Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) Paired with Language Therapy in the Treatment of Primary Progressive Aphasia: An Exploratory Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Eligibility: Inclusion and Exclusion Criteria
2.3. Literature Selection and Data Extraction
2.4. Data Analyses
3. Results
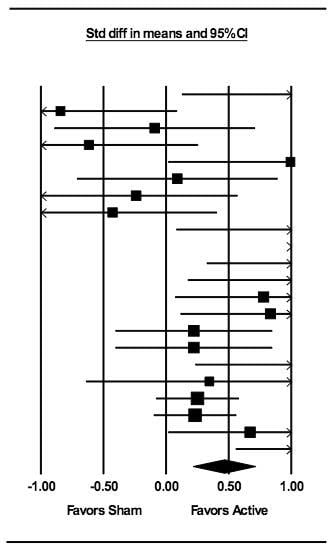
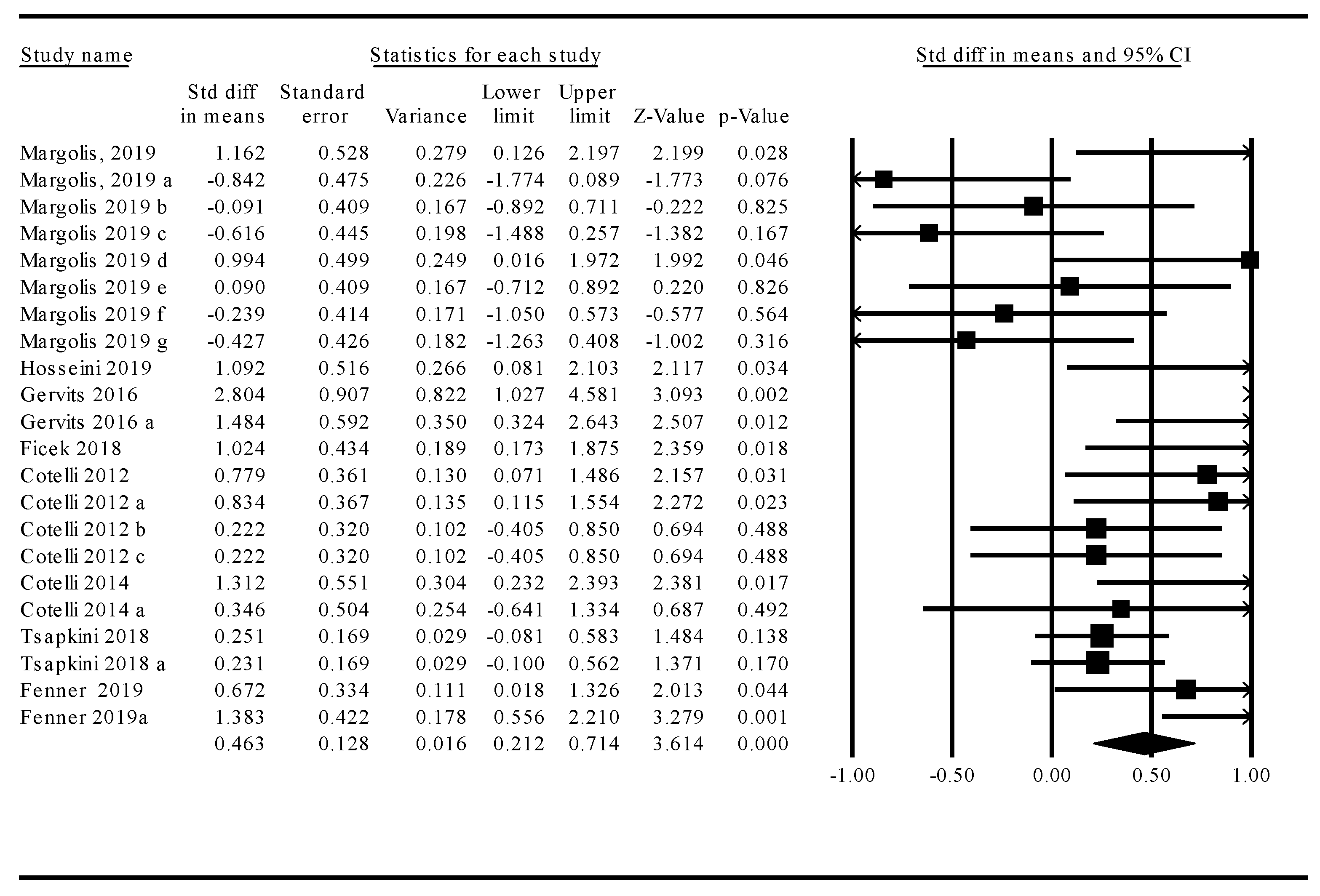
3.1. Meta-Analysis: Treatment Effects across tDCS and TMS Studies
3.2. Moderator Analyses
Stimulation Modality: tDCS Versus TMS
3.3. Language Function
3.4. Treated Versus Untreated Naming Stimuli
3.5. Stimulation Parameters: tDCS
3.5.1. Intensity
3.5.2. Stimulation Duration
3.5.3. Frequency of Treatment
3.6. Participant Demographics
3.6.1. Sex
3.6.2. Age
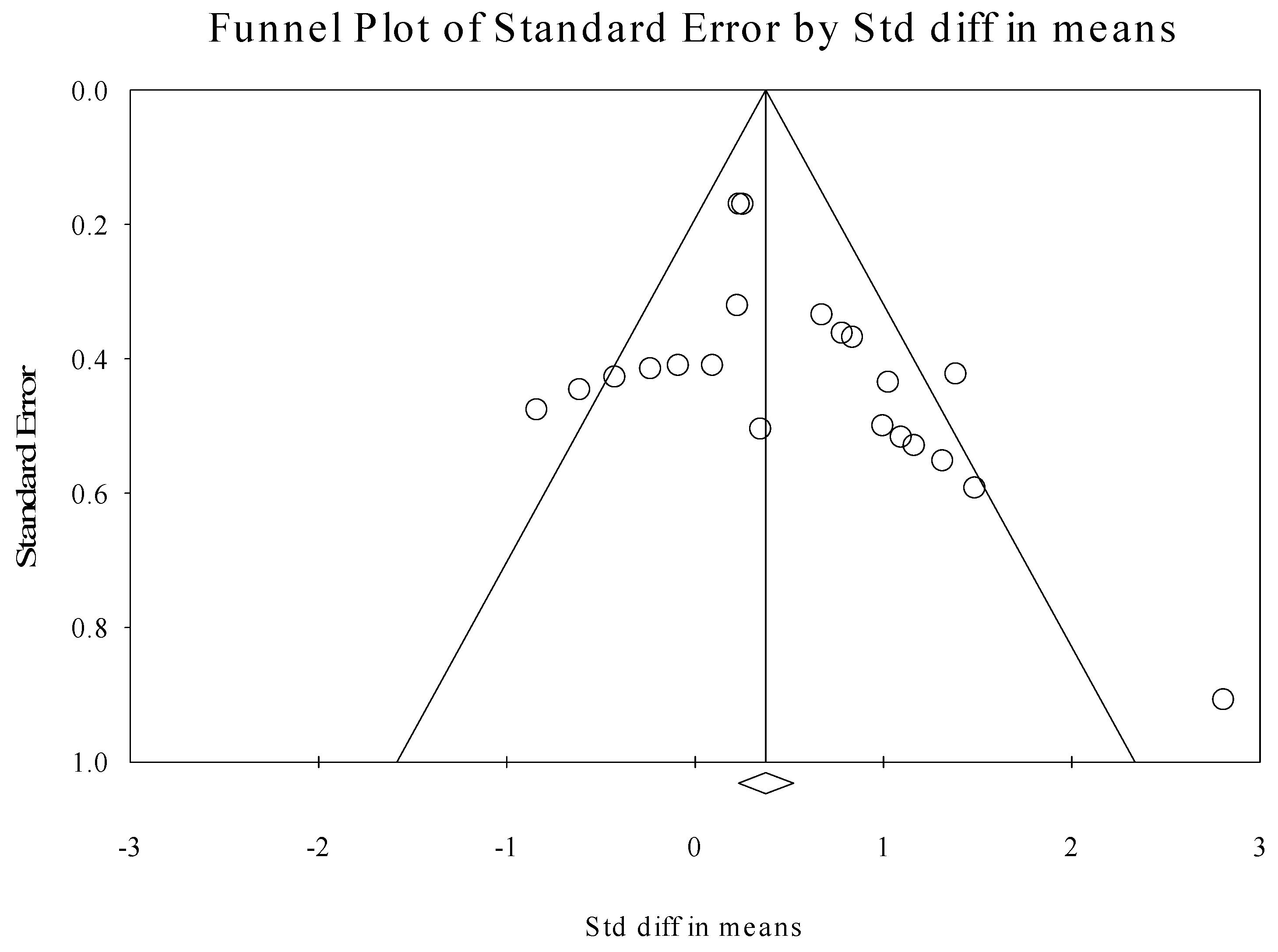
3.7. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goh, J.O.; Park, D.C. Neuroplasticity and cognitive aging: The scaffolding theory of aging and cognition. Restor. Neurol. Neurosci. 2009, 27, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.H. Neuroplasticity in the language system: Reorganization in post-stroke aphasia and in neuromodulation interventions. Restor. Neurol. Neurosci. 2016, 34, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Polanía, R.; Nitsche, M.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef]
- Norise, C.; Hamilton, R.H. Non-invasive Brain Stimulation in the Treatment of Post-stroke and Neurodegenerative Aphasia: Parallels, Differences, and Lessons Learned. Front. Hum. Neurosci. 2017, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikson, M.; Datta, A.; Elwassif, M. Establishing safety limits for transcranial direct current stimulation. Clin. Neurophysiol. 2009, 120, 1033–1034. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Chrysikou, E.G.; Hamilton, R.H. Noninvasive brain stimulation in the treatment of aphasia: Exploring interhemispheric relationships and their implications for neurorehabilitation. Restor. Neurol. Neurosci. 2011, 29, 375–394. [Google Scholar] [CrossRef]
- Medeiros, L.F.; De Souza, I.C.C.; Vidor, L.P.; De Souza, A.; Deitos, A.; Volz, M.S.; Fregni, F.; Caumo, W.; Torres, I.L. Neurobiological Effects of Transcranial Direct Current Stimulation: A Review. Front. Psychol. 2012, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sonmez, A.I.; Camsari, D.D.; Nandakumar, A.L.; Voort, J.L.V.; Kung, S.; Lewis, C.P.; Croarkin, P.E. Accelerated TMS for Depression: A systematic review and meta-analysis. Psychiatry Res. Neuroimaging 2019, 273, 770–781. [Google Scholar] [CrossRef]
- Gálvez, V.; Ho, K.-A.; Alonzo, A.; Martin, D.M.; George, D.; Loo, C.K. Neuromodulation Therapies for Geriatric Depression. Curr. Psychiatry Rep. 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Galletta, E.E.; Rao, P.R.; Barrett, A.M. Transcranial magnetic stimulation (TMS): Potential progress for language improvement in aphasia. Top. Stroke Rehabil. 2011, 18, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- Nardone, R.; Tezzon, F.; Höller, Y.; Golaszewski, S.; Trinka, E.; Brigo, F. Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurol. Scand. 2014, 129, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Pellicciari, M.C.; Miniussi, C. Transcranial Direct Current Stimulation in Neurodegenerative Disorders. J. ECT 2018, 34, 193–202. [Google Scholar] [CrossRef]
- De Aguiar, V.; Paolazzi, C.L.; Miceli, G. tDCS in post-stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex 2015, 63, 296–316. [Google Scholar] [CrossRef]
- Shah-Basak, P.P.; Wurzman, R.; Purcell, J.B.; Gervits, F.; Hamilton, R.H. Fields or flows? A comparative metaanalysis of transcranial magnetic and direct current stimulation to treat post-stroke aphasia. Restor. Neurol. Neurosci. 2016, 34, 537–558. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.-F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-Like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Alberici, A.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miozzo, A.; Padovani, A.; Miniussi, C.; Borroni, B. Prefrontal cortex rTMS enhances action naming in progressive non-fluent aphasia. Eur. J. Neurol. 2012, 19, 1404–1412. [Google Scholar] [CrossRef]
- Gervits, F.; Ash, S.; Coslett, H.B.; Rascovsky, K.; Grossman, M.; Hamilton, R.H. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang. 2016, 162, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Méndez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.E.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Montembeault, M.; Brambati, S.M.; Gorno-Tempini, M.L.; Migliaccio, R. Clinical, Anatomical, and Pathological Features in the Three Variants of Primary Progressive Aphasia: A Review. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 141st Annual Meeting of the American Neurological Association. Ann. Neurol. 2016, 80, S1–S265. [CrossRef] [PubMed]
- Tippett, D.C.; Hillis, A.E.; Tsapkini, K. Treatment of Primary Progressive Aphasia. Curr. Treat. Options Neurol. 2015, 17, 362. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.L.; Gorno-Tempini, M.L. The logopenic variant of primary progressive aphasia. Curr. Opin. Neurol. 2010, 23, 633–637. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Petesi, M.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miniussi, C.; Padovani, A.; Borroni, B. Treatment of Primary Progressive Aphasias by Transcranial Direct Current Stimulation Combined with Language Training. J. Alzheimer’s Dis. 2014, 39, 799–808. [Google Scholar] [CrossRef]
- Margolis, S.A.; Festa, E.K.; Papandonatos, G.D.; Korthauer, L.E.; Gonsalves, M.A.; Oberman, L.; Heindel, W.C.; Ott, B.R. A pilot study of repetitive transcranial magnetic stimulation in primary progressive aphasia. Brain Stimul. 2019, 12, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; McConathey, E.M.; Ungrady, M.; Grossman, M.; Coslett, H.B.; Hamilton, R.H. Proceedings #10: Transcranial Direct Current Stimulation Mediates Improvements in Verbal Fluency for Patients with Primary Progressive Aphasia. Brain Stimul. 2019, 12, e69–e71. [Google Scholar] [CrossRef]
- Tsapkini, K.; Webster, K.T.; Ficek, B.N.; Desmond, J.E.; Onyike, C.U.; Rapp, B.; Frangakis, C.E.; Hillis, A.E. Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2018, 4, 461–472. [Google Scholar] [CrossRef]
- Sierra, H.; Cordova, M.; Chen, C.-S.J.; Rajadhyaksha, M. Confocal Imaging–Guided Laser Ablation of Basal Cell Carcinomas: An Ex Vivo Study. J. Investig. Dermatol. 2015, 135, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Croot, K.; Nickels, L.; Laurence, F.; Manning, M. Impairment- and activity/participation-directed interventions in progressive language impairment: Clinical and theoretical issues. Aphasiology 2009, 23, 125–160. [Google Scholar] [CrossRef]
- Graham, K.S.; Patterson, K.; Pratt, K.H.; Hodges, J.R. Relearning and subsequent forgetting of semantic category exemplars in a case of semantic dementia. Neuropsychology 1999, 13, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Beeson, P.M.; King, R.M.; Bonakdarpour, B.; Henry, M.L.; Cho, H.; Rapcsak, S.Z. Positive Effects of Language Treatment for the Logopenic Variant of Primary Progressive Aphasia. J. Mol. Neurosci. 2011, 45, 724–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokel, R.; Rochon, E.; Anderson, N.D. Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychol. Rehabil. 2010, 20, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.L.; Meese, M.V.; Truong, S.; Babiak, M.C.; Miller, B.L.; Gorno-Tempini, M.L. Treatment for Apraxia of Speech in Nonfluent Variant Primary Progressive Aphasia. Behav. Neurol. 2013, 26, 77–88. [Google Scholar] [CrossRef]
- Jokel, R.; Rochon, E.; Leonard, C. Treating anomia in semantic dementia: Improvement, maintenance, or both? Neuropsychol. Rehabil. 2006, 16, 241–256. [Google Scholar] [CrossRef]
- Silvanto, J.; Muggleton, N.; Walsh, V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008, 12, 447–454. [Google Scholar] [CrossRef]
- Gill, J.; Shah-Basak, P.P.; Hamilton, R.H. It’s the Thought That Counts: Examining the Task-dependent Effects of Transcranial Direct Current Stimulation on Executive Function. Brain Stimul. 2015, 8, 253–259. [Google Scholar] [CrossRef]
- Teichmann, M.; Lesoil, C.; Godard, J.; Vernet, M.; Bertrand, A.; Levy, R.; Dubois, B.; Lemoine, L.; Truong, D.Q.; Bikson, M.; et al. Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann. Neurol. 2016, 80, 693–707. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Silvanto, J. Time course of the state-dependent effect of transcranial magnetic stimulation in the TMS-adaptation paradigm. Neurosci. Lett. 2008, 443, 82–85. [Google Scholar] [CrossRef]
- Nissim, N.R.; O’Shea, A.; Indahlastari, A.; Telles, R.; Richards, L.; Porges, E.; Cohen, R.; Woods, A.J. Effects of in-Scanner Bilateral Frontal tDCS on Functional Connectivity of the Working Memory Network in Older Adults. Front. Aging Neurosci. 2019, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cotelli, M.; Manenti, R.; Ferrari, C.; Gobbi, E.; Macis, A.; Cappa, S.F. Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in Primary Progressive Aphasia: A meta-analysis and systematic review. Neurosci. Biobehav. Rev. 2020, 108, 498–525. [Google Scholar] [CrossRef] [PubMed]
- Miniussi, C.; Cappa, S.F.; Cohen, L.G.; Floel, A.; Fregni, F.; Nitsche, M.A.; Oliveri, M.; Pascual-Leone, A.; Paulus, W.; Priori, A.; et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008, 1, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Manenti, R.; Rosini, S.; Calabria, M.; Brambilla, M.; Bisiacchi, P.S.; Zanetti, O.; Miniussi, C. Action and Object Naming in Physiological Aging: An rTMS Study. Front. Aging Neurosci. 2010, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaduman, A.; Göksun, T.; Chatterjee, A. Narratives of Focal Brain Injured Individuals: A Macro-Level Analysis. Neuropsychologia 2017, 99, 314–325. [Google Scholar] [CrossRef] [Green Version]
- Fricke, K.; Seeber, A.A.; Thirugnanasambandam, N.; Paulus, W.; Nitsche, M.A.; Rothwell, J.C. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2011, 105, 1141–1149. [Google Scholar] [CrossRef]
- Kronberg, G.; Bridi, M.; Abel, T.; Bikson, M.; Parra, L.C. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul. 2016, 10, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Naeser, M.A.; Martin, P.I.; Ho, M.; Treglia, E.; Kaplan, E.; Baker, E.H.; Pascual-Leon, A. Transcranial magnetic stimulation and aphasia research. Handb. Neuropsychol. Lang. 2012, 2, 679–699. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallee, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Coslett, H.B.; Thomas, A.L.; Faseyitan, O.; Benson, J.; Norise, C.; Hamilton, R.H. The right hemisphere is not unitary in its role in aphasia recovery. Cortex 2011, 48, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabré, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2011, 5, 175–195. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Drebing, D.; Hamilton, R. TMS and tDCS in post-stroke aphasia: Integrating novel treatment approaches with mechanisms of plasticity. Restor. Neurol. Neurosci. 2013, 31, 501–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, T.O.; Karabanov, A.N.; Hartwigsen, G.; Thielscher, A.; Siebner, H.R. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: Current approaches and future perspectives. NeuroImage 2016, 140, 4–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byeon, H. Meta-Analysis on the Effects of Transcranial Direct Current Stimulation on Naming of Elderly with Primary Progressive Aphasia. Int. J. Environ. Res. Public Health 2020, 17, 1095. [Google Scholar] [CrossRef] [Green Version]
- Kronberg, G.; Rahman, A.; Sharma, M.; Bikson, M.; Parra, L.C. Direct current stimulation boosts hebbian plasticity in vitro. Brain Stimul. 2020, 13, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Lafon, B.; Parra, L.C.; Bikson, M. Direct current stimulation boosts synaptic gain and cooperativity in vitro. J. Physiol. 2017, 595, 3535–3547. [Google Scholar] [CrossRef] [Green Version]
- Ross, T.P. The reliability of cluster and switch scores for the Controlled Oral Word Association Test. Arch. Clin. Neuropsychol. 2003, 18, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Mätzig, S.; Druks, J.; Masterson, J.; Vigliocco, G. Noun and verb differences in picture naming: Past studies and new evidence. Cortex 2009, 45, 738–758. [Google Scholar] [CrossRef]
- Galletta, E.E.; Goral, M. Response Time Inconsistencies in Object and Action Naming in Anomic Aphasia. Am. J. Speech-Lang. Pathol. 2018, 27, 477–484. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, U.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Ficek, B.N.; Wang, Z.; Zhao, Y.; Webster, K.T.; Desmond, J.E.; Hillis, A.E.; Frangakis, C.; Faria, A.V.; Caffo, B.; Tsapkini, K. The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage Clin. 2018, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Fenner, A.S.; Webster, K.T.; Ficek, B.N.; Frangakis, C.E.; Tsapkini, K. Written verb naming improves after tDCS over the left IFG in primary progressive aphasia. Front. Psychol. 2019, 10, 1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Wasserman, S.; Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis. J. Educ. Stat. 1988, 13, 75. [Google Scholar] [CrossRef]
- Grant, J.; Hunter, A. Measuring inconsistency in knowledgebases. J. Intell. Inf. Syst. 2006, 27, 159–184. [Google Scholar] [CrossRef]
- Thompson, S.; Higgins, J.P.T. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Nair, A.S. Publication bias—Importance of studies with negative results! Indian J. Anaesth. 2019, 63, 505–507. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef]
- Orwin, R.G. A Fail-Safe N for Effect Size in Meta-Analysis. J. Educ. Stat. 1983, 8, 157. [Google Scholar] [CrossRef]
- Kronberg, G.; Rahman, A.; Lafon, B.; Parra, L.C.; Bikson, M.; Sharma, M. Direct current stimulation boosts associative Hebbian synaptic plasticity and maintains its pathway specificity. Brain Stimul. 2019. [Google Scholar] [CrossRef] [Green Version]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Indahlastari, A.; Albizu, A.; O’Shea, A.; Forbes, M.A.; Nissim, N.R.; Kraft, J.N.; Evangelista, N.D.; Hausman, H.K.; Woods, A.J.; Initiative, A.D.N. Modeling transcranial electrical stimulation in the aging brain. Brain Stimul. 2020, 13, 664–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, S.; Towhidkhah, F. Computational human head models of tDCS: Influence of brain atrophy on current density distribution. Brain Stimul. 2018, 11, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, K.A.; Badran, B.W.; Devries, W.H.; Summers, P.M.; Kofmehl, E.; Li, X.; Borckardt, J.J.; Bikson, M.; George, M.S. Transcranial Electrical Stimulation Motor Threshold Combined with Reverse-Calculated Electric Field Modeling Can Determine Individualized tDCS Dosage. BioRxiv 2019, 2019, 798751. [Google Scholar] [CrossRef]
- Chhatbar, P.Y.; Kautz, S.A.; Takacs, I.; Rowland, N.C.; Revuelta, G.J.; George, M.S.; Bikson, M.; Feng, W. Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimul. 2018, 11, 727–733. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Bikson, M. Extending the parameter range for tDCS: Safety and tolerability of 4 mA stimulation. Brain Stimul. 2017, 10, 541–542. [Google Scholar] [CrossRef]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar]
- Samani, M.M.; Agboada, D.; Jamil, A.; Kuo, M.-F.; Nitsche, M.A. Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 2019, 119, 350–361. [Google Scholar] [CrossRef]
| Study | Modality | Study Design | Sample Size | PPA Variant | Sessions | Stimulation Parameters | Location of Stimulation | Concurrent Task | Primary Outcome Measure |
|---|---|---|---|---|---|---|---|---|---|
| [25] | tDCS | Between-subject study | 16 | naPPA | 10 | 2 mA for 25 min (30 s ramp up/down) | Anode: Left DLPFC (BA8/9); cathode: Right arm | Individualized speech therapy | Aachener Aphasie Test (AAT) naming subtest: mean correct response |
| [19] | tDCS | Open label pilot study | 6 | naPPA/lvPPA | 10 | 1.5 mA for 20 min (30 s ramp up/down) | Anode: Left fronto-temporal region (F7); cathode: Left occipito-parietal region (O1) | Narration of wordless picture books | Cookie theft picture task: elicited speech production from the Boston Naming Test; Language test for the reception of grammar (L-TROG): mean correct response |
| [61] | tDCS | Within-subject crossover | 24 | naPPA/lvPPA/svPPA | 15 | 2 mA tDCS for 20 min (active); 30 s (sham) | Anode: Left frontal lobe (F7); cathode: right cheek | Oral and written picture naming spelling therapy | Naming/spelling accuracy: percentage of correct response/correct letters |
| [28] | tDCS | Within-subject crossover | 36 | naPPA/lvPPA/svPPA | 15 | 2 mA for 20 min (30 s ramp up/down) | Anode: Left frontal lobe (F7); cathode: Right cheek | Written naming spelling therapy | Naming spelling accuracy: percentage of correct letters from treated versus untreated words |
| [62] | tDCS | Within-subject crossover | 11 | naPPA/lvPPA | 15 | 2 mA for 20 min (30 s ramp up/down) | Anode: Left frontal lobe (F7); cathode: Right cheek | Oral and written naming spelling treatment | Written naming performance: mean letter accuracy |
| [27] | tDCS | Within-subject crossover | 6 | naPPA | 10 | 1.5 mA for 20 min (30 s ramp up/down) | Anode: Left prefrontal region; cathode: Left occipital region | Category fluency | Category fluency: mean number of words |
| [18] | rTMS | Within-subject crossover | 10 | naPPA | 1 | rTMS: 20 Hz frequency at 90% MT; 500 ms; 84 trains | Left and Right DLPFC | Action/object naming | Action and object naming: mean correct response |
| [26] | rTMS | Within-subject crossover | 6 | naPPA | 2 | rTMS: 20 Hz frequency at 90% MT; 1000 ms; 84 trains | Left and Right DLPFC | Action/object naming task and Stroop task | Action and object naming: mean correct response; Stroop color-word accuracy |
| Study | Stimulation Modality | Sample Size | Mean Age | Education (Mean Year) | Percent Male | Disease Duration (Mean Year) |
|---|---|---|---|---|---|---|
| [25] | tDCS | 16 | 63.4 | 9.3 | 36 | 2 |
| [19] | tDCS | 6 | 66.2 | 16.3 | 17 | 4.2 |
| [61] | tDCS | 24 | 67.2 | - | 54 | 4.9 |
| [28] | tDCS | 36 | 67.9 | 16.3 | 55 | 5.8 |
| [62] | tDCS | 11 | 67.6 | - | 64 | 5 |
| [27] | tDCS | 3 | 67.0 | 14.3 | 33 | 4.8 |
| [18] | rTMS | 10 | 69.1 | - | 20 | 2.3 |
| [26] | rTMS | 6 | 67.0 | 15 | 67 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissim, N.R.; Moberg, P.J.; Hamilton, R.H. Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) Paired with Language Therapy in the Treatment of Primary Progressive Aphasia: An Exploratory Meta-Analysis. Brain Sci. 2020, 10, 597. https://doi.org/10.3390/brainsci10090597
Nissim NR, Moberg PJ, Hamilton RH. Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) Paired with Language Therapy in the Treatment of Primary Progressive Aphasia: An Exploratory Meta-Analysis. Brain Sciences. 2020; 10(9):597. https://doi.org/10.3390/brainsci10090597
Chicago/Turabian StyleNissim, Nicole R., Paul J. Moberg, and Roy H. Hamilton. 2020. "Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) Paired with Language Therapy in the Treatment of Primary Progressive Aphasia: An Exploratory Meta-Analysis" Brain Sciences 10, no. 9: 597. https://doi.org/10.3390/brainsci10090597
APA StyleNissim, N. R., Moberg, P. J., & Hamilton, R. H. (2020). Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) Paired with Language Therapy in the Treatment of Primary Progressive Aphasia: An Exploratory Meta-Analysis. Brain Sciences, 10(9), 597. https://doi.org/10.3390/brainsci10090597