Exposure to Commercial Cigarette Smoke Produces Psychomotor Sensitization via Hyperstimulation of Glutamate Response in the Dorsal Striatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Preparation of KCSCs
2.4. Administration of Nicotine and KCSCs
2.5. Experimental Designs
2.6. Open-Field Test
2.7. Surgery for Real-Time Glutamate Biosensing
2.8. In Vitro Calibration and In Vivo Real-Time Glutamate Biosensing
2.9. Statistics
3. Results
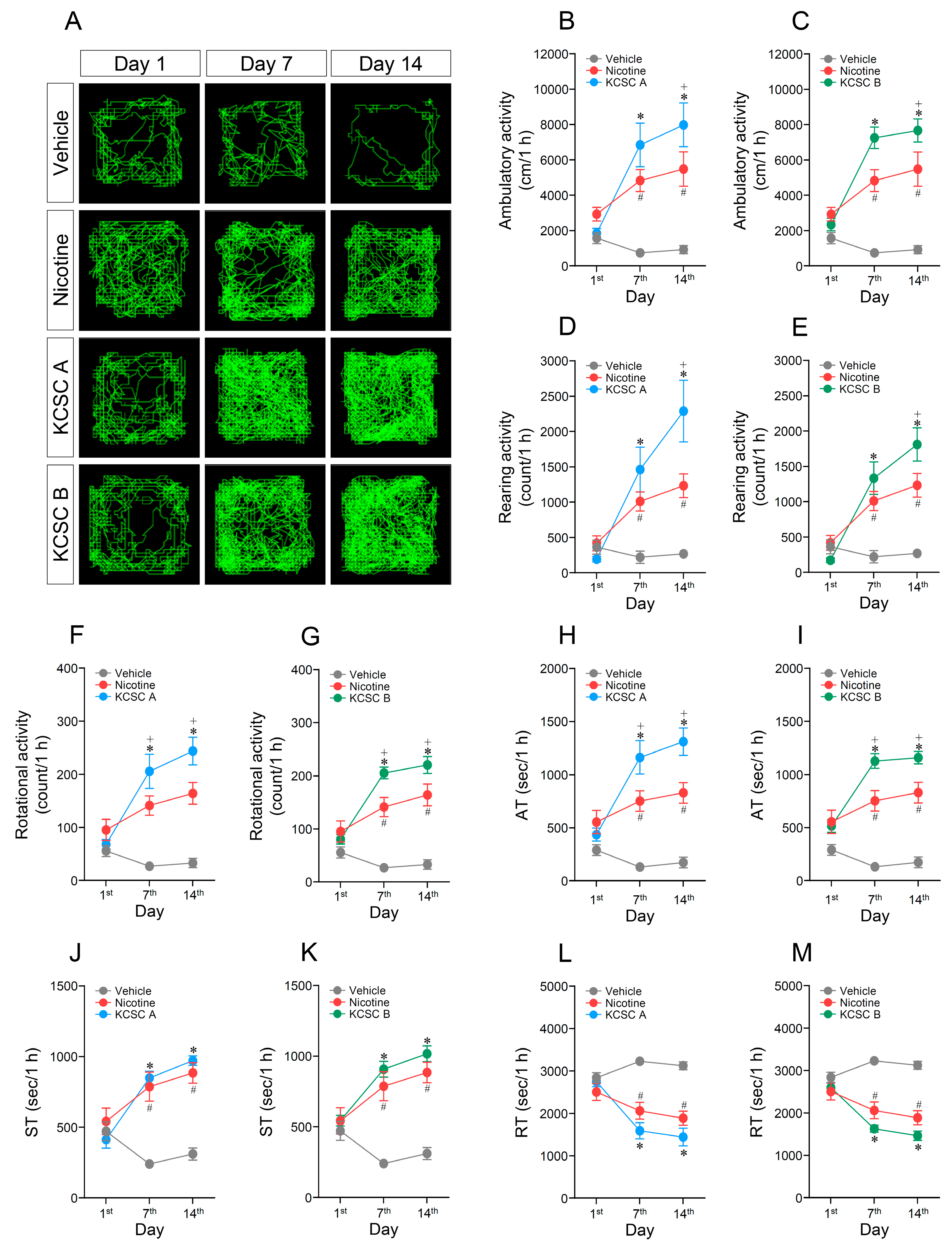
3.1. Repeated Administration of KCSCs Increased Ambulatory, Rearing, and Rotational Activities
3.2. Repeated Administration of KCSCs Increased Ambulatory and Stereotypy Time, but Decreased Resting Time in Behavioral States
3.3. Repeated Administration of KCSCs Induced Prolonged Increases in Ambulatory, Rearing, and Rotational Activities
3.4. Repeated Administration of KCSCs Induced Prolonged Increases in Ambulatory and Stereotypy Times, and Prolonged Decrease in Resting Time in Behavioral States
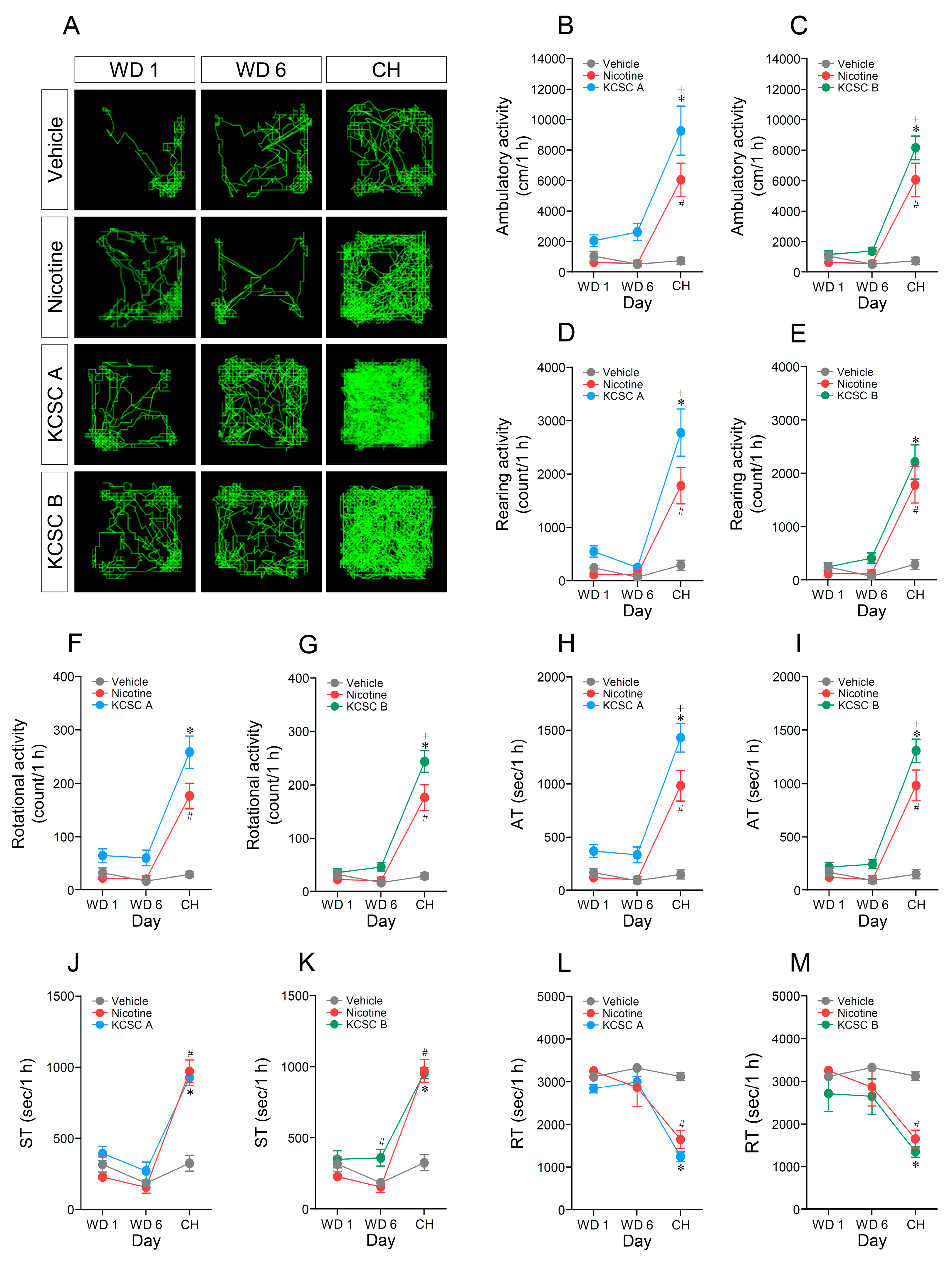
3.5. Challenge Administration of KCSCs after Withdrawal Periods Increased Ambulatory, Rearing, and Rotational Activities
3.6. Challenge Administration of KCSCs also Increased Ambulatory and Stereotypy Time, but Decreased Resting Time in Behavioral States
3.7. Challenge Administration of KCSCs Produced Prolonged Increases in Ambulatory, Rearing, and Rotational Activities
3.8. Challenge Administration of KCSCs Induced Prolonged Increases in Ambulatory and Stereotypy Times, and a Prolonged Decrease in Resting Time in Behavioral States
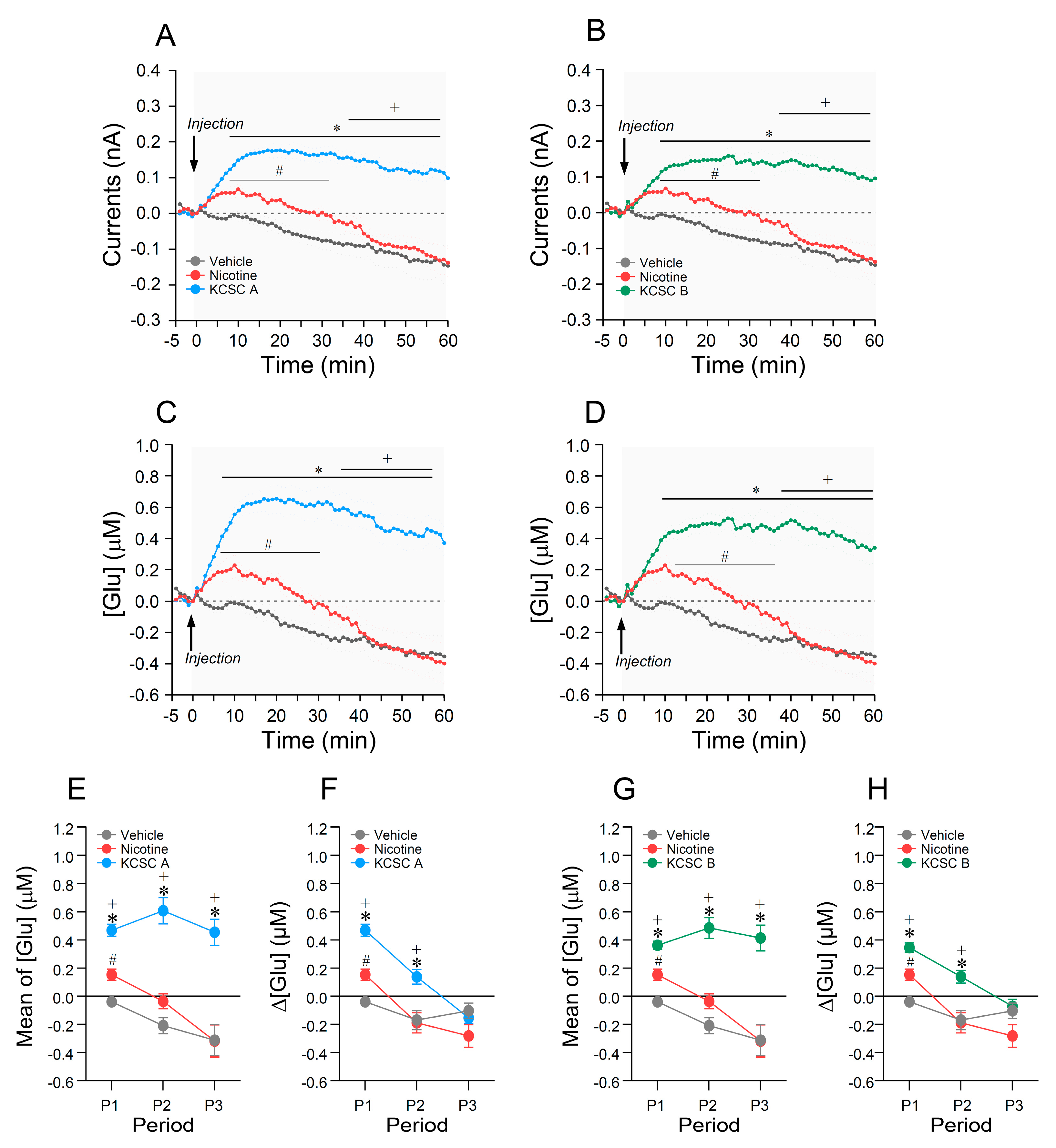
3.9. In Vitro Calibration of Real-Time Glutamate Biosensing
3.10. Repeated Administration of KCSCs Increased Glutamate Concentration in the Dorsal Striatum
3.11. Relations between Rates of Change in [Glu] in the Dorsal Striatum and Psychomotor Sensitization Following Repeated Administration of KCSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benowitz, N.L. Pharmacology of nicotine: Addiction and therapeutics. Annu. Rev. Pharm. Toxicol. 1996, 36, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clin. Pharm. Ther. 2008, 83, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.M.; Yasar, S.; Heishman, S.J.; Panlilio, L.V.; Henningfield, J.E.; Goldberg, S.R. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology 2004, 175, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, B.; Goldberg, S.R. Effects of nicotine in experimental animals and humans: An update on addictive properties. Handb. Exp. Pharm. 2009, 335–367. [Google Scholar] [CrossRef]
- Henley, B.M.; Williams, B.A.; Srinivasan, R.; Cohen, B.N.; Xiao, C.; Mackey, E.D.; Wold, B.J.; Lester, H.A. Transcriptional regulation by nicotine in dopaminergic neurons. Biochem. Pharm. 2013, 86, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, M.; Kiyatkin, E.A. Intravenous nicotine injection induces rapid, experience-dependent sensitization of glutamate release in the ventral tegmental area and nucleus accumbens. J. Neurochem. 2013, 127, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Mansvelder, H.D.; McGehee, D.S. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 2002, 53, 606–617. [Google Scholar] [CrossRef]
- Ryu, I.S.; Kim, J.; Seo, S.Y.; Yang, J.H.; Oh, J.H.; Lee, D.K.; Cho, H.W.; Yoon, S.S.; Seo, J.W.; Chang, S.; et al. Behavioral changes after nicotine challenge are associated with alpha7 nicotinic acetylcholine receptor-stimulated glutamate release in the rat dorsal striatum. Sci. Rep. 2017, 7, 15009. [Google Scholar] [CrossRef]
- Zhao-Shea, R.; Liu, L.; Soll, L.G.; Improgo, M.R.; Meyers, E.E.; McIntosh, J.M.; Grady, S.R.; Marks, M.J.; Gardner, P.D.; Tapper, A.R. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology 2011, 36, 1021–1032. [Google Scholar] [CrossRef]
- Djordjevic, M.V.; Doran, K.A. Nicotine content and delivery across tobacco products. Handb. Exp. Pharm. 2009, 61–82. [Google Scholar] [CrossRef]
- Jacob, P., 3rd; Yu, L.; Shulgin, A.T.; Benowitz, N.L. Minor tobacco alkaloids as biomarkers for tobacco use: Comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am. J. Public Health 1999, 89, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.Q.; Ding, L.; Tong, H.W.; Yong, G.P.; Zhou, X.Z.; Liu, S.M. Determination of Nicotine-Related Alkaloids in Tobacco and Cigarette Smoke by GC-FID. Chromatographia 2005, 62, 63–68. [Google Scholar] [CrossRef]
- Wu, W.; Ashley, D.L.; Watson, C.H. Determination of nicotine and other minor alkaloids in international cigarettes by solid-phase microextraction and gas chromatography/mass spectrometry. Anal. Chem. 2002, 74, 4878–4884. [Google Scholar] [CrossRef]
- Huang, H.Y.; Hsieh, S.H. Analyses of tobacco alkaloids by cation-selective exhaustive injection sweeping microemulsion electrokinetic chromatography. J. Chromatogr. A 2007, 1164, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Maciuk, A.; Moaddel, R.; Haginaka, J.; Wainer, I.W. Screening of tobacco smoke condensate for nicotinic acetylcholine receptor ligands using cellular membrane affinity chromatography columns and missing peak chromatography. J. Pharm. Biomed. Anal. 2008, 48, 238–246. [Google Scholar] [CrossRef][Green Version]
- Arnold, M.M.; Loughlin, S.E.; Belluzzi, J.D.; Leslie, F.M. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology 2014, 85, 293–304. [Google Scholar] [CrossRef]
- Caine, S.B.; Collins, G.T.; Thomsen, M.; Wright, C.; Lanier, R.K.; Mello, N.K. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp. Clin. Psychopharmacol. 2014, 22, 9–22. [Google Scholar] [CrossRef]
- Clemens, K.J.; Caille, S.; Stinus, L.; Cador, M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int. J. Neuropsychopharmacol. 2009, 12, 1355–1366. [Google Scholar] [CrossRef]
- Hall, B.J.; Wells, C.; Allenby, C.; Lin, M.Y.; Hao, I.; Marshall, L.; Rose, J.E.; Levin, E.D. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharm. Biochem. Behav. 2014, 120, 103–108. [Google Scholar] [CrossRef]
- Harris, A.C.; Tally, L.; Muelken, P.; Banal, A.; Schmidt, C.E.; Cao, Q.; LeSage, M.G. Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend. 2015, 153, 330–334. [Google Scholar] [CrossRef]
- Hoffman, A.C.; Evans, S.E. Abuse potential of non-nicotine tobacco smoke components: Acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob. Res. 2013, 15, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Talhout, R.; Opperhuizen, A.; van Amsterdam, J.G. Role of acetaldehyde in tobacco smoke addiction. Eur. Neuropsychopharmacol 2007, 17, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Brancato, A.; Plescia, F.; Marino, R.A.; Maniaci, G.; Navarra, M.; Cannizzaro, C. Involvement of dopamine D2 receptors in addictive-like behaviour for acetaldehyde. PLoS ONE 2014, 9, e99454. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.S.; Markou, A. The “stop” and “go” of nicotine dependence: Role of GABA and glutamate. Cold Spring Harb. Perspect. Med. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Semenova, S.; D’Souza, M.S.; Stoker, A.K.; Markou, A. Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology 2014, 76(Pt. B), 554–565. [Google Scholar] [CrossRef]
- Markou, A. Neurobiology of nicotine dependence. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3159–3168. [Google Scholar] [CrossRef]
- McGehee, D.S.; Heath, M.J.; Gelber, S.; Devay, P.; Role, L.W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 1995, 269, 1692–1696. [Google Scholar] [CrossRef]
- Schilstrom, B.; Fagerquist, M.V.; Zhang, X.; Hertel, P.; Panagis, G.; Nomikos, G.G.; Svensson, T.H. Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse 2000, 38, 375–383. [Google Scholar] [CrossRef]
- Ryu, I.S.; Kim, J.; Seo, S.Y.; Yang, J.H.; Oh, J.H.; Lee, D.K.; Cho, H.W.; Lee, K.; Yoon, S.S.; Seo, J.W.; et al. Repeated Administration of Cigarette Smoke Condensate Increases Glutamate Levels and Behavioral Sensitization. Front. Behav. Neurosci. 2018, 12, 47. [Google Scholar] [CrossRef]
- Parikh, V.; Naughton, S.X.; Shi, X.; Kelley, L.K.; Yegla, B.; Tallarida, C.S.; Rawls, S.M.; Unterwald, E.M. Cocaine-induced neuroadaptations in the dorsal striatum: Glutamate dynamics and behavioral sensitization. Neurochem. Int. 2014, 75, 54–65. [Google Scholar] [CrossRef]
- Kwak, H.-G.; Lim, H.-B. Inhibitory effects of Cnidium monnieri fruit extract on pulmonary inflammation in mice induced by cigarette smoke condensate and lipopolysaccharide. Chin. J. Nat. Med. 2014, 12, 641–647. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, J.E.; Jeong, M.H.; Choi, S.J.; Lee, K.; Chung, K.H. Comparative evaluation of the mutagenicity and genotoxicity of smoke condensate derived from Korean cigarettes. Environ. Health Toxicol. 2015, 30, e2015014. [Google Scholar] [CrossRef] [PubMed]
- International Organization of Standardization. Tobacco and Tobacco Products: Atmosphere for Conditioning and Testing (ISO 3402); International Organization of Standardization: Geneva, Switzerland, 1999; pp. 1–5. [Google Scholar]
- International Organization of Standardization. Cigarettes: Determination of Total and Nicotine-Free Dry Particulate Matter Using a Routine Analytical Smoking Machine (ISO 4387); International Organization of Standardization: Geneva, Switzerland, 2000; pp. 1–17. [Google Scholar]
- International Organization of Standardization. Routine Analytical Cigarette-Smoking Machine: Definitions and Standard Conditions (ISO 3308); International Organization of Standardization: Geneva, Switzerland, 2012; pp. 1–25. [Google Scholar]
- Wynder, E.L.; Hoffmann, D. Tobacco and health: A societal challenge. N. Engl. J. Med. 1979, 300, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Al Rashidi, M.; Shihadeh, A.; Saliba, N.A. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food Chem. Toxicol. 2008, 46, 3546–3549. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; An, Y.J.; Jo, S.; Lee, S.H.; Lee, S.J.; Choi, S.J.; Lee, K. Comparison of volatile organic compounds between cigarette smoke condensate (CSC) and extract (CSE) samples. Environ. Health Toxicol. 2018, 33, e2018012. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.G.; Balfour, D.J.; Benowitz, N.L.; Boyd, R.T.; Buccafusco, J.J.; Caggiula, A.R.; Craig, C.R.; Collins, A.C.; Damaj, M.I.; Donny, E.C.; et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 2007, 190, 269–319. [Google Scholar] [CrossRef]
- Wakabayashi, K.T.; Kiyatkin, E.A. Rapid changes in extracellular glutamate induced by natural arousing stimuli and intravenous cocaine in the nucleus accumbens shell and core. J. Neurophysiol. 2012, 108, 285–299. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Lee, J.Y.; Choi, M.J.; Choe, E.S.; Lee, Y.J.; Seo, J.W.; Yoon, S.S. Differential discriminative-stimulus effects of cigarette smoke condensate and nicotine in nicotine-discriminating rats. Behav. Brain Res. 2016, 306, 197–201. [Google Scholar] [CrossRef]
- Knackstedt, L.A.; Kalivas, P.W. Glutamate and reinstatement. Curr. Opin. Pharm. 2009, 9, 59–64. [Google Scholar] [CrossRef]
- Kalivas, P.W. Glutamate systems in cocaine addiction. Curr. Opin. Pharm. 2004, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Yager, L.M.; Garcia, A.F.; Wunsch, A.M.; Ferguson, S.M. The ins and outs of the striatum: Role in drug addiction. Neuroscience 2015, 301, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. Review. The incentive sensitization theory of addiction: Some current issues. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Wu, P.F.; Yang, Y.J.; Xiao, W.; Fan, J.; Liu, J.; Li, Y.L.; Luo, Y.; Hu, Z.L.; Jin, Y.; et al. Interactions between N-ethylmaleimide-sensitive factor and GluR2 in the nucleus accumbens contribute to the expression of locomotor sensitization to cocaine. J. Neurosci. 2014, 34, 3493–3508. [Google Scholar] [CrossRef]
- Steketee, J.D.; Kalivas, P.W. Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharm. Rev. 2011, 63, 348–365. [Google Scholar] [CrossRef]
- Vezina, P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004, 27, 827–839. [Google Scholar] [CrossRef]
- Herman, M.A.; Roberto, M. The addicted brain: Understanding the neurophysiological mechanisms of addictive disorders. Front. Integr. Neurosci. 2015, 9, 18. [Google Scholar] [CrossRef]
- D’Souza, M.S. Glutamatergic transmission in drug reward: Implications for drug addiction. Front. Neurosci. 2015, 9, 404. [Google Scholar] [CrossRef]
- Yang, J.H.; Sohn, S.; Kim, S.; Kim, J.; Oh, J.H.; Ryu, I.S.; Go, B.S.; Choe, E.S. Repeated nicotine exposure increases the intracellular interaction between ERK-mGluR5 in the nucleus accumbens more in adult than adolescent rats. Addict. Biol. 2020, e12913. [Google Scholar] [CrossRef]
- Wiley, J.L.; Marusich, J.A.; Thomas, B.F.; Jackson, K.J. Determination of behaviorally effective tobacco constituent doses in rats. Nicotine Tob. Res. 2015, 17, 368–371. [Google Scholar] [CrossRef]



| Types | Nicotine | Nornicotine | Cotinine | Anabasine | Anatabine | Myosmine | Norharmane | Harmane |
|---|---|---|---|---|---|---|---|---|
| KCSC A | 5640.0 (95.85) | 28.6 (0.49) | 6.9 (0.12) | 199.8 (3.40) | 6.1 (0.10) | 1.6 (0.02) | 0.9 (0.01) | 0.5 (0.01) |
| KCSC B | 3800.0 (95.80) | 18.7 (0.47) | 6.1 (0.15) | 136.7 (3.46) | 3.3 (0.08) | 0.9 (0.02) | 0.7 (0.01) | 0.4 (0.01) |
| Groups | A. Ambulatory Activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–20 Min | 20–40 Min | 40–60 Min | |||||||
| 14 days of repeated vehicle | 850.00 | ± | 180.93 | 151.67 | ± | 118.77 | 30.67 | ± | 8.08 |
| 14 days of repeated nicotine | 3840.50 | ± | 451.28 # | 997.33 | ± | 228.43 # | 773.67 | ± | 305.43 |
| 14 days of repeated KCSC A | 4124.50 | ± | 629.04 * | 1861.83 | ± | 298.11 *,+ | 1993.00 | ± | 344.85 *,+ |
| 14 days of repeated KCSC B | 4641.67 | ± | 561.08 * | 1578.33 | ± | 159.52 * | 1452.83 | ± | 64.61 *,+ |
| Groups | B. Rearing Activity | ||||||||
| 0–20 Min | 20–40 Min | 40–60 Min | |||||||
| 14 days of repeated vehicle | 210.83 | ± | 70.36 | 22.83 | ± | 20.27 | 7.00 | ± | 1.73 |
| 14 days of repeated nicotine | 952.17 | ± | 131.06 # | 299.83 | ± | 71.24 # | 191.67 | ± | 76.22 |
| 14 days of repeated KCSC A | 1052.00 | ± | 156.76 * | 612.33 | ± | 93.19 *,+ | 667.33 | ± | 111.59 *,+ |
| 14 days of repeated KCSC B | 905.50 | ± | 170.71 * | 460.67 | ± | 69.61 * | 445.17 | ± | 26.70 *,+ |
| Groups | C. Rotational Activity | ||||||||
| 0–20 Min | 20–40 Min | 40–60 Min | |||||||
| 14 days of repeated vehicle | 24.17 | ± | 5.63 | 6.00 | ± | 3.81 | 2.50 | ± | 0.56 |
| 14 days of repeated nicotine | 96.83 | ± | 6.89 # | 41.00 | ± | 7.29 # | 26.33 | ± | 9.63 |
| 14 days of repeated KCSC A | 107.33 | ± | 11.25 * | 68.17 | ± | 9.20 *,+ | 68.33 | ± | 7.83 *,+ |
| 14 days of repeated KCSC B | 121.00 | ± | 12.21 * | 51.67 | ± | 6.12 * | 48.17 | ± | 1.14 *,+ |
| Groups | A. Ambulatory Activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P1 (0–20 Min) | P2 (20–40 Min) | P3 (40–60 Min) | |||||||
| Vehicle challenge | 550.33 | ± | 118.12 | 137.83 | ± | 108.50 | 54.83 | ± | 23.88 |
| Nicotine challenge | 3510.50 | ± | 468.54 # | 1512.83 | ± | 337.40 # | 1037.33 | ± | 299.56 |
| KCSC A challenge | 4305.33 | ± | 634.05 * | 2471.33 | ± | 547.58 *,+ | 2496.00 | ± | 544.72 *,+ |
| KCSC B challenge | 4483.50 | ± | 446.57 * | 1986.00 | ± | 376.23 * | 1693.33 | ± | 293.24 *,+ |
| Groups | B. Rearing Activity | ||||||||
| P1 (0–20 Min) | P2 (20–40 Min) | P3 (40–60 Min) | |||||||
| Vehicle challenge | 254.00 | ± | 80.47 | 23.67 | ± | 20.16 | 12.00 | ± | 6.91 |
| Nicotine challenge | 1003.50 | ± | 190.27 # | 486.50 | ± | 107.50# | 291.83 | ± | 74.58 |
| KCSC A challenge | 1069.67 | ± | 136.39 * | 879.33 | ± | 154.75*,+ | 828.00 | ± | 162.69 *,+ |
| KCSC B challenge | 1051.17 | ± | 148.83 * | 626.67 | ± | 133.66* | 532.67 | ± | 101.85 *,+ |
| Groups | C. Rotational Activity | ||||||||
| P1 (0–20 Min) | P2 (20–40 Min) | P3 (40–60 Min) | |||||||
| Vehicle challenge | 22.33 | ± | 3.71 | 4.00 | ± | 2.25 | 2.83 | ± | 0.70 |
| Nicotine challenge | 89.83 | ± | 6.72 # | 51.83 | ± | 11.03 # | 34.67 | ± | 9.44 |
| KCSC A challenge | 109.50 | ± | 10.92 * | 73.83 | ± | 9.78 *,+ | 74.83 | ± | 11.48 *,+ |
| KCSC B challenge | 123.17 | ± | 8.60 * | 66.17 | ± | 10.20 * | 51.17 | ± | 6.58 *,+ |
| Groups | Mean Of [Glu] (Nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–20 Min | 20–40 Min | 40–60 Min | |||||||
| 14 days of repeated vehicle | −38.76 | ± | 30.12 | −208.02 | ± | 56.44 | −311.49 | ± | 110.71 |
| 14 days of repeated nicotine | 153.70 | ± | 39.56 # | −35.41 | ± | 52.93 | −317.70 | ± | 113.70 |
| 14 days of repeated KCSC A | 470.13 | ± | 42.72 *,+ | 608.43 | ± | 92.48 *,+ | 455.21 | ± | 92.60 *,+ |
| 14 days of repeated KCSC B | 346.11 | ± | 32.57 *,+ | 485.59 | ± | 73.82 * | 414.44 | ± | 91.82 *,+ |
| Groups | Δ[Glu] (Nm) | ||||||||
| 0–20 Min | 20–40 Min | 40–60 Min | |||||||
| 14 days of repeated vehicle | −38.76 | ± | 30.12 | −169.25 | ± | 67.53 | −103.47 | ± | 55.11 |
| 14 days of repeated nicotine | 153.70 | ± | 39.56 # | −189.11 | ± | 71.60 | −282.30 | ± | 80.12 |
| 14 days of repeated KCSC A | 470.13 | ± | 42.72 *,+ | 133.30 | ± | 51.65 *,+ | −153.22 | ± | 38.52 |
| 14 days of repeated KCSC B | 346.11 | ± | 32.57 *,+ | 139.47 | ± | 45.10 *,+ | −71.15 | ± | 48.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, I.S.; Kim, J.; Yang, J.H.; Seo, S.Y.; Sohn, S.; Kim, S.; Lee, K.; Seo, J.-W.; Choe, E.S. Exposure to Commercial Cigarette Smoke Produces Psychomotor Sensitization via Hyperstimulation of Glutamate Response in the Dorsal Striatum. Brain Sci. 2021, 11, 14. https://doi.org/10.3390/brainsci11010014
Ryu IS, Kim J, Yang JH, Seo SY, Sohn S, Kim S, Lee K, Seo J-W, Choe ES. Exposure to Commercial Cigarette Smoke Produces Psychomotor Sensitization via Hyperstimulation of Glutamate Response in the Dorsal Striatum. Brain Sciences. 2021; 11(1):14. https://doi.org/10.3390/brainsci11010014
Chicago/Turabian StyleRyu, In Soo, Jieun Kim, Ju Hwan Yang, Su Yeon Seo, Sumin Sohn, Sunghyun Kim, Kyuhong Lee, Joung-Wook Seo, and Eun Sang Choe. 2021. "Exposure to Commercial Cigarette Smoke Produces Psychomotor Sensitization via Hyperstimulation of Glutamate Response in the Dorsal Striatum" Brain Sciences 11, no. 1: 14. https://doi.org/10.3390/brainsci11010014
APA StyleRyu, I. S., Kim, J., Yang, J. H., Seo, S. Y., Sohn, S., Kim, S., Lee, K., Seo, J.-W., & Choe, E. S. (2021). Exposure to Commercial Cigarette Smoke Produces Psychomotor Sensitization via Hyperstimulation of Glutamate Response in the Dorsal Striatum. Brain Sciences, 11(1), 14. https://doi.org/10.3390/brainsci11010014





