Motor Preparation and Execution for Performance Difficulty: Centroparietal Beta Activation during the Effort Expenditure for Rewards Task as a Function of Motivation
Abstract
:1. Introduction
1.1. Beta Activity and Motor Functioning
1.2. Beta Activation and Motivation
1.3. Beta, Motor Functioning, and Motivation
1.4. The Present Study
2. Methods
2.1. Participants
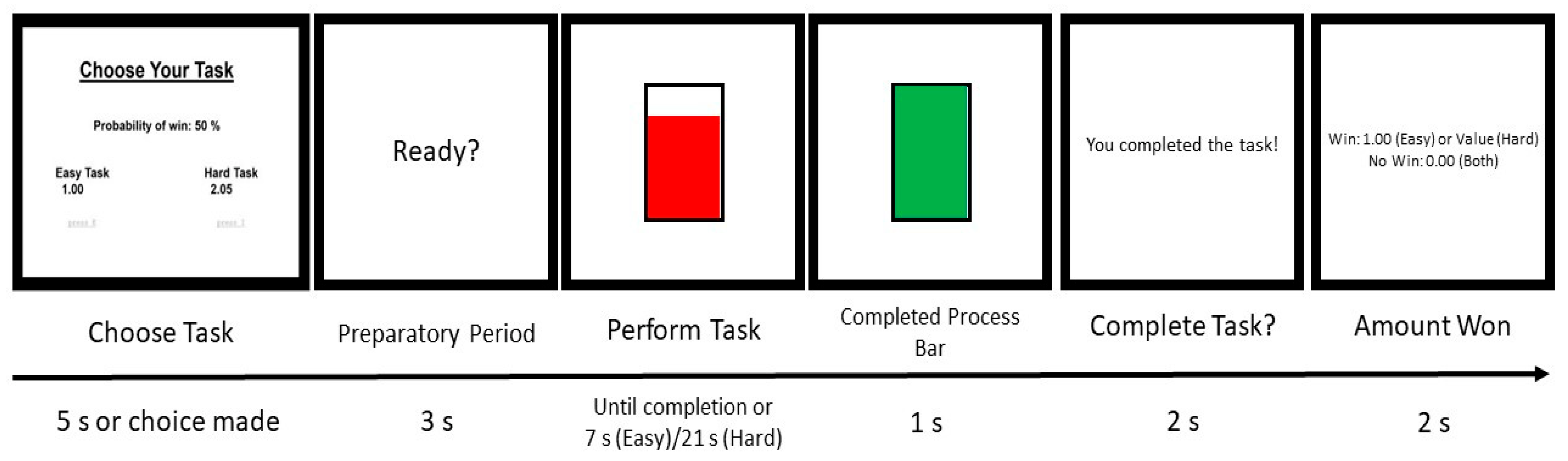
2.2. Procedures
2.3. EEG Assessment and Processing
2.4. Statistical Analyses
2.5. Beta Activation Analyses
3. Results
3.1. Preparatory Beta
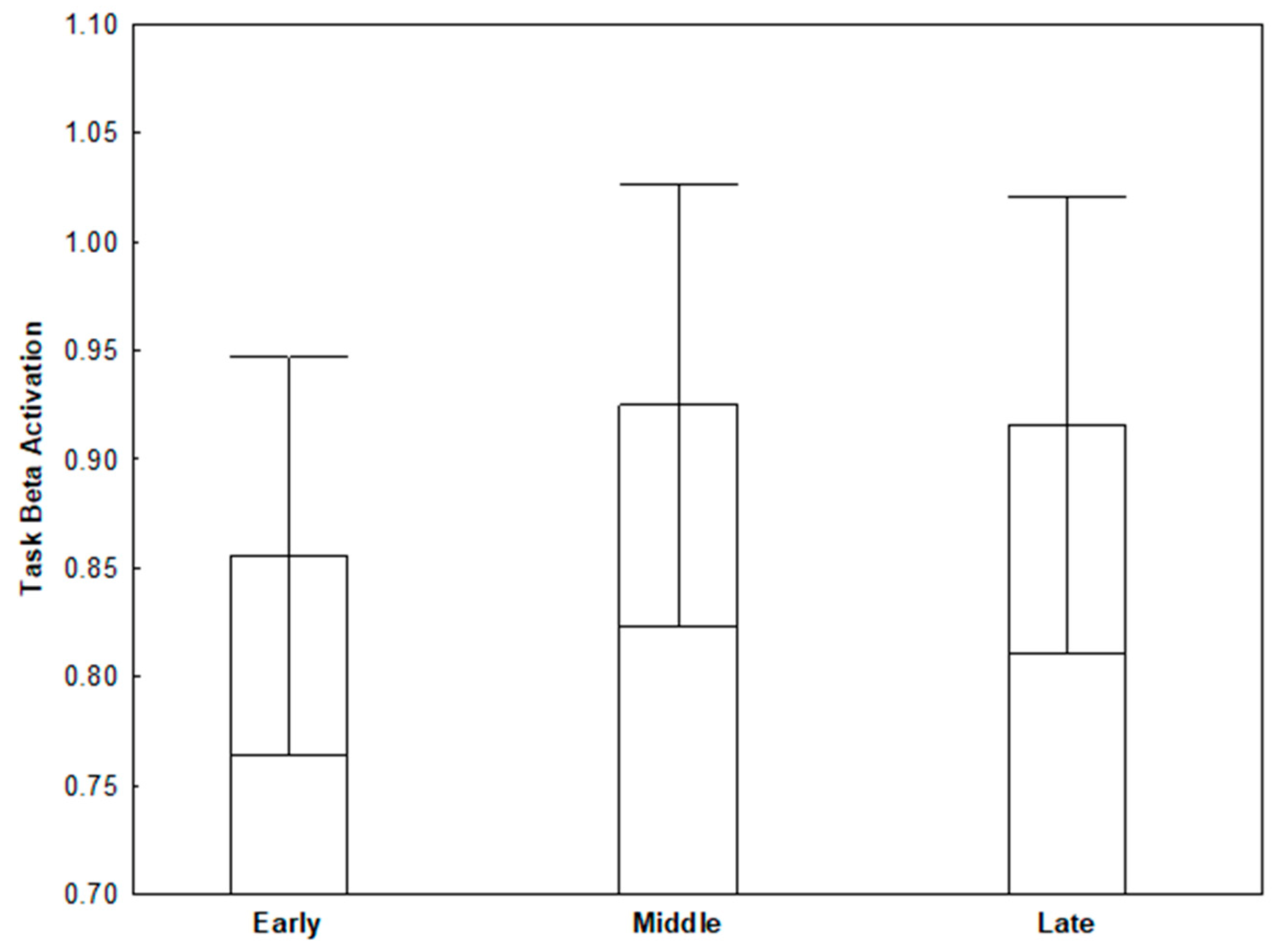
3.2. Task Beta
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuper, C.; Pfurtscheller, G. Event-related cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef]
- Gable, P.A.; Threadgill, A.H.; Adams, D.L. Neural activity underlying motor-action preparation and cognitive narrowing in approach-motivated goal states. Cogn. Affect. Behav. Neurosci. 2016, 16, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meadows, C.C.; Gable, P.A.; Lohse, K.R.; Miller, M.W. Motivation and motor cortical activity can independently affect motor performance. Neuroscience 2016, 339, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Threadgill, A.H.; Gable, P.A. Resting beta activation and trait motivation: Neurophysiological markers of motivated motor-action preparation. Int. J. Psychophysiol. 2018, 127, 46–51. [Google Scholar] [CrossRef]
- Kurzban, R. The sense of effort. Curr. Opin. Psychol. 2016, 7, 67–70. [Google Scholar] [CrossRef]
- Inzlicht, M.; Shenhav, A.; Olivola, C. The Effort Paradox: Effort Is Both Costly and Valued. Trends Cogn. Sci. 2018, 22, 337–349. [Google Scholar] [CrossRef]
- Caviola, S.; Carey, E.; Mammarella, I.C.; Szucs, D. Stress, Time Pressure, Strategy Selection and Math Anxiety in Mathematics: A Review of the Literature. Front. Psychol. 2017, 8, 1488. [Google Scholar] [CrossRef] [Green Version]
- Luttenberger, S.; Wimmer, S.; Paechter, M. Spotlight on math anxiety. Psychol. Res. Behav. Manag. 2018, 11, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Liu, T.; Szarkowski, R.; Rios, C.; Ashe, J.; He, B. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: An EEG and fMRI study of motor imagery and movements. NeuroImage 2010, 49, 2596–2606. [Google Scholar] [CrossRef] [Green Version]
- Van Wijk, B.C.M.; Daffertshofer, A.; Roach, N.; Praamstra, P. A role of beta oscillatory synchrony in biasing response competition? Cereb. Cortex 2009, 19, 1294–1302. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting Cortical Activity at Beta-Band Frequencies Slows Movement in Humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef] [Green Version]
- Wach, C.; Krause, V.; Moliadze, V.; Paulus, W.; Schnitzler, A.; Pollok, B. Effects of 10 Hz and 20 Hz transcranial alternating current stimulation (tACS) on otor functions and motor cortical excitability. Behav. Brain Res. 2013, 241, 1–6. [Google Scholar] [CrossRef]
- Tzagarakis, C.; Ince, N.F.; Leuthold, A.C.; Pellizzer, G. Beta-Band Activity during Motor Planning Reflects Response Uncertainty. J. Neurosci. 2010, 30, 11270–11277. [Google Scholar] [CrossRef]
- Chen, R.; Yaseen, Z.; Cohen, L.G.; Hallett, M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann. Neurol. 1998, 44, 317–325. [Google Scholar] [CrossRef]
- Jenkinson, N.; Brown, P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011, 34, 611–618. [Google Scholar] [CrossRef]
- Kuhn, A.A.; Kempf, F.; Brucke, C.; Gaynor Doyle, L.; Martinez-Torres, I.; Brown, P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. 2008, 28, 6165–6173. [Google Scholar] [CrossRef] [Green Version]
- Schulz, W. Dopamine reward prediction error coding. Dialogues Clin. Neurosci. 2016, 18, 23–32. [Google Scholar]
- Tobler, P.N.; Fiorillo, C.D.; Schultz, W. Adaptive Coding of Reward Value by Dopamine Neurons. Science 2005, 307, 1642–1645. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.M.; Kühn, A.A.; Hariz, M.; Kupsch, A.; Schneider, G.H.; Brown, P. Levadopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson’s Disease. Eur. J. Neurosci. 2005, 21, 1403–1412. [Google Scholar] [CrossRef]
- Babiloni, C.; Del Percio, C.; Vecchio, F.; Sebastiano, F.; Di Gennaro, G.; Quarato, P.P.; Morace, R.; Pavone, L.; Soricelli, A.; Noce, G.; et al. Alpha, beta, and gamma electrocorticographic rhythms in somatosensory, motor, premotor, and prefrontal cortical areas differ in movement execution and observation in humans. Clin. Neurophysiol. 2016, 127, 641–654. [Google Scholar] [CrossRef]
- Cunnington, R.; Windischberger, C.; Deecke, L.; Moser, E. The preparation and execution of self-initiated and externally-triggered movement: A study of event-related fMRI. NeuroImage 2002, 15, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Sanes, J.N.; Donoghue, J.P. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc. Natl. Acad. Sci. USA 1993, 90, 4470–4474. [Google Scholar] [CrossRef] [Green Version]
- Gable, P.A.; Harmon-Jones, E. The motivational dimensional model of affect: Implications for breadth of attention, memory, and cognitive categorization. Cogn. Emot. 2010, 24, 332–337. [Google Scholar] [CrossRef]
- Brehm, J.W.; Self, E.A. The intensity of motivation. Ann. Rev. Psychol. 1989, 40, 109–131. [Google Scholar] [CrossRef]
- Wright, R.A. Brehm’s theory of motivation as a model of effort and cardiovascular response. In The Psychology of Action: Linking Cognition and Motivation to Behavior; Gollwitzer, P.M., Bargh, J.A., Eds.; The Guilford Press: New York, NY, USA, 1996; pp. 424–453. [Google Scholar]
- Silvestrini, N.; Gendolla, G. Affect and cognitive control: Insights from research on effort mobilization. Int. J. Psychophysiol. 2019, 143, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Meyniel, F.; Pessiglione, M. Better get back to work: A role for motor beta desynchronization in incentive motivation. J. Neurosci. 2014, 34, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, R.A.; Miller, M.W.; Gable, P.A. Neural and Attentional Correlates of Intrinsic Motivation Resulting from Social Performance Expectancy. Neuroscience 2019, 416, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Salenius, S.; Schnitzler, A.; Salmelin, R.; Jousmäki, V.; Hari, R. Modulation of Human Cortical Rolandic Rhythms during Natural Sensorimotor Tasks. NeuroImage 1997, 5, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.; Salenius, S.; Salmelin, R.; Jousmäki, V.; Hari, R. Involvement of Primary Motor Cortex in Motor Imagery: A Neuromagnetic Study. NeuroImage 1997, 6, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J. Personal. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Wendel, C.J.; Wilhelm, R.A.; Gable, P.A. Individual Differences in Motivation and Impulsivity Link Resting Frontal Alpha Asymmetry and Motor Beta Activation. Biol. Psychol. 2021, 162, 108088. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Zalaudek, K.; Neuper, C. Event-related beta synchronization after wrist, finger and thumb movement. Electroencephalogr. Clin. Neurophysiol. Mot. Control. 1998, 109, 154–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Bressler, S.; Ding, M. Response preparation and inhibition: The role of the cortical sensorimotor beta rhythm. Neuroscience 2008, 156, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Stancák, A.; Pfurtscheller, G. Event-related desynchronisation of central beta-rhythms during brisk and slow self-paced finger movements of dominant and nondominant hand. Cogn. Brain Res. 1996, 4, 171–183. [Google Scholar] [CrossRef]
- McFarland, D.J.; Miner, L.A.; Vaughan, T.M.; Wolpaw, J. Mu and Beta Rhythm Topographies During Motor Imagery and Actual Movements. Brain Topogr. 2000, 12, 177–186. [Google Scholar] [CrossRef]
- Carver, C.S.; Scheier, M.F. Control theory: A useful conceptual framework for personality–social, clinical, and health psychology. Psychol. Bull. 1982, 92, 111–135. [Google Scholar] [CrossRef]
- Threadgill, A.H. From Preparation to Assessment: Exploring the Neural Substrates of Approach-Motivated Goal Pursuit. Doctoral Dissertation, UA Campus Repository, University of Alabama, Tuscaloosa, AL, USA, 2019. Available online: https://ir.ua.edu/bitstream/handle/123456789/6519/file_1.pdf?sequence=1 (accessed on 13 October 2021).
- Thürmer, J.L.; Scheier, M.F.; Carver, C.S. On the mechanics of goal striving: Experimental evidence of coasting and shifting. Motiv. Sci. 2020, 6, 266–274. [Google Scholar] [CrossRef]
- Heller, W.; Levy, J. Perception and expression of emotion in right-handers and left-handers. Neuropsychologia 1981, 19, 263–272. [Google Scholar] [CrossRef]
- Chapman, L.J.; Chapman, J.P. The measurement of handedness. Brain Cogn. 1987, 6, 175–183. [Google Scholar] [CrossRef]
- Treadway, M.T.; Buckholtz, J.W.; Schwartzman, A.N.; Lambert, W.E.; Zald, D.H. Worth the ‘EEfRT’? The Effort Expenditure for Rewards Task as an Objective Measure of Motivation and Anhedonia. PLoS ONE 2009, 4, e6598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inquisit 4.0.10. [Computer Software]. 2015. Available online: http://www.millisecond.com (accessed on 28 April 2021).
- Semlitsch, H.V.; Anderer, P.; Schuster, P.; Presslich, O. A Solution for Reliable and Valid Reduction of Ocular Artifacts, Applied to the P300 ERP. Psychophysiology 1986, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Jackson, D.C.; Larson, C.L. Human electroencephalography. In Handbook of Psychophysiology, 2nd ed.; Cacioppo, J.T., Tassinary, L.G., Berntson, G.G., Eds.; Cambridge University Press: New York, NY, USA, 2000; pp. 27–52. [Google Scholar]
- Muthukumaraswamy, S.D.; Johnson, B.W.; McNair, N.A. Mu rhythm modulation during observation of an object-directed grasp. Cogn. Brain Res. 2004, 19, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C.; Brunner, C.; da Silva, F.L. Beta rebound after different types of motor imagery in man. Neurosci. Lett. 2005, 378, 156–159. [Google Scholar] [CrossRef]
- Kwak, S.K.; Kim, J.H. Statistical data representation: Management of missing values and outliers. Korean J. Anesthesiol. 2017, 70, 407–411. [Google Scholar] [CrossRef]
- Lee, I.A.; Preacher, K.J. Calculation for the Test of the Difference between Two Dependent Correlations with One Variable in Common [Computer Software]. September 2013. Available online: http://quantpsy.org (accessed on 10 October 2021).
- Steiger, J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980, 87, 245–251. [Google Scholar] [CrossRef]
- Chen, C.C.; Litvak, V.; Gilbertson, T.; Kühn, A.; Lu, C.S.; Lee, S.T.; Tsai, C.H.; Tisch, S.; Limousin, P.; Hariz, M.; et al. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Exp. Neurol. 2007, 205, 214–221. [Google Scholar] [CrossRef]
- Wang, Z.; Lukowski, S.L.; Hart, S.A.; Lyons, I.M.; Thompson, L.A.; Kovas, Y.; Mazzocco, M.M.M.; Plomin, R.; Petrill, S.A. Is Math Anxiety Always Bad for Math Learning? The Role of Math Motivation. Psychol. Sci. 2015, 26, 1863–1876. [Google Scholar] [CrossRef] [Green Version]
- Paechter MMacher, D.; Martskvishvili, K.; Wimmer, S.; Papousek, I. Mathematics anxiety and statistics anxiety. Shared but also unshared components and antagonistic contributions to performance in statistics. Front. Psychol. Educ. Psychol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Skaalvik, E.M. Mathematics anxiety and coping strategies among middle school students: Relations with students’ achievement goal orientations and level of performance. Soc. Psychol. Educ. 2018, 21, 709–723. [Google Scholar] [CrossRef]
- Schneider, M.; Leuchs, L.; Czisch, M.; Sämann, P.G.; Spoormaker, V.I. Disentangling reward anticipation with simultaneous pupillometry/fMRI. NeuroImage 2018, 178, 11–22. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Ros, T.; Theberge, J.; Frewen, P.A.; Kluetsch, R.; Densmore, M.; Calhoun, V.D.; Lanius, R.A. Mind over chatter: Plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. NeuroImage 2013, 65, 324–335. [Google Scholar] [CrossRef]
- Massullo, C.; Carbone, G.A.; Farina, B.; Panno, A.; Capriotti, C.; Giacchini, M.; Machado, S.; Budde, H.; Murillo-Rodríguez, E.; Imperatori, C. Dysregulated brain salience within a triple network model in high trait anxiety individuals: A pilot EEG functional connectivity study. Int. J. Psychophysiol. 2020, 157, 61–69. [Google Scholar] [CrossRef]
- Wang, W.; Ho RL, M.; Gatto, B.; van der Veen, S.M.; Underation, M.K.; Thomas, J.S.; Antony, A.B.; Coomes, S.A. Cortical dynamics of movement-evoked pain in chronic low back pain. J. Physiol. 2021, 599, 289–305. [Google Scholar] [CrossRef]
- Kim, J.A.; Bosma, R.L.; Hemington, K.S.; Rogachov, A.; Osborne, N.R.; Cheng, J.C.; Oh, J.; Crawley, A.P.; Dunkley, B.T.; Davis, K.D. Neuropathic pain and pain interference are linked to alpha-band slowing and reduced beta-band magnetoencephalography activity within the dynamic pain connectome in patients with multiple sclerosis. Pain 2019, 160, 187–197. [Google Scholar] [CrossRef]
- Shtark, M.B.; Kozlova, L.I.; Bezmaternykh, D.D.; Mel’Nikov, M.Y.; Savelov, A.; Sokhadze, E.M. Neuroimaging Study of Alpha and Beta EEG Biofeedback Effects on Neural Networks. Appl. Psychophysiol. Biofeedback 2018, 43, 169–178. [Google Scholar] [CrossRef]
- Kisler, L.B.; Kim, J.A.; Hemington, K.S.; Rogachov, A.; Cheng, J.C.; Bosma, R.L.; Osborne, N.R.; Dunkley, B.T.; Inman, R.D.; Davis, K.D. Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. NeuroImage Clin. 2020, 26, 102241. [Google Scholar] [CrossRef]
- Mirabella, G. Should I stay or should I go? Conceptual underpinnings of goal-directed actions. Front. Syst. Neurosci. 2014, 8, 1–21. [Google Scholar] [CrossRef] [Green Version]

| Easy Trials | Total Points | Percent Easy | Easy Success Rate | Easy Task Duration |
|---|---|---|---|---|
| Preparatory beta | −0.32 ** | 0.15 | 0.05 | 0.28 † |
| Task beta | −0.30 ** | 0.11 | 0.11 | 0.17 |
| Hard Trials | Total points | Percent hard | Hard success rate | Hard task duration |
| Preparatory beta | −0.38 * | −0.04 | −0.40 * | 0.30 ** |
| Early Task Beta | −0.25 † | −0.10 | −0.24 † | 0.16 |
| Middle Task Beta | −0.31 ** | −0.18 | −0.33 ** | 0.16 |
| Late Task Beta | −0.28 † | −0.18 | −0.32 ** | 0.22 |
| Task Beta: (Late–Early) | −0.14 | −0.21 | −0.27 † | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilhelm, R.A.; Threadgill, A.H.; Gable, P.A. Motor Preparation and Execution for Performance Difficulty: Centroparietal Beta Activation during the Effort Expenditure for Rewards Task as a Function of Motivation. Brain Sci. 2021, 11, 1442. https://doi.org/10.3390/brainsci11111442
Wilhelm RA, Threadgill AH, Gable PA. Motor Preparation and Execution for Performance Difficulty: Centroparietal Beta Activation during the Effort Expenditure for Rewards Task as a Function of Motivation. Brain Sciences. 2021; 11(11):1442. https://doi.org/10.3390/brainsci11111442
Chicago/Turabian StyleWilhelm, Ricardo A., A. Hunter Threadgill, and Philip A. Gable. 2021. "Motor Preparation and Execution for Performance Difficulty: Centroparietal Beta Activation during the Effort Expenditure for Rewards Task as a Function of Motivation" Brain Sciences 11, no. 11: 1442. https://doi.org/10.3390/brainsci11111442
APA StyleWilhelm, R. A., Threadgill, A. H., & Gable, P. A. (2021). Motor Preparation and Execution for Performance Difficulty: Centroparietal Beta Activation during the Effort Expenditure for Rewards Task as a Function of Motivation. Brain Sciences, 11(11), 1442. https://doi.org/10.3390/brainsci11111442






