Effects of Physical Exercise on Cognition and Telomere Length in Healthy Older Women
Abstract
:1. Introduction
2. Materials and Methods
 L of each specific primer, 10
L of each specific primer, 10  L of the FastStart SYBR Green MasterMix and 7
L of the FastStart SYBR Green MasterMix and 7  L of water. The total amount of DNA used for each reaction was 10 ng in 2
L of water. The total amount of DNA used for each reaction was 10 ng in 2  L. The amplification program was as follows: 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s, 52 °C for 30 s and 60 °C for 1 min.
L. The amplification program was as follows: 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s, 52 °C for 30 s and 60 °C for 1 min.3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakian, V.A. Telomeres: Beginning to understand the end. Science 1995, 270, 1601–1607. [Google Scholar] [CrossRef]
- Blackburn, E.H. Structure and function of telomeres. Nature Publishing Group. Nature 1991, 353, 412–414. [Google Scholar]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell 1985, 43 Pt 1, 405–413. [Google Scholar] [CrossRef]
- Zhao, J.; Miao, K.; Wang, H.; Ding, H.; Wang, D.W. Association between telomere length and type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2013, 8, e79993. [Google Scholar] [CrossRef]
- Huzen, J.; Wong, L.S.M.; Van Veldhuisen, D.J.; Samani, N.J.; Zwinderman, A.H.; Codd, V.; Cawthon, R.M.; Benus, G.F.J.D.; Van Der Horst, I.C.C.; Navis, G.; et al. Telomere length loss due to smoking and metabolic traits. J. Intern. Med. 2014, 275, 155–163. [Google Scholar] [CrossRef]
- Best, J.R.; Davis, J.C.; Liu-Ambrose, T. Longitudinal analysis of physical performance, functional status, physical activity, and mood in relation to executive function in older adults who fall. J. Am. Geriatr. Soc. 2015, 63, 1112–1120. [Google Scholar] [CrossRef]
- Canela, A.; Vera, E.; Klatt, P.; Blasco, M.A. High-throughput telomere length quantification by FISH and its application to human population studies. Proc. Natl. Acad. Sci. USA 2007, 104, 5300–5305. [Google Scholar] [CrossRef] [Green Version]
- Mundstock, E.; Zatti, H.; Louzada, F.M.; Oliveira, S.G.; Guma, F.T.; Paris, M.M.; Rueda, A.B.; Machado, D.G.; Stein, R.T.; Jones, M.H.; et al. Effects of physical activity in telomere length: Systematic review and meta-analysis. Ageing Res. Rev. 2015, 22, 72–80. [Google Scholar] [CrossRef]
- Du, M.; Prescott, J.; Kraft, P.; Han, J.; Giovannucci, E.; Hankinson, S.E.; De Vivo, I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am. J. Epidemiol. 2012, 175, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Salthouse, T.A. Selective review of cognitive aging. J. Int. Neuropsychol. Soc. 2010, 16, 754–760. [Google Scholar] [CrossRef]
- Lezak, M.; Howieson, D.; Bigler, E.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Oberlin, L.; Verstynen, T.; Burzynska, A.; Voss, M.; Prakash, R.; Chaddock-Heyman, L. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. Neuroimage 2016, 131, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic Fitness Reduces Brain Tissue Loss in Aging Humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M176–M180. [Google Scholar] [CrossRef] [Green Version]
- Windle, G.; Hughes, D.; Linck, P.; Russell, I.; Woods, B. Is exercise effective in promoting mental well-being in older age? A systematic review. Aging Ment. Health 2010, 14, 652–669. [Google Scholar] [CrossRef]
- Luo, M.S.; Chui, E.W.T.; Li, L.W. The Longitudinal Associations between Physical Health and Mental Health among Older Adults. Aging Ment. Health 2020, 24, 1990–1998. [Google Scholar] [CrossRef]
- Formenti, D.; Cavaggioni, L.; Duca, M.; Trecroci, A.; Rapelli, M.; Alberti, G.; Komar, J.; Iodice, P. Acute Effect of Exercise on Cognitive Performance in Middle-Aged Adults: Aerobic Versus Balance. J. Phys. Act. Health 2020, 17, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, K.; Hayashi, Y.; Sakai, T.; Yahiro, T.; Tanaka, K.; Nishihira, Y. Acute Effects of Aerobic Exercise on Cognitive Function in Older Adults. J. Gerontol. Psychol. Sci. 2009, 64B, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.K.; Tsai, C.L.; Huang, C.C.; Wang, C.C.; Chu, I.H. Effects of acute resistance exercise on cognition in late middle-aged adults: General or specific cognitive improvement? J. Sci. Med. Sport 2014, 17, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Wang, Q.; Ting, N.S.; Brenner, D.R.; Conroy, S.M.; McIntyre, J.B.; Mickle, A.; Courneya, K.S.; Beattie, T. Effect of a 12-month exercise intervention on leukocyte telomere length: Results from the ALPHA Trial. Cancer Epidemiol. 2018, 56, 67–74. [Google Scholar] [CrossRef]
- Voss, M.W.; Weng, T.B.; Narayana-Kumanan, K.; Cole, R.C.; Wharff, C.; Reist, L.; DuBose, L.; Schmidt, P.G.; Sigurdsson, G.; Mills, J.A.; et al. Acute exercise effects predict training change in cognition and connectivity. Med. Sci. Sports Exerc. 2021, 52, 131–140. [Google Scholar] [CrossRef]
- Zhou, Y.; Hambly, B.D.; McLachlan, C.S. FTO associations with obesity and telomere length. J. Biomed. Sci. 2017, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; Nordfjall, K.; Mangino, M.; Balasubramanyam, M.; De Meyer, T.; Hendricks, A.E.; Giltay, E.J.; Hunt, S.C.; et al. Body mass index is negatively associated with telomere length: A collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 2018, 108, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Escobar, V.; Ezquerra, J.; Seva Díaz, A. El Mini Examen Cognoscitivo. Un test sencillo y práctico para detectar alteraciones intelectuales en pacientes médicos. Rev. Psiquiatr. Psicol. Médica 1979, 7, 198–202. [Google Scholar]
- Golden, C.J. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting Co.: Chicago, IL, USA, 1978. [Google Scholar]
- Bixby, W.R.; Spalding, T.W.; Haufler, A.J.; Deeny, S.P.; Mahlow, P.T.; Zimmerman, J.B.; Hatfield, B.D. The unique relation of physical activity to executive function in older men and women. Med. Sci. Sports Exerc. 2007, 39, 1408–1416. [Google Scholar] [CrossRef]
- Valgimigli, S.; Padovani, R.; Budriesi, C.; Leone, M.E.; Lugli, D.; Nichelli, P. The Stroop test: A normative Italian study on a paper version for clinical use. G. Ital. Psicol. 2010, 37, 945–956. [Google Scholar] [CrossRef]
- Brugnolo, A.; De Carli, F.; Accardo, J.; Amore, M.; Bosia, L.E.; Bruzzaniti, C.; Cappa, S.F.; Cocito, L.; Colazzo, G.; Ferrara, M.; et al. An updated Italian normative dataset for the Stroop color word test (SCWT). Neurol. Sci. 2016, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Reitan, R. Trail Making Test. Manual for Administration and Scoring; Reitan Neuropsychology Laboratory: Tucson, AZ, USA, 1992. [Google Scholar]
- Bruno, S.; Herrera Sanchez, M.B.; Pasquino, C.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G. Human Liver-Derived Stem Cells Improve Fibrosis and Inflammation Associated with Nonalcoholic Steatohepatitis. Stem Cells Int. 2019, 2019, 6351091. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Dutta, P.; Chard, N.; Wu, Y.; Chen, Q.H.; Chen, G.; Vadgama, J. A novel curcumin analog inhibits canonical and non-canonical functions of telomerase through STAT3 and NF-κB inactivation in colorectal cancer cells. Oncotarget 2019, 10, 4516–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OMS. Descriptive Note. 2020. Available online: https://www.who.int/es/news-room/fact-sheets/detail/physical-activity (accessed on 2 September 2021).
- Lin, S.; Yang, Y.; Qi, Q.; Wei, L.; Jing, N.; Jie, Z.; Xia, L.; Shifu, X. The beneficial effect of physical exercise on cognitive function in a non-dementia aging Chinese population. Front Aging Neurosci. 2019, 11, 238. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef]
- Spartano, N.L.; Demissie, S.; Himali, J.J.; Dukes, K.A.; Murabito, J.M.; Vasan, R.S.; Beiser, A.S.; Seshadri, S. Accelerometer-determined physical activity and cognitive function in middle-aged and older adults from two generations of the Framingham Heart Study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 618–626. [Google Scholar] [CrossRef]
- Albinet, C.T.; Abou-Dest, A.; André, N.; Audiffren, M. Executive functions improvement following a 5-month aquaerobics program in older adults: Role of cardiac vagal control in inhibition performance. Biol. Psychol. 2016, 115, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Mekari, S.; Dupuy, O.; Martins, R.; Evans, K.; Kimmerly, D.S.; Fraser, S.; Neyedli, H.F. The effects of cardiorespiratory fitness on executive function and prefrontal oxygenation in older adults. GeroScience 2019, 41, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Boucard, G.K.; Albinet, C.T.; Bugaiska, A.; Bouquet, C.A.; Clarys, D.; Audiffren, M. Impact of physical activity on executive functions in aging: A selective effect on inhibition among old adults. J. Sport Exerc. Psychol. 2012, 34, 808–827. [Google Scholar] [CrossRef]
- Denham, J.; Nelson, C.P.; O’Brien, B.J.; Nankervis, S.A.; Denniff, M.; Harvey, J.T.; Marques, F.Z.; Codd, V.; Zukowska-Szczechowska, E.; Samani, N.J.; et al. Longer Leukocyte Telomeres Are Associated with Ultra-Endurance Exercise Independent of Cardiovascular Risk Factors. PLoS ONE 2013, 8, e69377. [Google Scholar] [CrossRef]
- Mathur, S.; Ardestani, A.; Parker, B.; Cappizzi, J.; Polk, D.; Thompson, P.D. Telomere length and cardiorespiratory fitness in marathon runners. J. Investig. Med. 2013, 61, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Shadyab, A.H.; LaMonte, M.J.; Kooperberg, C.; Reiner, A.P.; Carty, C.L.; Manini, T.M.; Hou, L.; Di, C.; Macera, C.A.; Gallo, L.C.; et al. Leisure-time physical activity and leukocyte telomere length among older women. Exp. Gerontol. 2017, 95, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, P.; Fisher, R.; Kallings, L.; Svenson, U.; Roos, G.; Hellénius, M.L. Stand up for health—Avoiding sedentary behaviour might lengthen your telomeres: Secondary outcomes from a physical activity RCT in older people. Br. J. Sports Med. 2014, 48, 1407–1409. [Google Scholar] [CrossRef]
- Dimauro, I.; Scalabrin, M.; Fantini, C.; Grazioli, E.; Valls, M.R.B.; Mercatelli, N.; Parisi, A.; Sabatini, S.; Di Luigi, L.; Caporossi, D. Resistance training and redox homeostasis: Correlation with age-associated genomic changes. Redox Biol. 2016, 10, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2019, 40, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Hagstrom, A.D.; Denham, J. The effect of resistance training on telomere length in women recovering from breast cancer. J. Funct. Morphol. Kinesiol. 2018, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Furtado, C.L.; Ramos, F.K.P.; Kogure, G.S.; Santana-Lemos, B.A.; Ferriani, R.A.; Calado, R.T.; Dos Reis, R.M. A Nonrandomized Trial of Progressive Resistance Training Intervention in Women with Polycystic Ovary Syndrome and Its Implications in Telomere Content. Reprod. Sci. 2016, 23, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Nickels, M.; Mastana, S.; Hunter, D.; Denniff, M.; Codd, V.; Akam, E. The effect of a 12-week resistance training intervention on leukocyte telomere length. Heliyon 2020, 6, e04151. [Google Scholar] [CrossRef] [PubMed]
- Svenson, U.; Nordfjäll, K.; Baird, D.; Roger, L.; Osterman, P.; Hellenius, M.L.; Roos, G. Blood cell telomere length is a dynamic feature. PLoS ONE 2011, 6, e21485. [Google Scholar] [CrossRef]
- Mathur, M.B.; Epel, E.; Kind, S.; Desai, M.; Parks, C.G.; Sandler, D.P.; Khazeni, N. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immun. 2016, 54, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.Y.; Huang, Y.C.; Hung, C.F. Shortened telomere length in patients with depression: A meta-analytic study. J. Psychiatr. Res. 2016, 76, 84–93. [Google Scholar] [CrossRef] [PubMed]

| Statistical Descriptions | Control Group N = 33 | Experimental Group N = 41 | p-Value |
|---|---|---|---|
| Age (years) | Mean (SD) | Mean (SD) | 0.138 |
| 71.21 (4.329) | 72.70 (4.13) | ||
| Weight (kg) | Mean (SD) | Mean (SD) | |
| Pre | 62.72 (8.49) | 64.98 (8.41) | 0.258 |
| Post | 63.13 (8.40) | 64.70 (8.85) | 0.438 |
| %Fat (%) | Mean (SD) | Mean (SD) | |
| Pre | 38.73 (5.52) | 43.07 (3.60) | <0.001 |
| Post | 39.37 (5.45) | 42. 81 (4.93) | 0.006 |
| Variables | Time 0 | Time 1 | p Value | ||
|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Mental Status | |||||
| MMSE (max of 30) | 28.64 (1.50) | 27.67 (1.72) | 27.64 (1.75) | 28.40 (1.50) | <0.0001 |
| Attention | |||||
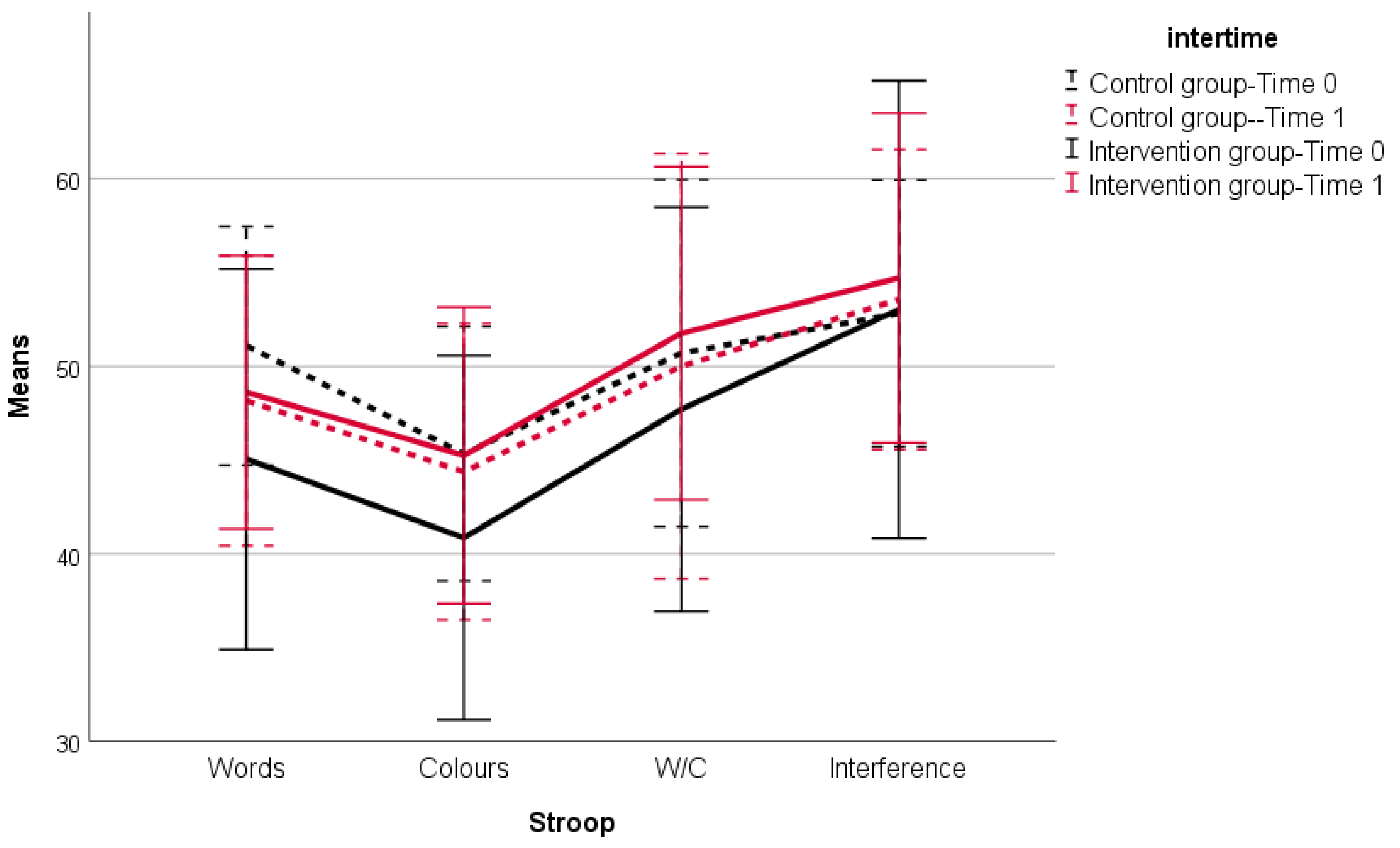
| Stroop Color–Word Test (number of items properly named in 45 s) | |||||
| Word reading | 50.78 (6.21) | 45.40 (10.02) | 48.16 (7.72) | 48.50 (7.34) | <0.0001 * |
| Color naming | 45.22 (6.86) | 40.70 (9.78) | 44.38 (7.91) | 45.20 (8.01) | 0.015 * |
| Word/Color | 50.97 (9.25) | 47.32 (10.63) | 50.00 (11.34) | 51.63 (8.96) | 0.200 * |
| Naming with interference | 53.31 (6.61) | 52.55 (11.97) | 53.56 (8.00) | 54.70 (8.79) | >0.999 * |
| Executive Functions | |||||
| Trail Making A (Seconds) | 56.19 (25.63) | 81.68 (35.74) | 67.25 (28.95) | 63.80 (22.46) | <0.001 * |
| Trail Making B (Seconds) | 151.38 (85.23) | 217.02 (108.52) | 178.44 (106.75) | 171.22 (79.02) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-González, J.L.; Sánchez-Rodríguez, J.L.; Martín-Vallejo, J.; Martel-Martel, A.; González-Sarmiento, R. Effects of Physical Exercise on Cognition and Telomere Length in Healthy Older Women. Brain Sci. 2021, 11, 1417. https://doi.org/10.3390/brainsci11111417
Sánchez-González JL, Sánchez-Rodríguez JL, Martín-Vallejo J, Martel-Martel A, González-Sarmiento R. Effects of Physical Exercise on Cognition and Telomere Length in Healthy Older Women. Brain Sciences. 2021; 11(11):1417. https://doi.org/10.3390/brainsci11111417
Chicago/Turabian StyleSánchez-González, Juan Luis, Juan Luis Sánchez-Rodríguez, Javier Martín-Vallejo, Abel Martel-Martel, and Rogelio González-Sarmiento. 2021. "Effects of Physical Exercise on Cognition and Telomere Length in Healthy Older Women" Brain Sciences 11, no. 11: 1417. https://doi.org/10.3390/brainsci11111417
APA StyleSánchez-González, J. L., Sánchez-Rodríguez, J. L., Martín-Vallejo, J., Martel-Martel, A., & González-Sarmiento, R. (2021). Effects of Physical Exercise on Cognition and Telomere Length in Healthy Older Women. Brain Sciences, 11(11), 1417. https://doi.org/10.3390/brainsci11111417








