The Effects of Auditory Feedback Gait Training Using Smart Insole on Stroke Patients
Abstract
1. Introduction
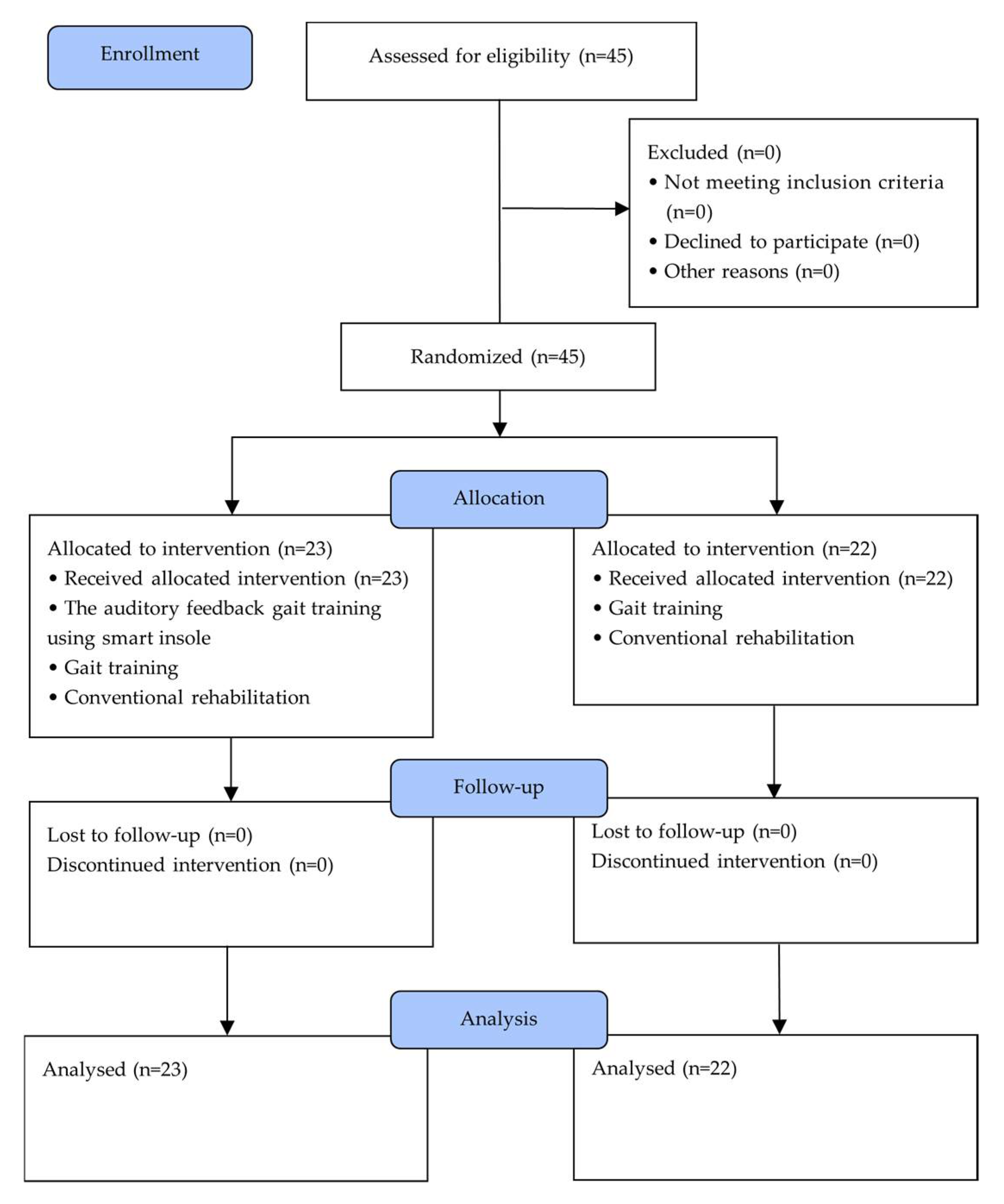
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedure
2.3. Experimeantal Method
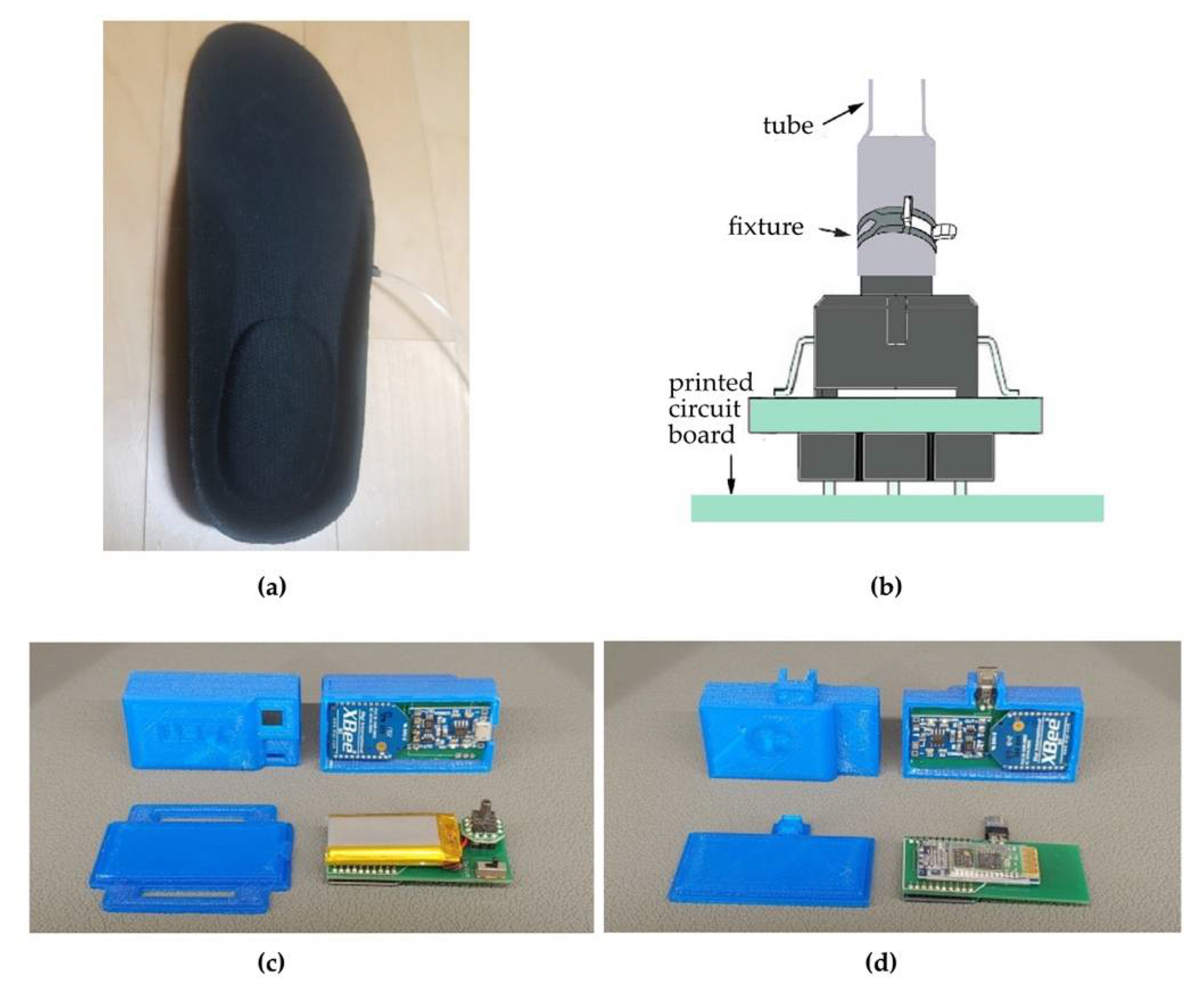
2.3.1. Insole Auditory Feedback
2.3.2. Auditory Feedback Gait Training Using the Smart Insole (AFGT)
2.3.3. Conventional Rehabilitation Training
2.3.4. General Gait Training (GGT)
2.4. Assessment Tools and Data Collection
2.4.1. Gait Variables
2.4.2. Gait Symmetry
2.4.3. Dynamic Balance
- Timed up and Go (TUG)
- 2.
- Berg Balance Scale (BBS)
2.4.4. Modified Barthel Index (MBI)
2.4.5. Mini Mental State Examination-Korea Version (MMSE-K)
2.5. Data Analysis
3. Results
3.1. Gait
3.1.1. Temporal Gait Parameter
3.1.2. Spatial Gait Parameter
3.1.3. Symmetry of Gait
3.2. Dynamic Balance
3.3. Activities of Daily Living (ADL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gebruers, N.; Vanroy, C.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Monitoring of physical activity after stroke: A systematic review of accelerometry-based measures. Arch. Phys. Med. Rehabil. 2010, 91, 288–297. [Google Scholar] [CrossRef]
- Bays, C.L. Quality of life of stroke survivors: A research synthesis. The Journal of neuroscience nursing. J. Am. Assoc. Neurosci. Nurses 2001, 33, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, C.K.; Bowden, M.G.; Neptune, R.R.; Kautz, S.A. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch. Phys. Med. Rehabil. 2007, 88, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.; Edwards, S.; Freeman, J. Associated reactions: Their value in clinical practice? Physiother. Res. Int. J. Res. Clin. Phys. Ther. 1998, 3, 151–152. [Google Scholar] [CrossRef]
- Forghany, S.; Tyson, S.; Nester, C.; Preece, S.; Jones, R. Foot posture after stroke: Frequency, nature and clinical significance. Clin. Rehabil. 2011, 25, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.B.; Schmitz, T.J.; Fulk, G. Physical Rehabilitation; F.A. Davis Company: Philadelphia, PA, USA, 2019. [Google Scholar]
- Adamczyk, P.G.; Kuo, A.D. Mechanical and energetic consequences of rolling foot shape in human walking. J. Exp. Biol. 2013, 216, 2722–2731. [Google Scholar] [CrossRef]
- Chisholm, A.E.; Perry, S.D.; McIlroy, W.E. Inter-limb centre of pressure symmetry during gait among stroke survivors. Gait Posture 2011, 33, 238–243. [Google Scholar] [CrossRef]
- Marigold, D.S.; Eng, J.J.; Timothy Inglis, J. Modulation of ankle muscle postural reflexes in stroke: Influence of weight-bearing load. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 2789–2797. [Google Scholar] [CrossRef][Green Version]
- Brady, K.; Garcia, T. Constraint-induced movement therapy (CIMT): Pediatric applications. Dev. Disabil. Res. Rev. 2009, 15, 102–111. [Google Scholar] [CrossRef]
- Etoom, M.; Hawamdeh, M.; Hawamdeh, Z.; Alwardat, M.; Giordani, L.; Bacciu, S.; Scarpini, C.; Foti, C. Constraint-induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: Systematic review and meta-analysis. Int. J. Rehabil. Research. Int. Z. Rehabilitationsforschung. Rev. Int. Rech. Readapt. 2016, 39, 197–210. [Google Scholar] [CrossRef]
- Kim, J.S.; Nam, C.H.; Kim, J.Y.; Gye, J.W.; Hong, S.P.; Kim, M.H.; Park, B.C. Objective Evaluation of the Effect of Q-Switched Nd:YAG (532 nm) Laser on Solar Lentigo by Using a Colorimeter. Ann. Dermatol. 2015, 27, 326–328. [Google Scholar] [CrossRef]
- Seo, M.; Shin, M.-J.; Park, T.S.; Park, J.-H. Clinometric Gait Analysis Using Smart Insoles in Patients with Hemiplegia After Stroke: Pilot Study. JMIR Mhealth Uhealth 2020, 8, e22208. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.J.; Parker, J.; McCullagh, P.; Zheng, H.; Nugent, C.; Black, N.D.; Mawson, S. A Personalized Self-Management Rehabilitation System for Stroke Survivors: A Quantitative Gait Analysis Using a Smart Insole. JMIR Rehabil. Assist. Technol. 2016, 3, e11. [Google Scholar] [CrossRef]
- Truong, P.H.; Lee, J.; Kwon, A.-R.; Jeong, G.-M. Stride Counting in Human Walking and Walking Distance Estimation Using Insole Sensors. Sensors 2016, 16, 823. [Google Scholar] [CrossRef] [PubMed]
- Hegde, N.; Bries, M.; Swibas, T.; Melanson, E.; Sazonov, E. Automatic Recognition of Activities of Daily Living Utilizing Insole-Based and Wrist-Worn Wearable Sensors. IEEE J. Biomed. Health Inform. 2018, 22, 979–988. [Google Scholar] [CrossRef]
- Munoz-Organero, M.; Parker, J.; Powell, L.; Mawson, S. Assessing Walking Strategies Using Insole Pressure Sensors for Stroke Survivors. Sensors 2016, 16, 1631. [Google Scholar] [CrossRef]
- Farid, L.; Jacobs, D.; Do Santos, J.; Simon, O.; Gracies, J.-M.; Hutin, E. FeetMe Monitor-connected insoles are a valid and reliable alternative for the evaluation of gait speed after stroke. Top. Stroke Rehabil. 2021, 28, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.C.; Rao, N.; Muthukrishnan, S.; Aruin, A.S. A textured insole improves gait symmetry in individuals with stroke. Disabil. Rehabil. 2018, 40, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.E.; Soraghan, C.J.; Matthews, F.; Markham, C. A concept for extending the applicability of constraint-induced movement therapy through motor cortex activity feedback using a neural prosthesis. Comput. Intell. Neurosci. 2007, 2007, 51363. [Google Scholar] [CrossRef] [PubMed]
- Khoo, I.H.; Marayong, P.; Krishnan, V.; Balagtas, M.; Rojas, O.; Leyba, K. Real-time biofeedback device for gait rehabilitation of post-stroke patients. Biomed. Eng. Lett. 2017, 7, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H. The Influence of Auditory-Feedback Device Using Wearable Air-Pressure Insole on Spatiotemporal Gait Symmetry in Chronic Hemplegia; Sahm Yook University: Seoul, Korea, 2019. [Google Scholar]
- Webster, K.E.; Wittwer, J.E.; Feller, J.A. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture 2005, 22, 317–321. [Google Scholar] [CrossRef]
- van Bloemendaal, M.; Beelen, A.; Kleissen, R.F.M.; Geurts, A.C.; Nollet, F.; Bus, S.A. Concurrent validity and reliability of a low-cost gait analysis system for assessment of spatiotemporal gait parameters. J. Rehabil. Med. 2019, 51, 456–463. [Google Scholar] [CrossRef]
- Hsu, A.-L.; Tang, P.-F.; Jan, M.-H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003, 84, 1185–1193. [Google Scholar] [CrossRef]
- In, T.S.; Song, C.S. The Effects of Whole Body Vibration on Knee Extensor Strength, and Balance and Walking Ability with Chronic Stroke. J. Korean Soc. Phys. Med. 2010, 5, 675–683. [Google Scholar]
- Downs, S.; Marquez, J.; Chiarelli, P. The Berg Balance Scale has high intra- and inter-rater reliability but absolute reliability varies across the scale: A systematic review. J. Physiother. 2013, 59, 93–99. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kyu, P.B.; Shin, H.S.; Kang, Y.K.; Pyun, S.B.; Paik, N.J.; Kim, S.H.; Kim, T.H.; Han, T.R. Development of the Korean Version of Modified Barthel Index (K-MBI): Multi-center Study for Subjects with Stroke. J. Korean Acad. Rehabil. Med. 2007, 31, 283–297. [Google Scholar]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef]
- Schmitt, J.S.; Di Fabio, R.P. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. J. Clin. Epidemiol. 2004, 57, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- de Vet, H.C.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol 2006, 59, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.M.; Pei, Y.-C.; Hong, W.-H.; Chung, C.-Y.; Lau, Y.-C.; Chen, C.P. Foot contact pattern analysis in hemiplegic stroke patients: An implication for neurologic status determination11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabil. 2004, 85, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mukaino, M.; Ohtsuka, K.; Otaka, Y.; Tanikawa, H.; Matsuda, F.; Tsuchiyama, K.; Yamada, J.; Saitoh, E. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int. J. Rehabil. Research. Int. Z. Rehabilitationsforschung. Rev. Int. Rech. Readapt. 2020, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Changes in gait symmetry and velocity after stroke: A cross-sectional study from weeks to years after stroke. Neurorehabilit. Neural Repair 2010, 24, 783–790. [Google Scholar] [CrossRef]
- Nadeau, S. Understanding Spatial and Temporal Gait Asymmetries in Individuals Post Stroke. Int. J. Phys. Med. Rehabil. 2014, 2. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Hu, S.; Zhu, B.; Han, F.; Zhao, X. Brunnstrom Stage Automatic Evaluation for Stroke Patients by Using Multi-Channel sEMG. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]
- Sim, A.J.; Redler, G.; Peacock, J.; Naso, C.; Wasserman, S.; McNitt, K.B.; Hoffe, S.E.; Johnstone, P.A.S.; Harrison, L.B.; Rosenberg, S.A. Harnessing COVID-Driven Technical Innovations for Improved Multi-Disciplinary Cancer Care in the Post-COVID Era: The Virtual Patient Room. Cancer Control. J. Moffitt Cancer Cent. 2020, 27, 1073274820964800. [Google Scholar] [CrossRef]
- Turns, L.J.; Neptune, R.R.; Kautz, S.A. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch. Phys. Med. Rehabil. 2007, 88, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Yelnik, A.; Bonan, I. Clinical tools for assessing balance disorders. Neurophysiol. Clin. Clin. Neurophysiol. 2008, 38, 439–445. [Google Scholar] [CrossRef]
- Faria, C.D.C.d.M.; Teixeira-Salmela, L.F.; Nadeau, S. Effects of the direction of turning on the timed up & go test with stroke subjects. Top. Stroke Rehabil. 2009, 16, 196–206. [Google Scholar]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Crea, S.; Cipriani, C.; Donati, M.; Carrozza, M.C.; Vitiello, N. Providing Time-Discrete Gait Information by Wearable Feedback Apparatus for Lower-Limb Amputees: Usability and Functional Validation. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 250–257. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef]
- Ngueleu, A.M.; Blanchette, A.K.; Bouyer, L.; Maltais, D.; McFadyen, B.J.; Moffet, H.; Batcho, C.S. Design and Accuracy of an Instrumented Insole Using Pressure Sensors for Step Count. Sensors 2019, 19, 984. [Google Scholar] [CrossRef]
- Arndt, A. Correction for sensor creep in the evaluation of long-term plantar pressure data. J. Biomech. 2003, 36, 1813–1817. [Google Scholar] [CrossRef]
- Koch, M.; Lunde, L.-K.; Ernst, M.; Knardahl, S.; Veiersted, K.B. Validity and reliability of pressure-measurement insoles for vertical ground reaction force assessment in field situations. Appl. Ergon. 2016, 53, 44–51. [Google Scholar] [CrossRef]
- Razak, A.H.A.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot plantar pressure measurement system: A review. Sensors 2012, 12, 9884–9912. [Google Scholar] [CrossRef]
- Woodburn, J.; Helliwell, P.S. Obervations on the F-scan in-shoe pressure measuring with. Clin. Biomech. 1997, 12, S16. [Google Scholar] [CrossRef]
- Smith, B.T.; Coiro, D.J.; Finson, R.; Betz, R.R.; McCarthy, J. Evaluation of force-sensing resistors for gait event detection to trigger electrical stimulation to improve walking in the child with cerebral palsy. IEEE Eng. Med. Biol. Soc. 2002, 10, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gouwanda, D.; Gopalai, A.A. A robust real-time gait event detection using wireless gyroscope and its application on normal and altered gaits. Med. Eng. Phys. 2015, 37, 219–225. [Google Scholar] [CrossRef]
- Liedtke, C.; Fokkenrood, S.A.W.; Menger, J.T.; van der Kooij, H.; Veltink, P.H. Evaluation of instrumented shoes for ambulatory assessment of ground reaction forces. Gait Posture 2007, 26, 39–47. [Google Scholar] [CrossRef] [PubMed]
- van Meulen, F.B.; Weenk, D.; van Asseldonk, E.H.F.; Schepers, H.M.; Veltink, P.H.; Buurke, J.H. Analysis of Balance during Functional Walking in Stroke Survivors. PLoS ONE 2016, 11, e0166789. [Google Scholar] [CrossRef]
| AFGTG (n = 23) | GGTG (n = 22) | χ2/t | p | |
|---|---|---|---|---|
| Age (year) | 61.78 ± 8.06 | 64.23 ± 0.57 | 0.875 | 0.386 |
| Height (cm) | 164.65 ± 7.49 | 162.45 ± 9.13 | 0.885 | 0.381 |
| Weight (kg) | 61.12 ± 7.61 | 61.47 ± 8.95 | 0.142 | 0.888 |
| BMI (point) | 22.51 ± 2.15 | 23.2 ± 2.00 | 1.111 | 0.273 |
| Duration of stroke (month) | 14.57 ± 7.24 | 16.86 ± 7.19 | 1.068 | 0.291 |
| MMSE-K | 25.78 ± 1.31 | 25.68 ± 0.99 | 0.289 | 0.774 |
| Gender (male/female) | 14/9 | 13/9 | 0.015 | 0.903 |
| Paretic side (right/left) | 12/11 | 16/6 | 2.021 | 0.155 |
| Stroke type (infarction/hemorrhage) | 16/7 | 13/9 | 0.538 | 0.463 |
| AFGTG (n = 23) | GGTG (n = 22) | Time F(p) | Interaction F(p) | MDC MDC% | ||
|---|---|---|---|---|---|---|
| Velocity (cm/s) | Pre | 29.65 ± 15.18 | 30.72 ± 14.20 | |||
| Post | 34.62 ± 16.88 | 32.63 ± 16.04 | 33.987 | 6.739 | 2.58 | |
| Pre-Post | 4.98 ± 4.47 † | 1.91 ± 3.35 † | (0.000) | (0.013) | 51.91 | |
| Cadence (step/min) | Pre | 55.46 ± 13.15 | 56.21 ± 12.75 | |||
| Post | 59.98 ± 17.94 | 58.52 ± 16.61 | 10.546 | 1.113 | ||
| Pre-Post | 4.52 ± 7.96 † | 2.30 ± 5.94 | (0.000) | (0.297) | ||
| Stride time (sec) | Pre | 2.36 ± 0.99 | 2.33 ± 1.01 | |||
| Post | 2.26 ± 1.05 | 2.29 ± 1.04 | 4.044 | 0.021 | ||
| Pre-Post | −0.10 ± 0.28 | −0.04 ± 0.17 | (0.051) | (0.344) | ||
| Affected side Step time (sec) | Pre | 1.36 ± 0.50 | 1.35 ± 0.50 | |||
| Post | 1.23 ± 0.52 | 1.33 ± 0.51 | 12.976 | 5.188 | 0.09 | |
| Pre-Post | −0.12 ± 0.16 † | −0.03 ± 0.12 | (0.001) | (0.028) | 73.62 | |
| Unaffected side Step time (sec) | Pre | 1.01 ± 0.50 | 0.98 ± 0.52 | |||
| Post | 1.03 ± 0.53 | 0.97 ± 0.55 | 0.092 | 0.511 | ||
| Pre-Post | 0.02 ± 0.16 | −0.01 ± 0.12 | (0.763) | (0.479) | ||
| Unaffected side Sing limb support time (sec) | Pre | 0.53 ± 0.14 | 0.57 ± 0.15 | |||
| Post | 0.54 ± 0.14 | 0.57 ± 0.16 | 0.163 | 0.002 | ||
| Pre-Post | 0.00 ± 0.05 | 0.00 ± 0.07 | (0.668) | (0.961) | ||
| Affected side Sing limb support time (sec) | Pre | 0.39 ± 0.14 | 0.40 ± 0.14 | |||
| Post | 0.44 ± 0.14 | 0.40 ± 0.14 | 3.377 | 7.021 | 0.03 | |
| Pre-Post | 0.05 ± 0.05 † | 0.00 ± 0.07 | (0.073) | (0.011) | 61.43 | |
| Double limb support (%) | Pre | 57.70 ± 14.39 | 55.29 ± 13.73 | |||
| Post | 52.28 ± 15.86 | 54.15 ± 12.79 | 40.633 | 17.361 | 2.28 | |
| Pre-Post | −5.41 ± 3.95 † | −1.13 ± 2.82 | (0.000) | (0.000) | 42.14 |
| AFGTG (n = 23) | GGTG (n = 22) | Time F(p) | Interaction F(p) | MDC MDC% | ||
|---|---|---|---|---|---|---|
| Stride length (cm) | Pre | 62.26 ± 21.97 | 64.25 ± 20.77 | |||
| Post | 67.31 ± 18.66 | 65.11 ± 18.84 | 12.337 | 6.179 | 3.76 | |
| Pre-Post | 5.05 ± 6.50 † | 0.86 ± 4.59 | (0.001) | (0.017) | 74.35 | |
| Affected side Step length (cm) | Pre | 33.27 ± 12.74 | 34.08 ± 12.11 | |||
| Post | 34.48 ± 10.44 | 34.13 ± 10.29 | 1.138 | 0.978 | ||
| Pre-Post | 1.21 ± 3.94 | 0.05 ± 3.93 | (0.292) | (0.328) | ||
| Unaffected side Step length (cm) | Pre | 28.99 ± 9.56 | 30.17 ± 9.02 | |||
| Post | 32.83 ± 8.76 | 30.99 ± 9.02 | 24.960 | 10.510 | 2.03 | |
| Pre-Post | 3.84 ± 3.51 † | 0.82 ± 2.68 | (0.000) | (0.002) | 52.75 | |
| Affected side gait line (cm) | Pre | 22.71 ± 3.8 | 22.4 ± 3.59 | |||
| Post | 24.69 ± 4.47 | 22.61 ± 2.99 | 7.678 | 5.028 | 0.69 | |
| Pre-Post | 1.98 ± 1.20 † | 0.21 ± 3.59 | (0.008) | (0.030) | 34.87 |
| AFGTG (n = 23) | GGTG (n = 22) | Time F(p) | Interaction F(p) | MDC MDC% | ||
|---|---|---|---|---|---|---|
| Gait symmetry on step length (score) | Pre | 0.19 ± 0.10 | 0.17 ± 0.11 | |||
| Post | 0.12 ± 0.08 | 0.20 ± 0.19 | 1.090 | 4.218 | 0.06 | |
| Pre-Post | −0.07 ± 0.10 † | 0.02 ± 0.17 | (0.302) | (0.046) | 91.66 | |
| Gait symmetry on step time (score) | Pre | 0.40 ± 0.23 | 0.46 ± 0.29 | |||
| Post | 0.23 ± 0.11 | 0.49 ± 0.43 | 3.377 | 7.021 | 0.12 | |
| Pre-Post | −0.16 ± 0.20 † | 0.03 ± 0.28 | (0.073) | (0.011) | 71.83 | |
| Gait symmetry on single stance time (score) | Pre | 0.39 ± 0.14 | 0.40 ± 0.14 | |||
| Post | 0.44 ± 0.14 | 0.40 ± 0.14 | 7.025 | 6.853 | 0.03 | |
| Pre-Post | 0.05 ± 0.05 † | 0.00 ± 0.07 | (0.011) | (0.012) | 61.43 |
| AFGTG (n = 23) | GGTG (n = 22) | Time F(p) | Interaction F(p) | MDC MDC% | ||
|---|---|---|---|---|---|---|
| TUG (sec) | Pre | 31.76 ± 7.08 | 32.53 ± 6.74 | |||
| Post | 27.59 ± 5.24 | 30.81 ± 6.54 | 35.633 | 6.152 | 1.76 | |
| Pre-Post | −4.16 ± 3.05 † | −1.72 ± 3.55 † | (0.000) | (0.017) | 42.37 | |
| BBS (point) | Pre | 29.42 ± 9.39 | 29.48 ± 8.95 | |||
| Post | 33.48 ± 8.55 | 31.28 ± 8.30 | 37.997 | 5.632 | 1.61 | |
| Pre-Post | 4.07 ±2.78 † | 1.81 ± 3.58 † | (0.000) | (0.022) | 39.47 | |
| MBI (score) | Pre | 48.68 ± 11.40 | 51.14 ± 12.26 | |||
| Post | 55.85 ± 14.18 | 52.79 ± 12.54 | 52.187 | 20.415 | 2.79 | |
| Pre-Post | 7.18 ± 4.83 † | 1.65 ± 12.54 † | (0.000) | (0.000) | 38.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jung, S.; Song, C. The Effects of Auditory Feedback Gait Training Using Smart Insole on Stroke Patients. Brain Sci. 2021, 11, 1377. https://doi.org/10.3390/brainsci11111377
Kim J, Jung S, Song C. The Effects of Auditory Feedback Gait Training Using Smart Insole on Stroke Patients. Brain Sciences. 2021; 11(11):1377. https://doi.org/10.3390/brainsci11111377
Chicago/Turabian StyleKim, Junghyun, Sangwoo Jung, and Changho Song. 2021. "The Effects of Auditory Feedback Gait Training Using Smart Insole on Stroke Patients" Brain Sciences 11, no. 11: 1377. https://doi.org/10.3390/brainsci11111377
APA StyleKim, J., Jung, S., & Song, C. (2021). The Effects of Auditory Feedback Gait Training Using Smart Insole on Stroke Patients. Brain Sciences, 11(11), 1377. https://doi.org/10.3390/brainsci11111377






