Sleep-Related Rhythmic Movement Disorder in Young Children with Down Syndrome: Prevalence and Clinical Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Participant Recruitment
2.3. Measures
2.3.1. Questionnaires
- A demographic questionnaire comprising age, gender, ethnicity, parent occupation, highest parental education level attained, and geographical location based on postcode.
- The Child’s Sleep Habits questionnaire (CSHQ), consisting of 33 items, which can be subdivided into eight subscales: bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep-disordered breathing, and daytime sleepiness. Items are rated on a 3-point scale according to frequency of occurrence from usually to rarely. It is based on common clinical symptom presentations of the most prevalent paediatric diagnoses according to the ICSD. Psychometric properties show satisfactory test–retest reliability for both normal and clinical populations [17]. The CSHQ has previously been used to assess sleep problems in children with DS [10,18]. A total score threshold of 41 and above detects clinical sleep problems with a sensitivity of 0.80 and specificity of 0.72.
- A life events questionnaire, with 21 items, which focuses on recent life events [19] to ascertain any negative life events (i.e., death of an immediate family member, parental divorce, etc.) that may impact the child’s behaviour. In a typical adult population, 7–8 events are reported.
- The Strengths and Difficulties Questionnaire (SDQ), a 25-item behavioural screening tool divided into 5 sub-scales: emotional symptoms, conduct problems, hyperactivity, peer problems and a pro-social scale. Psychometric properties indicate good reliability and validity [20]. The SDQ was previously used in children with DS [21]. Age-appropriate versions were used. A total difficulties score of 0–13 (aged 4 years and older) or 0–12 (aged 2–4 years) is considered normal, and 14–16 and 13–15, respectively, are considered borderline.
- The Quantitative Checklist for Autism in Toddlers (QCHAT-10), a 10-item screening instrument for ASD in children under four years old. The QCHAT demonstrates good psychometric properties and external validity in discriminating between autism and developmental delay in children [22], with a recommended clinical threshold score of 3 [23].
2.3.2. Home Videosomnography
2.3.3. Actigraphy
- A.
- Sleep period = total number of minutes from sleep-onset to morning awakening time;
- B.
- Sleep minutes = total number of minutes scored as sleep during the sleep period—excludes any periods of wakefulness;
- C.
- Sleep efficiency (%) = proportion of minutes scored as sleep during the sleep period;
- D.
- Sleep onset latency = minutes taken from lights-out to sleep onset.
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Prevalence
- 1.
- Maximal prevalence
- 2.
- Likely prevalence
- 3.
- Minimal prevalence
3.2. Demographics
3.3. Actigraphy
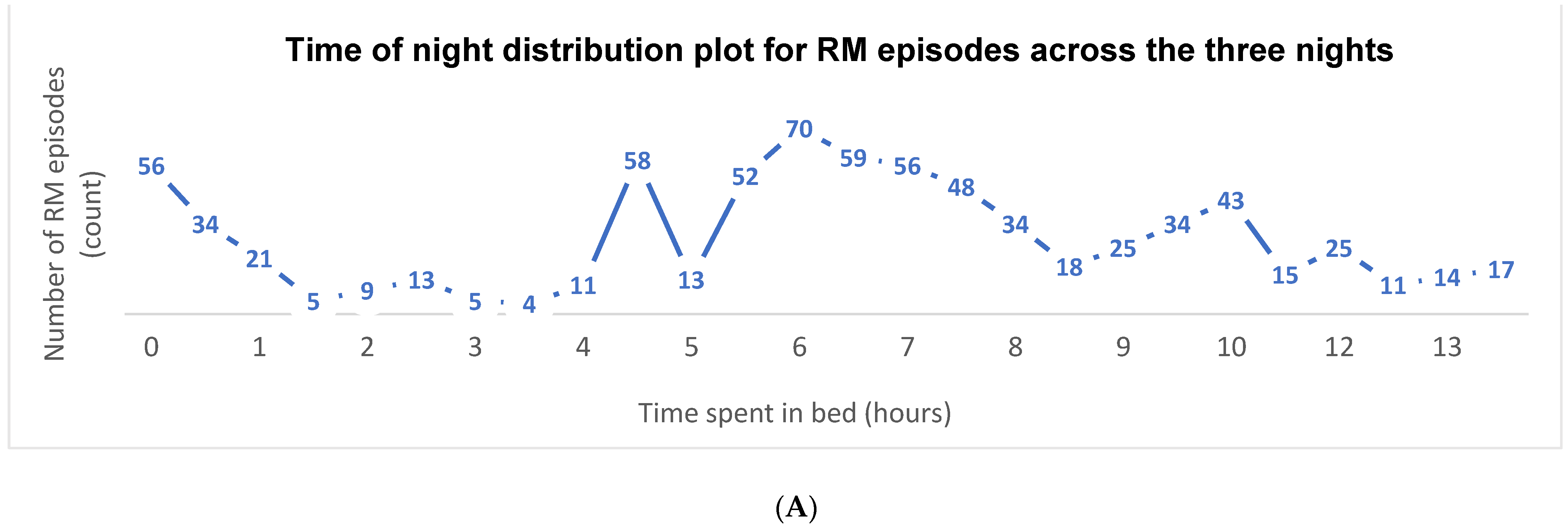
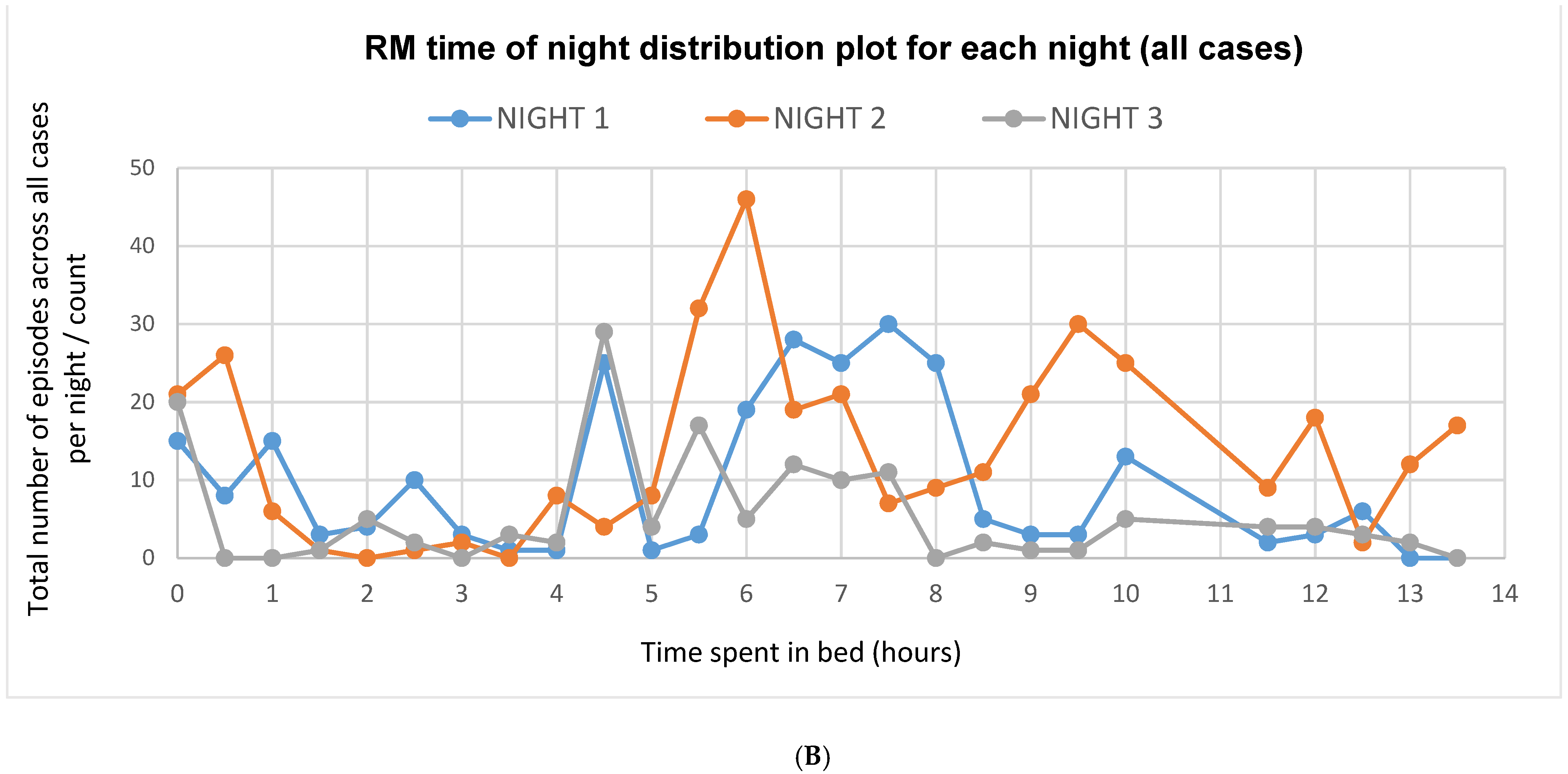
3.4. Video Scoring
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Gogo, E.; Van Sluijs, R.M.; Cheung, T.; Gaskell, C.; Jones, L.; Alwan, N.A.; Hill, C.M. Objectively confirmed prevalence of sleep-related rhythmic movement disorder in pre-school children. Sleep Med. 2018, 53, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Gwyther, A.R.; Walters, A.S.; Hill, C.M. Rhythmic movement disorder in childhood: An integrative review. Sleep Med. Rev. 2017, 35, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Sallustro, F.; Atwell, C.W. Body rocking, head banging, and head rolling in normal children. J. Pediatr. 1978, 93, 704–708. [Google Scholar] [CrossRef]
- Beckett, C.; Bredenkamp, D.; Castle, J.; Groothues, C.; O’Connor, T.G.; Rutter, M.L. Behavior Patterns Associated with Institutional Deprivation: A Study of Children Adopted from Romania. J. Dev. Behav. Pediatr. 2002, 23, 297–303. [Google Scholar] [CrossRef]
- Congenital Anomaly Statistics 2018. In Service NCAaRDR; Public Health England: London, UK, 2020; p. 32.
- Levanon, A.; Tarasiuk, A.; Tal, A. Sleep characteristics in children with Down syndrome. J. Pediatr. 1999, 134, 755–760. [Google Scholar] [CrossRef]
- Maris, M.; Verhulst, S.; Wojciechowski, M.; Van de Heyning, P.; Boudewyns, A. Prevalence of Obstructive Sleep Apnea in Children with Down Syndrome. Sleep 2016, 39, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Yau, S.; Pickering, R.M.; Gringras, P.; Elphick, H.; Evans, H.J.; Farquhar, M.; Martin, J.; Joyce, A.; Reynolds, J.; Kingshott, R.N.; et al. Sleep in infants and toddlers with Down syndrome compared to typically developing peers: Looking beyond snoring. Sleep Med. 2019, 63, 88–97. [Google Scholar] [CrossRef]
- Carter, M.; McCaughey, E.; Annaz, D.; Hill, C.M. Sleep problems in a Down syndrome population. Arch. Dis. Child. 2009, 94, 308–310. [Google Scholar] [CrossRef]
- DelRosso, L.M. Case 71—A 5-year-old boy with repetitive nocturnal movements. In Pediatric Sleep Pearls; DelRosso, L.M., Berry, R.B., Beck, S.E., Wagner, M.H., Marcus, C.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–241. [Google Scholar]
- Horne, R.S.; Wijayaratne, P.; Nixon, G.M.; Walter, L.M. Sleep and sleep disordered breathing in children with down syndrome: Effects on behaviour, neurocognition and the cardiovascular system. Sleep Med. Rev. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Nisbet, L.C.; Phillips, N.N.; Hoban, T.F.; O’Brien, L.M. Characterization of a sleep architectural phenotype in children with Down syndrome. Sleep Breath. 2014, 19, 1065–1071. [Google Scholar] [CrossRef]
- Joyce, A.; Elphick, H.; Farquhar, M.; Gringras, P.; Evans, H.; Bucks, R.; Kreppner, J.; Kingshott, R.; Martin, J.; Reynolds, J.; et al. Obstructive Sleep Apnoea Contributes to Executive Function Impairment in Young Children with Down Syndrome. Behav. Sleep Med. 2020, 18, 611–621. [Google Scholar] [CrossRef]
- Edgin, J.O.; Tooley, U.; Demara, B.; Nyhuis, C.; Anand, P.; Spanò, G. Sleep Disturbance and Expressive Language Development in Preschool-Age Children with Down Syndrome. Child Dev. 2015, 86, 1984–1998. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.M.; Evans, H.J.; Elphick, H.; Farquhar, M.; Pickering, R.M.; Kingshott, R.; Martin, J.; Reynolds, J.; Joyce, A.; Rush, C.; et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med. 2016, 27-28, 99–106. [Google Scholar] [CrossRef]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef]
- Shott, S.R.; Amin, R.; Chini, B.; Heubi, C.; Hotze, S.; Akers, R. Obstructive Sleep Apnea. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 432. [Google Scholar] [CrossRef] [Green Version]
- Brugha, T.S.; Bebbington, P.; Tennant, C.; Hurry, J. The List of Threatening Experiences: A subset of 12 life event categories with considerable long-term contextual threat. Psychol. Med. 1985, 15, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Goodman, R. Psychometric Properties of the Strengths and Difficulties Questionnaire. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 1337–1345. [Google Scholar] [CrossRef]
- Warner, G.; Moss, J.; Smith, P.; Howlin, P. Autism characteristics and behavioural disturbances in ~500 children with Down’s syndrome in England and Wales. Autism Res. 2014, 7, 433–441. [Google Scholar] [CrossRef]
- Ruta, L.; Chiarotti, F.; Arduino, G.M.; Apicella, F.; Leonardi, E.; Maggio, R.; Carrozza, C.; Chericoni, N.; Costanzo, V.; Turco, N.; et al. Validation of the Quantitative Checklist for Autism in Toddlers in an Italian Clinical Sample of Young Children With Autism and Other Developmental Disorders. Front Psychiatry 2019, 10, 488. [Google Scholar] [CrossRef] [Green Version]
- Allison, C.; Auyeung, B.; Baron-Cohen, S. Toward brief “Red Flags” for autism screening: The Short Autism Spectrum Quotient and the Short Quantitative Checklist for Autism in toddlers in 1000 cases and 3000 controls [corrected]. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 202–212.e7. [Google Scholar] [CrossRef]
- Rutter, M.; Bailey, A.; Lord, C. The Social Communication Questionnaire; Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Magyar, C.I.; Pandolfi, V.; Dill, C.A. An Initial Evaluation of the Social Communication Questionnaire for the Assessment of Autism Spectrum Disorders in Children with Down Syndrome. J. Dev. Behav. Pediatr. 2012, 33, 134–145. [Google Scholar] [CrossRef]
- Hyde, M.; O’Driscoll, D.M.; Binette, S.; Galang, C.; Tan, S.K.; Verginis, N.; Davey, M.J.; Horne, R.S.C. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J. Sleep Res. 2007, 16, 213–216. [Google Scholar] [CrossRef]
- Sadeh, A.; Hauri, P.J.; Kripke, D.F.; Lavie, P. The Role of Actigraphy in the Evaluation of Sleep Disorders. Sleep 1995, 18, 288–302. [Google Scholar] [CrossRef]
- Vrugt, D.T.; Pederson, D.R. The effects of vertical rocking frequencies on the arousal level in two-month-old infants. Child Dev. 1973, 44, 205–209. [Google Scholar] [CrossRef]
- Chirakalwasan, N.; Hassan, F.; Kaplish, N.; Fetterolf, J.; Chervin, R. Near resolution of sleep related rhythmic movement disorder after CPAP for OSA. Sleep Med. 2009, 10, 497–500. [Google Scholar] [CrossRef]
- Groswasser, J.; Sottiaux, M.; Rebuffat, E.; Simon, T.; Vandeweyer, M.; Kelmanson, I.; Blum, D.; Kahn, A. Reduction in obstructive breathing events during body rocking: A controlled polygraphic study in preterm and full-term infants. Pediatrics 1995, 96, 64–68. [Google Scholar]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Spicuzza, L.; Caruso, D.; Di Maria, G. Obstructive sleep apnoea syndrome and its management. Ther. Adv. Chronic Dis. 2015, 6, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Acebo, C.; Sadeh, A.; Seifer, R.; Tzischinsky, O.; Wolfson, A.R.; Hafer, A.; Carskadon, M.A. Estimating Sleep Patterns with Activity Monitoring in Children and Adolescents: How Many Nights Are Necessary for Reliable Measures? Sleep 1999, 22, 95–103. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Hoffman, E.K.; Stansberry, E.; Shaffer, R. Convergent validity of actigraphy with polysomnography and parent reports when measuring sleep in children with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 281–291. [Google Scholar] [CrossRef]
- Gall, M.; Kohn, B.; Wiesmeyr, C.; van Sluijs, R.; Wilhelm, E.; Rondei, Q.; Jäger, L.; Achermann, P.; Landolt, H.-P.; Jenni, O.G.; et al. A Novel Approach to Assess Sleep-Related Rhythmic Movement Disorder in Children Using Automatic 3D Analysis. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
- Kushida, C.A.; Chang, A.; Gadkary, C.; Guilleminault, C.; Carrillo, O.; Dement, W.C. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001, 2, 389–396. [Google Scholar] [CrossRef]




| Variable | Cases n = 8 Mean (SD) | Controls n = 8 Mean (SD) | Significance Level | ||
|---|---|---|---|---|---|
| Age in months | 54.75 (21.24) | 56.00 (20.68) | p = 0.91 | ||
| Gender | 4M:4F | 4M:4F | p = 1.00 | ||
| Ethnicity | White British | n = 6 | White British | n = 7 | p = 0.58 |
| Black British | n = 1 | Black British | n = 1 | ||
| Asian British | n = 1 | Asian British | n = 0 | ||
| Parent educational level | N/A | n = 1 | N/A | n = 0 | p = 0.21 |
| GCSE * | n = 0 | GCSE * | n = 1 | ||
| A-Level | n = 2 | A-Level | n = 5 | ||
| Degree or higher | n = 5 | Degree or higher | n = 2 | ||
| CSHQ total sleep disturbance score | 53.88 (5.44) | 54.50 (7.76) | p = 0.86 | ||
| Total Recent Life Events Score | 2.13 (1.81) | 2.88 (2.30) | p = 0.48 | ||
| Total SDQ Difficulties Score | 11.75 (4.59) | 13.63 (2.07) | p = 0.31 | ||
| Total QCHAT-10 score ** | 0.50 (0.71) | 4.00 (2.65) | p = 0.18 | ||
| Total SCQ score *** | 9.33 (3.08) | 8.20 (3.03) | p = 0.56 | ||
| Participant | Age (months) | Gender | Number of Episodes per Night (Mean, (Range)) | Duration of Episodes in Seconds (Mean, (Range)) | Total Time Spent in RMs in Minutes per Night (Mean, (Range)) | Semiology | |||
|---|---|---|---|---|---|---|---|---|---|
| Movement Types | Range of Frequencies of Rhythmic Movements (Hz) | Impact against Object (i.e., Mattress/Cushioning Apparatus) | Vocalisation | ||||||
| A | 54 | F | 48.0, (39–58) | 65.2, (1–723) | 52.2, (38.1–65.9) | Head banging, body rocking | 0.20–1.17 | Yes | Yes |
| B | 18 | F | 12.3, (5–19) | 14.0, (3–98) | 2.8, (2.6–3.1) | Leg banging, hand banging, arm waving | 0.30–2.00 | Yes | No |
| C | 66 | F | 7.3, (5–9) | 27.1, (4–77) | 3.2, (2.2–3.9) | Rubbing against hands/toy | 0.36–0.81 | No | No |
| D | 30 | M | 11.7, (9–13) | 14.6, (3–55) | 2.8, (2.1–3.5) | Head banging and rolling, body rocking, leg banging and rolling, arm banging, hand banging | 0.31–2.00 | Yes | No |
| E | 75 | M | 71.3, (36–94) | 51.1, (2–388) | 60.8, (11.7–104.3) | Head banging, body rocking, bouncing | 0.26–1.50 | Yes | No |
| F | 74 | M | 59.7, (31–85) | 41.4, (2–263) | 41.2, (18.5–58.7) | Head-banging | 0.60–2.50 | Yes | No |
| G | 50 | F | 44.7, (5–121) | 26.8, (3–239) | 20.0, (0.5–58.1) | Lateral hip movements | 0.31–1.00 | No | No |
| H | 71 | M | 8.0, (3–13) | 156.5, (4–849) | 20.9, (5.7–46.0) | Body rolling | 0.59–1.53 | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, C.; Wood, I.; Gwyther, A.; Basnet, S.; Gaskell, C.; Gringras, P.; Elphick, H.; Evans, H.; Hill, C.M. Sleep-Related Rhythmic Movement Disorder in Young Children with Down Syndrome: Prevalence and Clinical Features. Brain Sci. 2021, 11, 1326. https://doi.org/10.3390/brainsci11101326
Kose C, Wood I, Gwyther A, Basnet S, Gaskell C, Gringras P, Elphick H, Evans H, Hill CM. Sleep-Related Rhythmic Movement Disorder in Young Children with Down Syndrome: Prevalence and Clinical Features. Brain Sciences. 2021; 11(10):1326. https://doi.org/10.3390/brainsci11101326
Chicago/Turabian StyleKose, Ceren, Izabelle Wood, Amy Gwyther, Susiksha Basnet, Chloe Gaskell, Paul Gringras, Heather Elphick, Hazel Evans, and Catherine M. Hill. 2021. "Sleep-Related Rhythmic Movement Disorder in Young Children with Down Syndrome: Prevalence and Clinical Features" Brain Sciences 11, no. 10: 1326. https://doi.org/10.3390/brainsci11101326
APA StyleKose, C., Wood, I., Gwyther, A., Basnet, S., Gaskell, C., Gringras, P., Elphick, H., Evans, H., & Hill, C. M. (2021). Sleep-Related Rhythmic Movement Disorder in Young Children with Down Syndrome: Prevalence and Clinical Features. Brain Sciences, 11(10), 1326. https://doi.org/10.3390/brainsci11101326





