When, How, and to What Extent Are Individuals with Unresponsive Wakefulness Syndrome Able to Progress? Neurobehavioral Progress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Data Analysis
3. Results
3.1. Participants
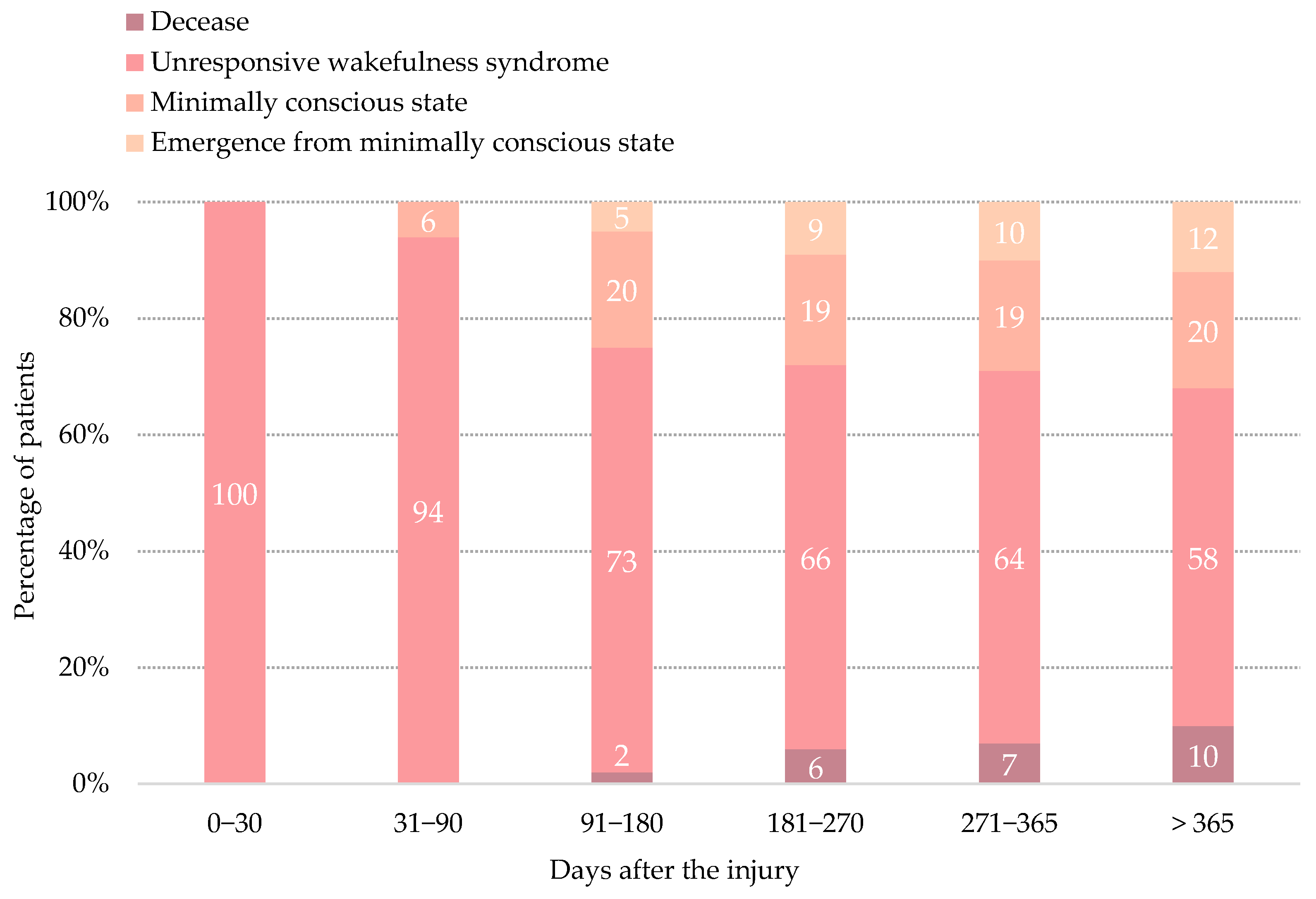
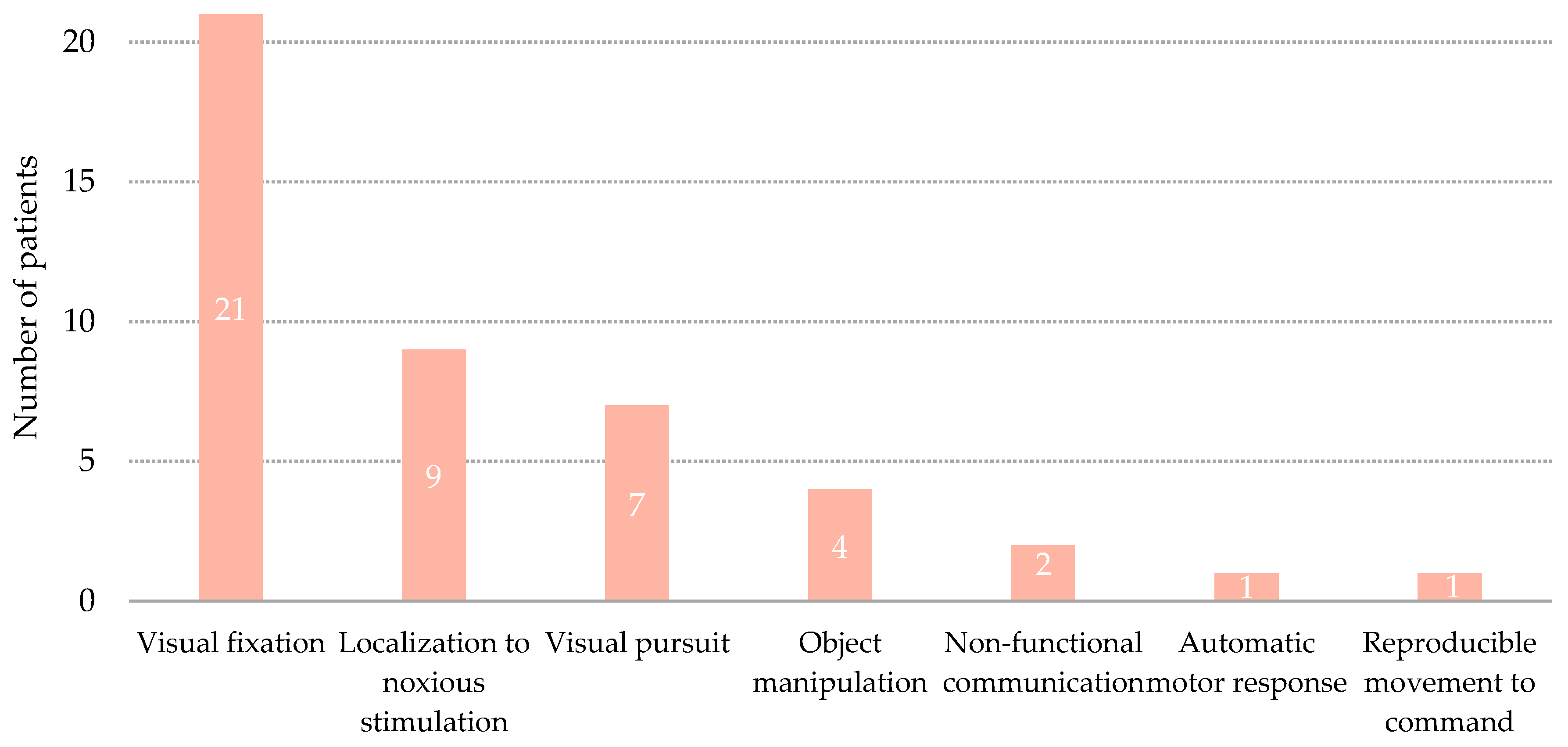
3.2. Transition to Minimally Conscious State
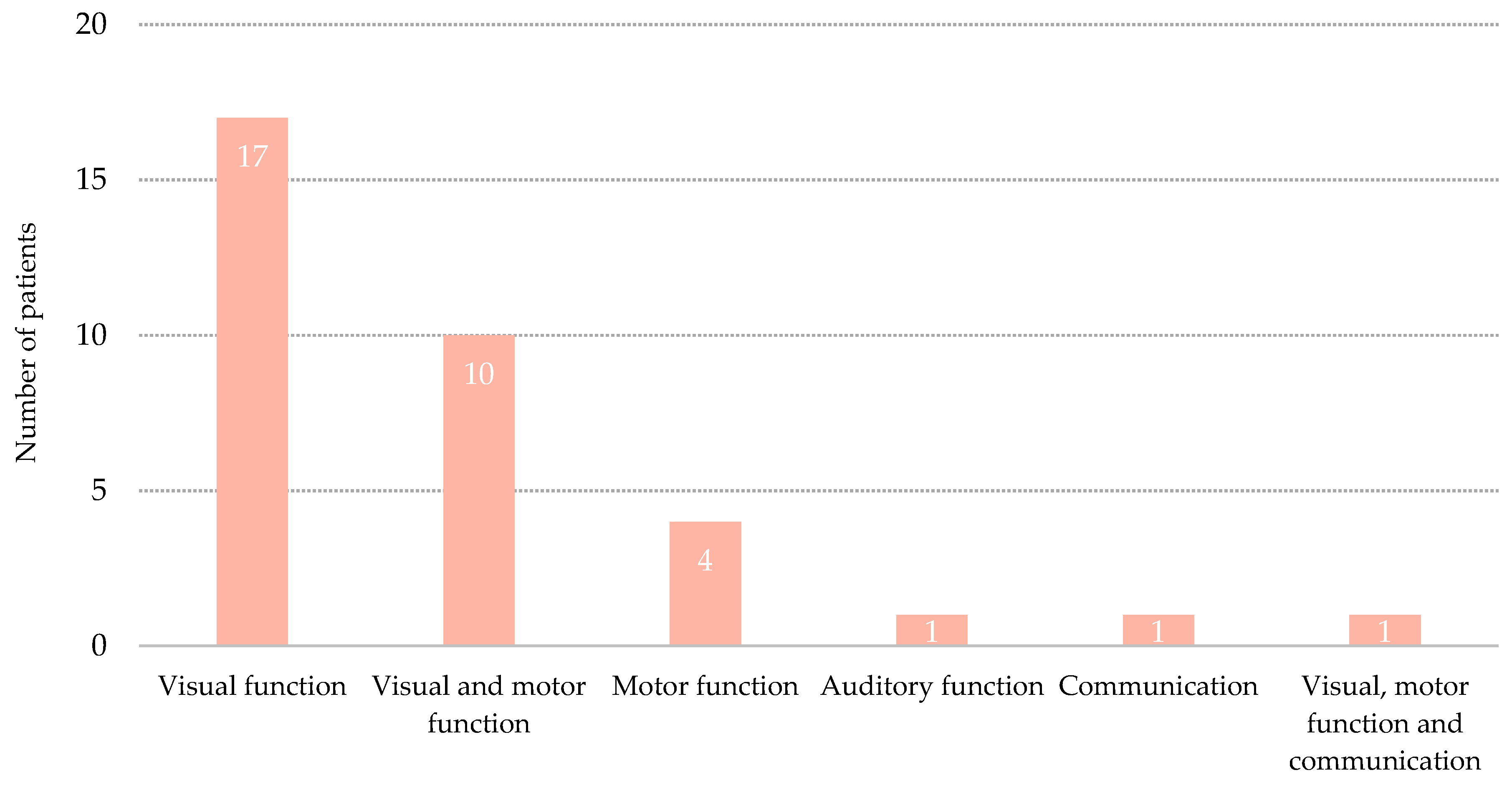
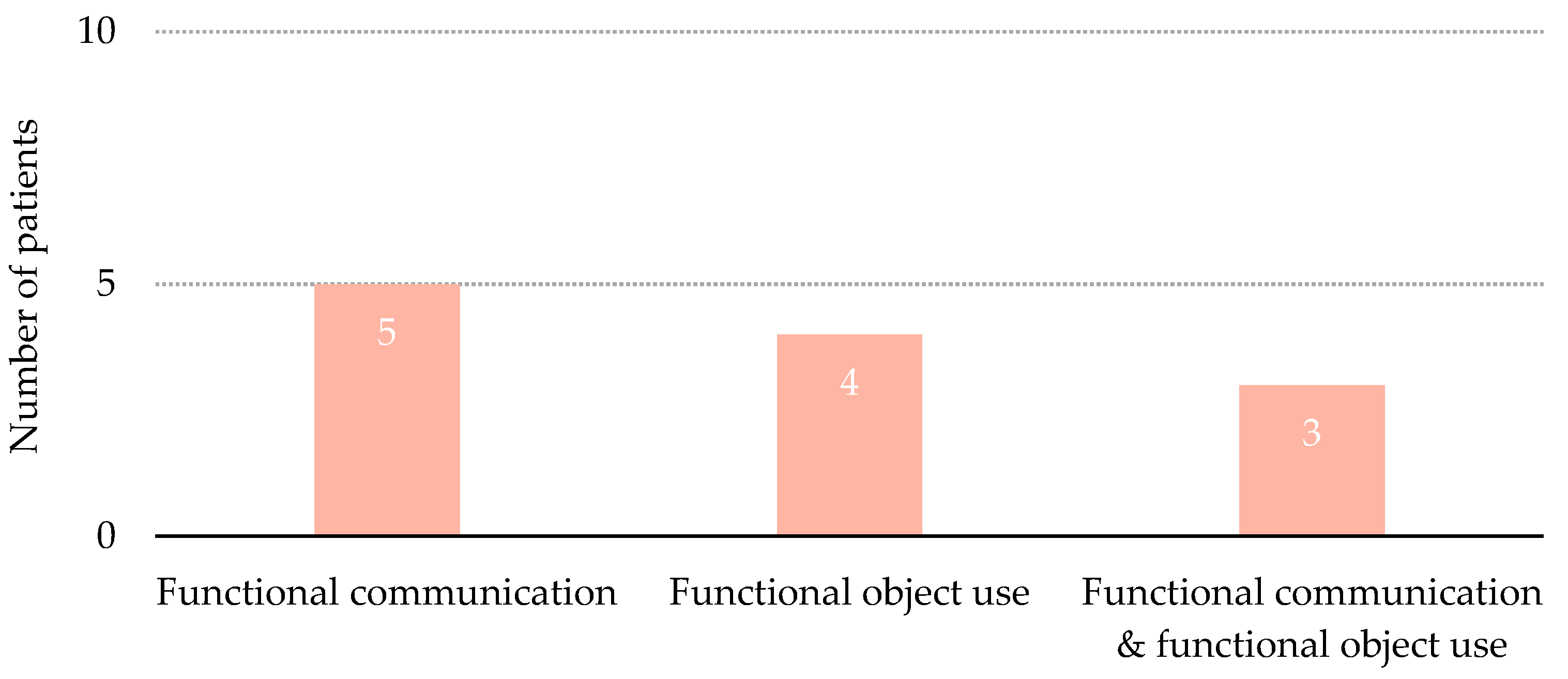
3.3. Emergence from Minimally Conscious State
3.4. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Multi-Society Task Force on PVS. Medical Aspects of the Persistent Vegetative State. N. Engl. J. Med. 1994, 330, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Bender, A.; Diserens, K.; Van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef] [Green Version]
- Jennett, B.; Plum, F. Persistent vegetative state after brain damage. RN 1972, 35, 734–737. [Google Scholar] [CrossRef]
- Casarett, D.; Fishman, J.M.; MacMoran, H.J.; Pickard, A.; Asch, D.A. Epidemiology and prognosis of coma in daytime television dramas. Br. Med. J. 2005, 331, 1537–1539. [Google Scholar] [CrossRef] [Green Version]
- Septien, S.; Rubin, M.A. Disorders of Consciousness: Ethical Issues of Diagnosis, Treatment, and Prognostication. Semin. Neurol. 2018, 38, 548–554. [Google Scholar] [CrossRef]
- Aspen Consensus Conference Workgroup on the Vegetative and Minimally Conscious States Draft Consensus Statement; Aspen: Hoboken, NJ, USA, 1996.
- Giacino, J.T.; Kalmar, K. The vegetative and minimally conscious states: A comparison of clinical features and functional outcome. J. Head Trauma Rehabil. 1997, 12, 36–51. [Google Scholar] [CrossRef]
- Burke, W.J. The minimally conscious state: Definition and diagnostic criteria [1] (multiple letters). Neurology 2002, 59, 1473–1474. [Google Scholar] [CrossRef]
- Farisco, M.; Laureys, S.; Evers, K. The Intrinsic Activity of the Brain and its Relation to Levels and Disorders of Consciousness. Mind Matter 2017, 15, 197–219. [Google Scholar]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; Von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Sazbon, L.; Groswasser, Z. Outcome in 134 patients with prolonged posttraumatic unawareness. Part 1: Parameters determining late recovery of consciousness. J. Neurosurg. 1990, 72, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Comprehensive systematic review update summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; The American Congress of Rehabilitation Medicine; And the. Neurology 2018, 91, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarà, M.; Sacco, S.; Cipolla, F.; Onorati, P.; Scoppetta, C.; Albertini, G.; Carolei, A. An unexpected recovery from permanent vegetative state. Brain Inj. 2007, 21, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Dhamapurkar, S.K.; Rose, A.; Florschutz, G.; Wilson, B.A. The natural history of continuing improvement in an individual after a long period of impaired consciousness: The story of I.J. Brain Inj. 2016, 30, 230–236. [Google Scholar] [CrossRef]
- Faran, S.; Vatine, J.J.; Lazary, A.; Ohry, A.; Birbaumer, N.; Kotchoubey, B. Late recovery from permanent traumatic vegetative state heralded by event-related potentials. J. Neurol. Neurosurg. Psychiatry 2006, 77, 998–1000. [Google Scholar] [CrossRef] [Green Version]
- Childs, N.L.; Mercer, W.N. Late Improvement in Consciousness after Post-Traumatic Vegetative State. N. Engl. J. Med. 1996, 334, 24–25. [Google Scholar] [CrossRef]
- McMillan, T.M.; Herbert, C.M. Further recovery in a potential treatment withdrawal case 10 years after brain injury. Brain Inj. 2004. [Google Scholar] [CrossRef]
- Sancisi, E.; Battistini, A.; Stefano, C.D.; Simoncini, L.; Simoncini, L.; Montagna, P.; Piperno, R. Late recovery from post-traumatic vegetative state. Brain Inj. 2009, 23, 163–166. [Google Scholar] [CrossRef]
- Illman, N.A.; Crawford, S. Late-recovery from “permanent” vegetative state in the context of severe traumatic brain injury: A case report exploring objective and subjective aspects of recovery and rehabilitation. Neuropsychol. Rehabil. 2018. [Google Scholar] [CrossRef]
- De Tanti, A.; Saviola, D.; Basagni, B.; Cavatorta, S.; Chiari, M.; Casalino, S.; De Bernardi, D.; Galvani, R. Recovery of consciousness after 7 years in vegetative state of non-traumatic origin: A single case study. Brain Inj. 2016. [Google Scholar] [CrossRef]
- Naro, A.; Calabrò, R.S.; Pollicino, P.; Lombardo, C.; Bramanti, P. Unexpected recovery from a vegetative state or misdiagnosis? Lesson learned from a case report. NeuroRehabilitation 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpino, M.; Lolli, F.; Hakiki, B.; Atzori, T.; Lanzo, G.; Sterpu, R.; Portaccio, E.; Romoli, A.M.; Morrocchesi, A.; Amantini, A.; et al. Prognostic value of post-acute EEG in severe disorders of consciousness, using American Clinical Neurophysiology Society terminology. Neurophysiol. Clin. 2019, 49, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.M. The central role of prognosis in clinical decision making. JAMA J. Am. Med. Assoc. 2012, 307, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Demertzi, A.; Ledoux, D.; Bruno, M.A.; Vanhaudenhuyse, A.; Gosseries, O.; Soddu, A.; Schnakers, C.; Moonen, G.; Laureys, S. Attitudes towards end-of-life issues in disorders of consciousness: A European survey. J. Neurol. 2011, 258, 1058–1065. [Google Scholar] [CrossRef]
- Katz, D.I.; Polyak, M.; Coughlan, D.; Nichols, M.; Roche, A. Natural history of recovery from brain injury after prolonged disorders of consciousness: Outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog. Brain Res. 2009, 177, 73–88. [Google Scholar] [CrossRef]
- Luauté, J.; Maucort-Boulch, D.; Tell, L.; Quelard, F.; Sarraf, T.; Iwaz, J.; Boisson, D.; Fischer, C. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 2010, 75, 246–252. [Google Scholar] [CrossRef]
- Steppacher, I.; Kaps, M.; Kissler, J. Will time heal? A long-term follow-up of severe disorders of consciousness. Ann. Clin. Transl. Neurol. 2014, 1, 401–408. [Google Scholar] [CrossRef]
- Estraneo, A.; De Bellis, F.; Masotta, O.; Loreto, V.; Fiorenza, S.; Lo Sapio, M.; Trojano, L. Demographical and clinical indices for long-term evolution of patients in vegetative or in minimally conscious state. Brain Inj. 2019, 33, 1633–1639. [Google Scholar] [CrossRef]
- Estraneo, A.; Moretta, P.; Loreto, V.; Lanzillo, B.; Santoro, L.; Trojano, L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology 2010, 75, 239–245. [Google Scholar] [CrossRef]
- Estraneo, A.; Moretta, P.; Loreto, V.; Lanzillo, B.; Cozzolino, A.; Saltalamacchia, A.; Lullo, F.; Santoro, L.; Trojano, L. Predictors of recovery of responsiveness in prolonged anoxic vegetative state. Neurology 2013, 80, 464–470. [Google Scholar] [CrossRef]
- Estraneo, A.; Moretta, P.; Loreto, V.; Santoro, L.; Trojano, L. Clinical and neuropsychological long-term outcomes after late recovery of responsiveness: A case series. Arch. Phys. Med. Rehabil. 2014, 95, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, E.; Morrocchesi, A.; Romoli, A.M.; Hakiki, B.; Taglioli, M.P.; Lippi, E.; Di Renzone, M.; Grippo, A.; Macchi, C. Score on Coma Recovery Scale-Revised at admission predicts outcome at discharge in intensive rehabilitation after severe brain injury. Brain Inj. 2018, 32, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estraneo, A.; Fiorenza, S.; Magliacano, A.; Formisano, R.; Mattia, D.; Grippo, A.; Romoli, A.M.; Angelakis, E.; Cassol, H.; Thibaut, A.; et al. Multicenter prospective study on predictors of short-term outcome in disorders of consciousness. Neurology 2020, 95, e1488–e1499. [Google Scholar] [CrossRef] [PubMed]
- Noé, E.; Olaya, J.; Colomer, C.; Moliner, B.; Ugart, P.; Rodriguez, C.; Llorens, R.; Ferri, J. Current validity of diagnosis of permanent vegetative state: A longitudinal study in a sample of patients with altered states of consciousness. Neurologia 2017. [Google Scholar] [CrossRef]
- Whyte, J.; Katz, D.; Long, D.; DiPasquale, M.C.; Polansky, M.; Kalmar, K.; Giacino, J.; Childs, N.; Mercer, W.; Novak, P.; et al. Predictors of outcome in prolonged posttraumatic disorders of consciousness and assessment of medication effects: A multicenter study. Arch. Phys. Med. Rehabil. 2005, 86, 453–462. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, J.H.; Kim, A.R.; Park, M.; Kim, T.W. Neurobehavioral recovery in patients who emerged from prolonged disorder of consciousness: A retrospective study. BMC Neurol. 2020, 20. [Google Scholar] [CrossRef]
- Chen, W.G.; Li, R.; Zhang, Y.; Hao, J.H.; Du, J.B.; Guo, A.S.; Song, W.Q. Recovery from prolonged disorders of consciousness: A dual-center prospective cohort study in China. World J. Clin. Cases 2020, 8, 2520–2529. [Google Scholar] [CrossRef]
- Cavinato, M.; Freo, U.; Ori, C.; Zorzi, M.; Tonin, P.; Piccione, F.; Merico, A. Post-acute P300 predicts recovery of consciousness from traumatic vegetative state. Brain Inj. 2009, 23, 973–980. [Google Scholar] [CrossRef]
- Sarà, M.; Pistoia, F.; Pasqualetti, P.; Sebastiano, F.; Onorati, P.; Rossini, P.M. Functional isolation within the cerebral cortex in the vegetative state: A nonlinear method to predict clinical outcomes. Neurorehabil. Neural Repair 2011, 25, 35–42. [Google Scholar] [CrossRef]
- Lucca, L.F.; Lofaro, D.; Pignolo, L.; Leto, E.; Ursino, M.; Cortese, M.D.; Conforti, D.; Tonin, P.; Cerasa, A. Outcome prediction in disorders of consciousness: The role of coma recovery scale revised. BMC Neurol. 2019, 19. [Google Scholar] [CrossRef] [Green Version]
- Bagnato, S.; Boccagni, C.; Sant’Angelo, A.; Fingelkurts, A.A.; Fingelkurts, A.A.; Galardi, G. Longitudinal Assessment of Clinical Signs of Recovery in Patients with Unresponsive Wakefulness Syndrome after Traumatic or Nontraumatic Brain Injury. J. Neurotrauma 2017, 34, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Aidinoff, E.; Elkayam, K.; Oximitny, A.; Groswasser, Z.; Gelernter, I.; Catz, A. Consciousness recovery after various periods in vegetative state. Brain Inj. 2020, 34, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice guideline update recommendations summary: Disorders of consciousness. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Martens, G.; Bodien, Y.; Sheau, K.; Christoforou, A.; Giacino, J.T. Which behaviours are first to emerge during recovery of consciousness after severe brain injury? Ann. Phys. Rehabil. Med. 2020, 63, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Pape, T.L.B.; Tang, C.; Guernon, A.; Lundgren, S.; Blahnik, M.; Wei, Y.; Querubin, M.; Zollman, F.; Soneru, I. Predictive Value of the Disorders of Consciousness Scale (DOCS). PM R 2009, 1, 152–161. [Google Scholar] [CrossRef]
- Noé, E.; Olaya, J.; Navarro, M.D.; Noguera, P.; Colomer, C.; García-Panach, J.; Rivero, S.; Moliner, B.; Ferri, J. Behavioral recovery in disorders of consciousness: A prospective study with the Spanish version of the coma recovery scale-revised. Arch. Phys. Med. Rehabil. 2012, 93. [Google Scholar] [CrossRef]
- Bagnato, S.; Minafra, L.; Bravatà, V.; Boccagni, C.; Sant’Angelo, A.; Castiglione, A.; Andriolo, M.; Lucca, L.F.; De Tanti, A.; Pistarini, C.; et al. Brain-derived neurotrophic factor (Val66Met) polymorphism does not influence recovery from a post-traumatic vegetative state: A blinded retrospective multi-centric study. J. Neurotrauma 2012, 29, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Arnaldi, D.; Terzaghi, M.; Cremascoli, R.; De Carli, F.; Maggioni, G.; Pistarini, C.; Nobili, F.; Moglia, A.; Manni, R. The prognostic value of sleep patterns in disorders of consciousness in the sub-acute phase. Clin. Neurophysiol. 2016, 127, 1445–1451. [Google Scholar] [CrossRef]
- Wang, F.; Di, H.; Hu, X.; Jing, S.; Thibaut, A.; Di Perri, C.; Huang, W.; Nie, Y.; Schnakers, C.; Laureys, S. Cerebral response to subject’s own name showed high prognostic value in traumatic vegetative state. BMC Med. 2015. [Google Scholar] [CrossRef] [Green Version]
- Noé, E.; Olaya, J.; Colomer, C.; Moliner, B.; Ugart, P.; Rodriguez, C.; Llorens, R.; Ferri, J. Validez actual del diagnóstico de «estado vegetativo permanente»: Estudio longitudinal en una muestra clínica de pacientes en estados alterados de conciencia. Neurología 2019. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Moons, K.G.M.; Van der Windt, D.A.; Hayden, J.A.; Perel, P.; Schroter, S.; Riley, R.D.; Hemingway, H.; Altman, D.G. Prognosis Research Strategy (PROGRESS) 3: Prognostic Model Research. PLoS Med. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaya, J.; Noé, E.; Navarro, M.D.; O’Valle, M.; Colomer, C.; Moliner, B.; Ippoliti, C.; Ferri, J.; Maza, A.; Llorens, R. When, How, and to What Extent Are Individuals with Unresponsive Wakefulness Syndrome Able to Progress? Functional Independence. Brain Sci. 2020, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.; Noé, E.; Lloréns, R. The Spanish version of the coma recovery scale-revised: Events on a correct timeline. Brain Inj. 2015, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotu, V.; Deshpande, B. Model Evaluation. In Predictive Analytics and Data Mining; Kotu, V., Deshpande, B., Eds.; Morgan Kaufmann: Boston, MA, USA, 2015; pp. 257–273. ISBN 978-0-12-801460-8. [Google Scholar]
- Greenwald, B.D.; Hammond, F.M.; Harrison-Felix, C.; Nakase-Richardson, R.; Howe, L.L.S.; Kreider, S. Mortality following Traumatic Brain Injury among Individuals Unable to Follow Commands at the Time of Rehabilitation Admission: A National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems Study. J. Neurotrauma 2015, 32, 1883–1892. [Google Scholar] [CrossRef] [Green Version]
- Pincherle, A.; Rossi, F.; Jöhr, J.; Dunet, V.; Ryvlin, P.; Oddo, M.; Schiff, N.; Diserens, K. Early discrimination of cognitive motor dissociation from disorders of consciousness: Pitfalls and clues. J. Neurol. 2020. [Google Scholar] [CrossRef]
- Cruse, D.; Chennu, S.; Chatelle, C.; Bekinschtein, T.A.; Fernández-Espejo, D.; Pickard, J.D.; Laureys, S.; Owen, A.M. Bedside detection of awareness in the vegetative state: A cohort study. Lancet 2011, 378, 2088–2094. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.M.; Vanhaudenhuyse, A.; Coleman, M.R.; Boly, M.; Pickard, J.D.; Tshibanda, L.; Owen, A.M.; Laureys, S. Willful Modulation of Brain Activity in Disorders of Consciousness. N. Engl. J. Med. 2010, 362, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.A.; Vanhaudenhuyse, A.; Schnakers, C.; Boly, M.; Gosseries, O.; Demertzi, A.; Majerus, S.; Moonen, G.; Hustinx, R.; Laureys, S. Visual fixation in the vegetative state: An observational case series PET study. BMC Neurol. 2010. [Google Scholar] [CrossRef] [Green Version]
| Patients Who Transitioned to MCS (n = 34) | Patients Who Remained in UWS (n = 66) | Significance | |
|---|---|---|---|
| Age (years) | 33.6 ± 15.0 | 39.8 ± 19.2 | NS (p = 0.100) |
| Sex (n, %) Women Men | 9 (26.5%) 25 (73.5%) | 20 (30.3%) 46 (69.7%) | NS (p = 0.689) |
| Education (years) | 10.9 ± 4.4 | 10.8 ± 5.4 | NS (p = 0.908) |
| Etiology (n, %) Traumatic Non-traumatic | 21 (61.8%) 13 (38.2%) | 19 (28.8%) 47 (71.2%) | p = 0.001 |
| Time since injury (days) | 104.6 ± 64.0 | 147.3 ± 91.9 | p = 0.017 |
| Coma Recovery Scale-Revised | 6.3 ± 1.4 | 5.4 ± 1.2 | p = 0.001 |
| Multivariable Logistic Regression Model | Cross-Validation Evaluation Metrics | |||||
|---|---|---|---|---|---|---|
| B (SE) | OR (95% CI) | p | Training | Validation | ||
| Accuracy (95% CI) | 0.79 (0.77–0.80) | 0.75 (0.67–0.83) | ||||
| Constant | −4.27 (1.30) | 0.01 (0.00–0.18) | 0.001 | Precision (95% CI) | 0.73 (0.70–0.76) | 0.67 (0.54–0.79) |
| Etiology 1 | 1.57 (0.51) | 4.78 (1.77–12.90) | 0.002 | Sensitivity (95% CI) | 0.60 (0.53–0.68) | 0.56 (0.36–0.76) |
| Time since injury | −0.01 (0.01) | 0.99 (0.98–1.00) | 0.007 | Specificity (95% CI) | 0.88 (0.86–0.91) | 0.85 (0.76–0.94) |
| Coma Recovery Scale-Revised | 0.70 (0.22) | 2.01 (1.32–3.08) | 0.001 | AUC (95% CI) | 0.82 (0.80–0.84) | 0.80 (0.76–0.84) |
| Patients | Characteristics | Admission | Progression from UWS to MCS | Emergence from MCS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Etiology | Age | Time Since Injury | CRS-R | Age | Time Since Injury | Time Since Admission | CRS-R | Signs | Age | Time Since Injury | Time Since Admission | CRS-R | Signs | |
| Patient 1 | M | Traumatic | 25 | 86 | 5 | 25 | 110 | 24 | 6 | V | 26 | 271 | 185 | 20 | C |
| Patient 2 | M | Anoxia | 14 | 38 | 7 | 14 | 76 | 38 | 10 | V | 14 | 104 | 66 | 22 | C |
| Patient 3 | M | Traumatic | 26 | 38 | 6 | 26 | 76 | 38 | 18 | M | 26 | 132 | 94 | 23 | C/FU |
| Patient 4 | M | Traumatic | 19 | 240 | 3 | 20 | 486 | 246 | 14 | V/M | 20 | 668 | 428 | 20 | C |
| Patient 5 | W | Traumatic | 31 | 146 | 7 | 31 | 178 | 32 | 8 | V | 32 | 346 | 200 | 19 | C/FU |
| Patient 6 | M | Traumatic | 31 | 62 | 8 | 31 | 89 | 27 | 10 | M | 31 | 172 | 110 | 18 | C/FU |
| Patient 7 | M | Traumatic | 25 | 213 | 8 | 25 | 250 | 37 | 10 | V/M | 25 | 401 | 188 | 16 | C |
| Patient 8 | M | Traumatic | 33 | 45 | 6 | 33 | 91 | 46 | 11 | V/M | 33 | 201 | 156 | 19 | FU |
| Patient 9 | M | Fat embolism | 19 | 45 | 6 | 19 | 86 | 41 | 10 | C | 19 | 99 | 54 | 12 | C |
| Patient 10 | M | Traumatic | 21 | 117 | 8 | 21 | 181 | 64 | 9 | V | 21 | 243 | 126 | 21 | FU |
| Patient 11 | M | Traumatic | 13 | 126 | 8 | 13 | 158 | 32 | 12 | V | 13 | 222 | 96 | 21 | FU |
| Patient 12 | M | Traumatic | 31 | 101 | 7 | 31 | 130 | 29 | 9 | V | 31 | 154 | 53 | 18 | FU |
| Patients Who Emerged from MCS (n = 12) | Patients Who Did not Emerge from MCS (n = 88) | Significance | |
|---|---|---|---|
| Age (years) | 24.5 ± 6.8 | 39.5 ± 18.4 | p = 0.006 |
| Sex (n, %) Women Men | 1 (8.3%) 11 (91.7%) | 28 (31.8%) 60 (68.2%) | NS (p = 0.093) |
| Education (years) | 10.2 ± 2.6 | 10.9 ± 5.3 | NS (p = 0.690) |
| Etiology (n, %) Traumatic Non-traumatic | 10 (83.3%) 2 (16.7%) | 30 (34.1%) 58 (65.9%) | p = 0.001 |
| Time since injury (days) | 105.2 ± 68.7 | 136.6 ± 87.2 | NS (p = 0.236) |
| Coma Recovery Scale-Revised | 6.6 ± 1.5 | 5.6 ± 1.3 | p = 0.014 |
| Multivariable Logistic Regression Model | Cross-Validation Evaluation Metrics | |||||
|---|---|---|---|---|---|---|
| B (SE) | OR (95% CI) | p | Training | Validation | ||
| Accuracy (95% CI) | 0.89 (0.87–0.90) | 0.88 (0.86–0.90) | ||||
| Constant | −4.76 (2.10) | 0.01 (0.00–0.53) | 0.024 | Precision (95% CI) | 0.50 (0.06–0.94) | 0.10 (0.00 *−0.30) |
| Age | −0.07 (0.04) | 0.93 (0.87–1.00) | 0.050 | Sensitivity (95% CI) | 0.08 (0.00–0.16) | 0.10 (0.00 *–0.30) |
| Etiology 1 | 2.04 (0.86) | 7.68 (1.42–41.52) | 0.018 | Specificity (95% CI) | 1.00 (0.99–1.00) | 0.99 (0.97–1.00 *) |
| Coma Recovery Scale-Revised | 0.58 (0.29) | 1.79 (1.02–3.14) | 0.041 | AUC (95% CI) | 0.86 (0.84–0.88) | 0.78 (0.68–0.89) |
| Patients Who Died (n = 10) | Patients Who Survive (n = 90) | Significance | |
|---|---|---|---|
| Age (years) | 57.2 ± 15.6 | 35.5 ± 17.0 | p < 0.001 |
| Sex (n, %) Women Men | 4 (40.0%) 6 (60.0%) | 25 (27.8%) 65 (72.2%) | NS (p = 0.419) |
| Education (years) | 12.4 ± 5.9 | 10.6 ± 5.0 | NS (p = 0.295) |
| Etiology (n, %) Traumatic Non-traumatic | 4 (40.0%) 6 (60.0%) | 36 (40.0%) 54 (60.0%) | NS (p = 1.000) |
| Time since injury (years) | 135.7 ± 110.1 | 132.5 ± 83.1 | NS (p = 0.911) |
| Coma Recovery Scale-Revised | 5.8 ± 1.3 | 5.0 ± 1.3 | NS (p = 0.081) |
| Multivariable Logistic Regression Model | Cross-Validation Evaluation Metrics | |||||
|---|---|---|---|---|---|---|
| B (SE) | OR (95% CI) | p | Training | Validation | ||
| Accuracy (95% CI) | 0.91 (0.90–0.91) | 0.90 (0.87–0.93) | ||||
| Precision (95% CI) | 0.60 (0.12–1.00 *) | 0.20 (0.00 *–0.59) | ||||
| Sensitivity (95% CI) | 0.08 (0.02–0.14) | 0.10 (0.00 *–0.30) | ||||
| Constant | −5.78 (1.37) | 0.00 (0.00–0.05) | <0.001 | Specificity (95% CI) | 1.00 (1.00–1.00) | 0.99 (0.97–1.00 *) |
| Age | 0.08 (0.02) | 1.08 (1.03–1.10) | 0.002 | AUC (95% CI) | 0.83 (0.79–0.87) | 0.78 (0.61–0.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noé, E.; Ferri, J.; Olaya, J.; Navarro, M.D.; O’Valle, M.; Colomer, C.; Moliner, B.; Ippoliti, C.; Maza, A.; Llorens, R. When, How, and to What Extent Are Individuals with Unresponsive Wakefulness Syndrome Able to Progress? Neurobehavioral Progress. Brain Sci. 2021, 11, 126. https://doi.org/10.3390/brainsci11010126
Noé E, Ferri J, Olaya J, Navarro MD, O’Valle M, Colomer C, Moliner B, Ippoliti C, Maza A, Llorens R. When, How, and to What Extent Are Individuals with Unresponsive Wakefulness Syndrome Able to Progress? Neurobehavioral Progress. Brain Sciences. 2021; 11(1):126. https://doi.org/10.3390/brainsci11010126
Chicago/Turabian StyleNoé, Enrique, Joan Ferri, José Olaya, María Dolores Navarro, Myrtha O’Valle, Carolina Colomer, Belén Moliner, Camilla Ippoliti, Anny Maza, and Roberto Llorens. 2021. "When, How, and to What Extent Are Individuals with Unresponsive Wakefulness Syndrome Able to Progress? Neurobehavioral Progress" Brain Sciences 11, no. 1: 126. https://doi.org/10.3390/brainsci11010126
APA StyleNoé, E., Ferri, J., Olaya, J., Navarro, M. D., O’Valle, M., Colomer, C., Moliner, B., Ippoliti, C., Maza, A., & Llorens, R. (2021). When, How, and to What Extent Are Individuals with Unresponsive Wakefulness Syndrome Able to Progress? Neurobehavioral Progress. Brain Sciences, 11(1), 126. https://doi.org/10.3390/brainsci11010126





