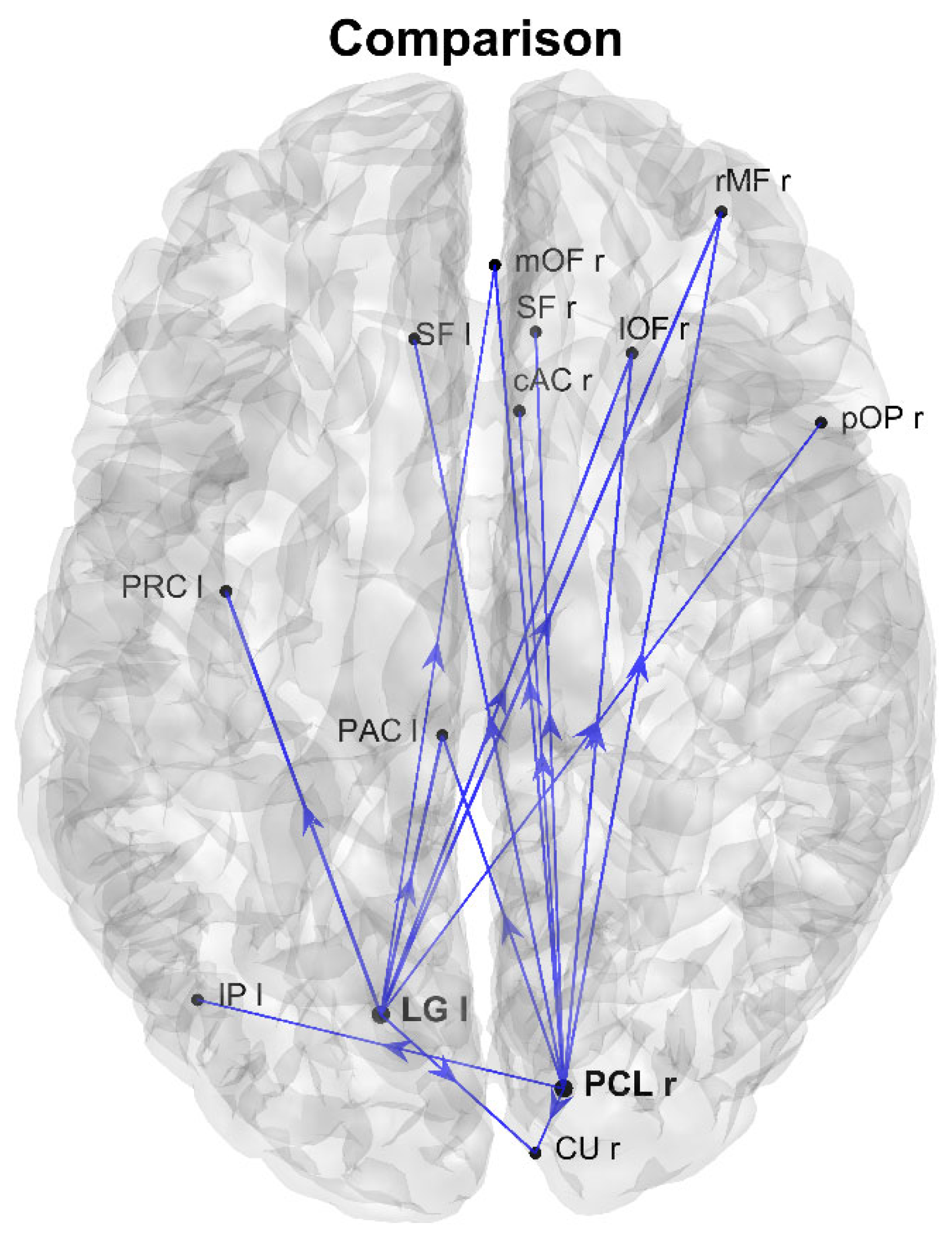
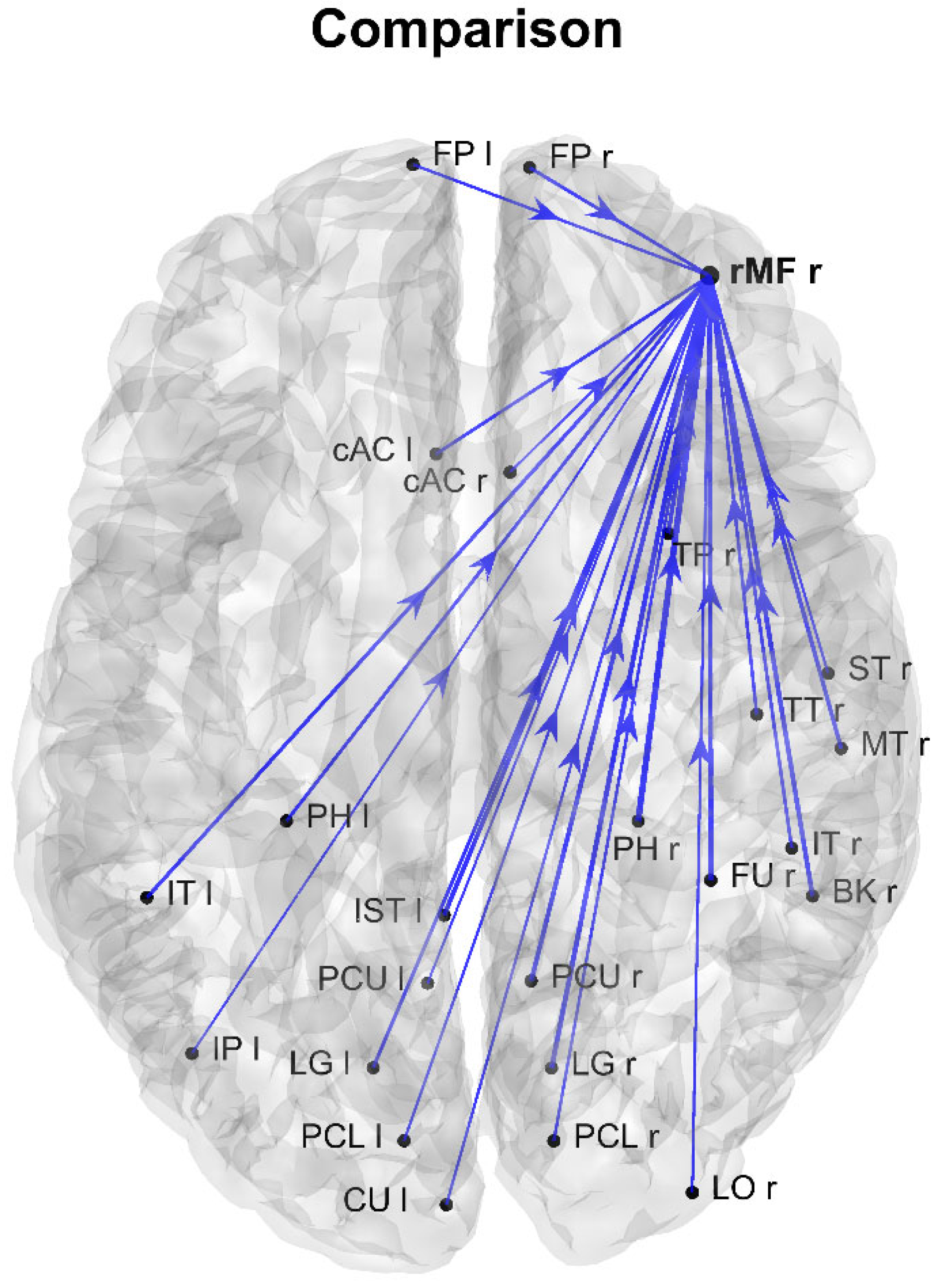
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Tanguay, P.E. Autism in DSM-5. AJP 2011, 168, 1142–1144. [Google Scholar] [CrossRef]
- Baker, J.P. Autism at 70—Redrawing the Boundaries. N. Engl. J. Med. 2013, 369, 1089–1091. [Google Scholar] [CrossRef] [Green Version]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Malesand Females, Scientists and Mathematicians. J. Autism Dev. Disord. 2001, 13. [Google Scholar]
- Bralten, J.; van Hulzen, K.J.; Martens, M.B.; Galesloot, T.E.; Arias Vasquez, A.; Kiemeney, L.A.; Buitelaar, J.K.; Muntjewerff, J.W.; Franke, B.; Poelmans, G. Autism Spectrum Disorders and Autistic Traits Share Genetics and Biology. Mol. Psychiatry 2018, 23, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Hoekstra, R.A.; Bartels, M.; Verweij, C.J.H.; Boomsma, D.I. Heritability of Autistic Traits in the General Population. Arch. Pediatr. Adolesc. Med. 2007, 161, 372–377. [Google Scholar] [CrossRef]
- Piven, J. Broader Autism Phenotype: Evidence from a Family History Study of Multiple-Incidence Autism Families. AJP 1997, 154, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Frith, U. Autism: Explaining the Enigma, 2nd ed.; Blackwell Publishing: Malden, MA, USA, 2003; ISBN 978-0-631-22900-1. [Google Scholar]
- Stewart, M.E.; Watson, J.; Allcock, A.-J.; Yaqoob, T. Autistic Traits Predict Performance on the Block Design. Autism 2009, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Alink, A.; Charest, I. Clinically Relevant Autistic Traits Predict Greater Reliance on Detail for Image Recognition. Sci. Rep. 2020, 10, 14239. [Google Scholar] [CrossRef] [PubMed]
- Goris, J.; Brass, M.; Cambier, C.; Delplanque, J.; Wiersema, J.R.; Braem, S. The Relation Between Preference for Predictability and Autistic Traits. Autism Res. 2020, 13, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Dimond, D.; Schuetze, M.; Smith, R.E.; Dhollander, T.; Cho, I.; Vinette, S.; Ten Eycke, K.; Lebel, C.; McCrimmon, A.; Dewey, D.; et al. Reduced White Matter Fiber Density in Autism Spectrum Disorder. Cereb. Cortex 2019, 29, 1778–1788. [Google Scholar] [CrossRef]
- Kessler, K.; Seymour, R.A.; Rippon, G. Brain Oscillations and Connectivity in Autism Spectrum Disorders (ASD): New Approaches to Methodology, Measurement and Modelling. Neurosci. Biobehav. Rev. 2016, 71, 601–620. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.M.; Wallace, M.T. Dysfunction of Sensory Oscillations in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2016, 68, 848–861. [Google Scholar] [CrossRef] [Green Version]
- Duffy, F.H.; Als, H. A Stable Pattern of EEG Spectral Coherence Distinguishes Children with Autism from Neuro-Typical Controls–A Large Case Control Study. BMC Med. 2012, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Kana, R.K.; Minshew, N.J. Functional and Anatomical Cortical Underconnectivity in Autism: Evidence from an FMRI Study of an Executive Function Task and Corpus Callosum Morphometry. Cereb. Cortex 2007, 17, 951–961. [Google Scholar] [CrossRef]
- Catarino, A.; Andrade, A.; Churches, O.; Wagner, A.P.; Baron-Cohen, S.; Ring, H. Task-Related Functional Connectivity in Autism Spectrum Conditions: An EEG Study Using Wavelet Transform Coherence. Mol. Autism 2013, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Just, M.A.; Keller, T.A.; Malave, V.L.; Kana, R.K.; Varma, S. Autism as a Neural Systems Disorder: A Theory of Frontal-Posterior Underconnectivity. Neurosci. Biobehav. Rev. 2012, 36, 1292–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rane, P.; Cochran, D.; Hodge, S.M.; Haselgrove, C.; Kennedy, D.; Frazier, J.A. Connectivity in Autism: A Review of MRI Connectivity Studies. Harv. Rev. Psychiatry 2015, 23, 223–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supekar, K.; Uddin, L.Q.; Khouzam, A.; Phillips, J.; Gaillard, W.D.; Kenworthy, L.E.; Yerys, B.E.; Vaidya, C.J.; Menon, V. Brain Hyperconnectivity in Children with Autism and Its Links to Social Deficits. Cell Rep. 2013, 5, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Keown, C.L.; Shih, P.; Nair, A.; Peterson, N.; Mulvey, M.E.; Müller, R.-A. Local Functional Overconnectivity in Posterior Brain Regions Is Associated with Symptom Severity in Autism Spectrum Disorders. Cell Rep. 2013, 5, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Amso, D.; Haas, S.; Tenenbaum, E.; Markant, J.; Sheinkopf, S.J. Bottom-Up Attention Orienting in Young Children with Autism. J. Autism Dev. Disord. 2014, 44, 664–673. [Google Scholar] [CrossRef]
- Karvelis, P.; Seitz, A.R.; Lawrie, S.M.; Seriès, P. Autistic Traits, but Not Schizotypy, Predict Increased Weighting of Sensory Information in Bayesian Visual Integration. eLife 2018, 7, e34115. [Google Scholar] [CrossRef]
- Takesaki, N.; Kikuchi, M.; Yoshimura, Y.; Hiraishi, H.; Hasegawa, C.; Kaneda, R.; Nakatani, H.; Takahashi, T.; Mottron, L.; Minabe, Y. The Contribution of Increased Gamma Band Connectivity to Visual Non-Verbal Reasoning in Autistic Children: A MEG Study. PLoS ONE 2016, 11, e0163133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Michmizos, K.; Tommerdahl, M.; Ganesan, S.; Kitzbichler, M.G.; Zetino, M.; Garel, K.-L.A.; Herbert, M.R.; Hämäläinen, M.S.; Kenet, T. Somatosensory Cortex Functional Connectivity Abnormalities in Autism Show Opposite Trends, Depending on Direction and Spatial Scale. Brain 2015, 138, 1394–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamashli, F.; Kozhemiako, N.; Khan, S.; Nunes, A.S.; McGuiggan, N.M.; Losh, A.; Joseph, R.M.; Ahveninen, J.; Doesburg, S.M.; Hämäläinen, M.S.; et al. Children with Autism Spectrum Disorder Show Altered Functional Connectivity and Abnormal Maturation Trajectories in Response to Inverted Faces. Autism Res. 2021, 14, 1101–1114. [Google Scholar] [CrossRef]
- Seymour, R.A.; Rippon, G.; Gooding-Williams, G.; Schoffelen, J.M.; Kessler, K. Dysregulated Oscillatory Connectivity in the Visual System in Autism Spectrum Disorder. Brain 2019, 142, 3294–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, M.; Kitajo, K.; Fukao, K.; Murai, T.; Yamaguchi, Y.; Funabiki, Y. Frontal Theta Activation during Motor Synchronization in Autism. Sci. Rep. 2017, 7, 15034. [Google Scholar] [CrossRef]
- Wang, J.; Barstein, J.; Ethridge, L.E.; Mosconi, M.W.; Takarae, Y.; Sweeney, J.A. Resting State EEG Abnormalities in Autism Spectrum Disorders. J. Neurodev. Disord. 2013, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Schröder, Y.; Hohmann, D.M.; Meller, T.; Evermann, U.; Pfarr, J.-K.; Jansen, A.; Kamp-Becker, I.; Grezellschak, S.; Nenadić, I. Associations of Subclinical Autistic-like Traits with Brain Structural Variation Using Diffusion Tensor Imaging and Voxel-Based Morphometry. Eur. Psychiatry 2021, 64, e27. [Google Scholar] [CrossRef]
- Hirose, K.; Miyata, J.; Sugihara, G.; Kubota, M.; Sasamoto, A.; Aso, T.; Fukuyama, H.; Murai, T.; Takahashi, H. Fiber Tract Associated with Autistic Traits in Healthy Adults. J. Psychiatr. Res. 2014, 59, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Barttfeld, P.; Amoruso, L.; Ais, J.; Cukier, S.; Bavassi, L.; Tomio, A.; Manes, F.; Ibanez, A.; Sigman, M. Organization of Brain Networks Governed by Long-Range Connections Index Autistic Traits in the General Population. J. Neurodev. Disord. 2013, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; MIT Press: Cambridge, MA, USA, 2014; ISBN 978-0-262-31956-0. [Google Scholar]
- Ricci, G.; Magosso, E.; Ursino, M. The Relationship between Oscillations in Brain Regions and Functional Connectivity: A Critical Analysis with the Aid of Neural Mass Models. Brain Sci. 2021, 11, 487. [Google Scholar] [CrossRef]
- Chicharro, D. On the Spectral Formulation of Granger Causality. Biol. Cybern. 2011, 105, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Woodbury-Smith, M.R.; Robinson, J.; Wheelwright, S.; Baron-Cohen, S. Screening Adults for Asperger Syndrome Using the AQ: A Preliminary Study of Its Dia.agnostic Validity in Clinical Practice. J. Autism Dev. Disord. 2005, 35, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ruzich, E.; Allison, C.; Smith, P.; Watson, P.; Auyeung, B.; Ring, H.; Baron-Cohen, S. Measuring Autistic Traits in the General Population: A Systematic Review of the Autism-Spectrum Quotient (AQ) in a Nonclinical Population Sample of 6900 Typical Adult Males and Females. Mol. Autism 2015, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Ruta, L.; Mazzone, D.; Mazzone, L.; Wheelwright, S.; Baron-Cohen, S. The Autism-Spectrum Quotient—Italian Version: A Cross-Cultural Confirmation of the Broader Autism Phenotype. J. Autism Dev. Disord. 2012, 42, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, e879716. [Google Scholar] [CrossRef]
- Gramfort, A.; Papadopoulo, T.; Olivi, E.; Clerc, M. OpenMEEG: Opensource Software for Quasistatic Bioelectromagnetics. BioMed Eng. OnLine 2010, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp. Clin. Pharmacol. 2002, 24 (Suppl. D), 5–12. [Google Scholar]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An Automated Labeling System for Subdividing the Human Cerebral Cortex on MRI Scans into Gyral Based Regions of Interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Ding, M.; Chen, Y.; Bressler, S.L. Granger Causality: Basic Theory and Application to Neuroscience. In Handbook of Time Series Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 437–460. ISBN 978-3-527-60997-0. [Google Scholar]
- O’Reilly, C.; Lewis, J.D.; Elsabbagh, M. Is Functional Brain Connectivity Atypical in Autism? A Systematic Review of EEG and MEG Studies. PLoS ONE 2017, 12, e0175870. [Google Scholar] [CrossRef]
- Kikinis, Z.; Fallon, J.H.; Niznikiewicz, M.; Nestor, P.; Davidson, C.; Bobrow, L.; Pelavin, P.E.; Fischl, B.; Yendiki, A.; McCarley, R.W.; et al. Gray Matter Volume Reduction in Rostral Middle Frontal Gyrus in Patients with Chronic Schizophrenia. Schizophr. Res. 2010, 123, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jao Keehn, R.J.; Nair, S.; Pueschel, E.B.; Linke, A.C.; Fishman, I.; Müller, R.-A. Atypical Local and Distal Patterns of Occipito-Frontal Functional Connectivity Are Related to Symptom Severity in Autism. Cereb. Cortex 2019, 29, 3319–3330. [Google Scholar] [CrossRef] [PubMed]
- Manjaly, Z.M.; Bruning, N.; Neufang, S.; Stephan, K.E.; Brieber, S.; Marshall, J.C.; Kamp-Becker, I.; Remschmidt, H.; Herpertz-Dahlmann, B.; Konrad, K.; et al. Neurophysiological Correlates of Relatively Enhanced Local Visual Search in Autistic Adolescents. NeuroImage 2007, 35, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Samson, F.; Mottron, L.; Soulières, I.; Zeffiro, T.A. Enhanced Visual Functioning in Autism: An ALE Meta-Analysis. Human Brain Mapp. 2012, 33, 1553–1581. [Google Scholar] [CrossRef] [PubMed]
- Michalareas, G.; Vezoli, J.; van Pelt, S.; Schoffelen, J.-M.; Kennedy, H.; Fries, P. Alpha-Beta and Gamma Rhythms Subserve Feedback and Feedforward Influences among Human Visual Cortical Areas. Neuron 2016, 89, 384–397. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.; Schwiedrzik, C.M.; Wibral, M.; Singer, W.; Melloni, L. Expecting to See a Letter: Alpha Oscillations as Carriers of Top-Down Sensory Predictions. Cereb. Cortex 2016, 26, 3146–3160. [Google Scholar] [CrossRef] [Green Version]
- Happé, F. Autism: Cognitive Deficit or Cognitive Style? Trends Cogn. Sci. 1999, 3, 216–222. [Google Scholar] [CrossRef]
- Joseph, R.M.; Keehn, B.; Connolly, C.; Wolfe, J.M.; Horowitz, T.S. Why Is Visual Search Superior in Autism Spectrum Disorder? Dev. Sci. 2009, 12, 1083–1096. [Google Scholar] [CrossRef]
- O’Riordan, M.A.; Plaisted, K.C.; Driver, J.; Baron-Cohen, S. Superior Visual Search in Autism. J. Exp. Psychol. Hum. Percept. Perform. 2001, 27, 719–730. [Google Scholar] [CrossRef]
- Romei, V.; Driver, J.; Schyns, P.G.; Thut, G. Rhythmic TMS over Parietal Cortex Links Distinct Brain Frequencies to Global versus Local Visual Processing. Curr. Biol. 2011, 21, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Gosselin, F.; Schyns, P.G. Perceptual Moments of Conscious Visual Experience Inferred from Oscillatory Brain Activity. Proc. Natl. Acad. Sci. USA 2006, 103, 5626–5631. [Google Scholar] [CrossRef] [Green Version]
- Ronconi, L.; Vitale, A.; Federici, A.; Pini, E.; Molteni, M.; Casartelli, L. Altered Neural Oscillations and Connectivity in the Beta Band Underlie Detail-Oriented Visual Processing in Autism. Neuroimage Clin. 2020, 28, 102484. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Wang, X.; Zhang, H.; Zhou, Y.; Chen, L.; Li, Y.; Wu, L. Increased EEG Coherence in Long-distance and Short-distance Connectivity in Children with Autism Spectrum Disorders. Brain Behav. 2020, 10, e01796. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Engell, A.D.; von dem Hagen, E.; Henson, R.N.A.; Calder, A.J. Autism Spectrum Traits Predict the Neural Response to Eye Gaze in Typical Individuals. NeuroImage 2012, 59, 3356–3363. [Google Scholar] [CrossRef]
- Bellato, A.; Arora, I.; Kochhar, P.; Hollis, C.; Groom, M.J. Atypical Electrophysiological Indices of Eyes-Open and Eyes-Closed Resting-State in Children and Adolescents with ADHD and Autism. Brain Sci. 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Mash, L.E.; Keehn, B.; Linke, A.C.; Liu, T.T.; Helm, J.L.; Haist, F.; Townsend, J.; Müller, R.-A. Atypical Relationships between Spontaneous EEG and FMRI Activity in Autism. Brain Connect. 2020, 10, 18–28. [Google Scholar] [CrossRef]

| ROI | Abbreviation | ROI | Abbreviation |
|---|---|---|---|
| Banks of Sup. Temp. Sulcus | BK | Parahippocampal | PH |
| Caudal Anterior Cingulate | cAC | Pars Opercularis | pOP |
| Caudal Middle Frontal | cMF | Pars Orbitalis | pOR |
| Cuneus | CU | Pars Triangularis | pTR |
| Entorhinal | EN | Pericalcarine | PCL |
| Frontal Pole | FP | Postcentral | POC |
| Fusiform | FU | Posterior Cingulate | PCG |
| Inferior Parietal | IP | Precentral | PRC |
| Inferior Temporal | IT | Precuneus | PCU |
| Insula | IN | Rostral Anterior Cingulate | rAC |
| Isthmus Cingulate | IST | Rostral Middle Frontal | rMF |
| Lateral Occipital | LO | Superior Frontal | SF |
| Lateral Orbitofrontal | IOF | Superior Parietal | SP |
| Lingual | LG | Superior Temporal | ST |
| Medial Orbitofrontal | mOF | Supramarginal | SMG |
| Middle Temporal | MT | Temporal Pole | TP |
| Paracentral | PAC | Transverse Temporal | TT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasi, L.; Magosso, E.; Ricci, G.; Ursino, M.; Romei, V. The Directionality of Fronto-Posterior Brain Connectivity Is Associated with the Degree of Individual Autistic Traits. Brain Sci. 2021, 11, 1443. https://doi.org/10.3390/brainsci11111443
Tarasi L, Magosso E, Ricci G, Ursino M, Romei V. The Directionality of Fronto-Posterior Brain Connectivity Is Associated with the Degree of Individual Autistic Traits. Brain Sciences. 2021; 11(11):1443. https://doi.org/10.3390/brainsci11111443
Chicago/Turabian StyleTarasi, Luca, Elisa Magosso, Giulia Ricci, Mauro Ursino, and Vincenzo Romei. 2021. "The Directionality of Fronto-Posterior Brain Connectivity Is Associated with the Degree of Individual Autistic Traits" Brain Sciences 11, no. 11: 1443. https://doi.org/10.3390/brainsci11111443
APA StyleTarasi, L., Magosso, E., Ricci, G., Ursino, M., & Romei, V. (2021). The Directionality of Fronto-Posterior Brain Connectivity Is Associated with the Degree of Individual Autistic Traits. Brain Sciences, 11(11), 1443. https://doi.org/10.3390/brainsci11111443








