Multiple Thrombectomies in the Same Patient within One Month: Case Report of a Patient with Trousseau Syndrome and Acute Ischemic Stroke
Abstract
1. Introduction
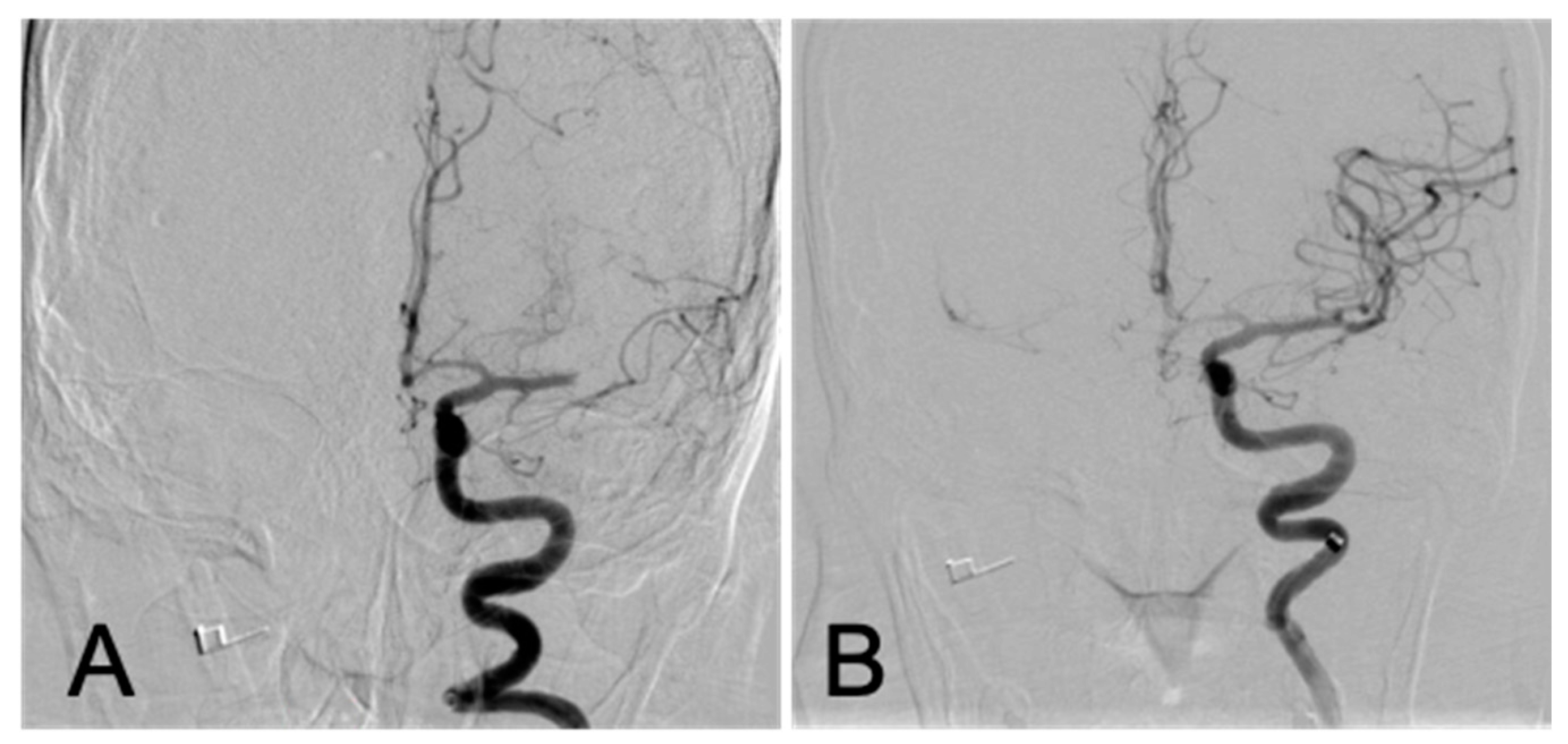
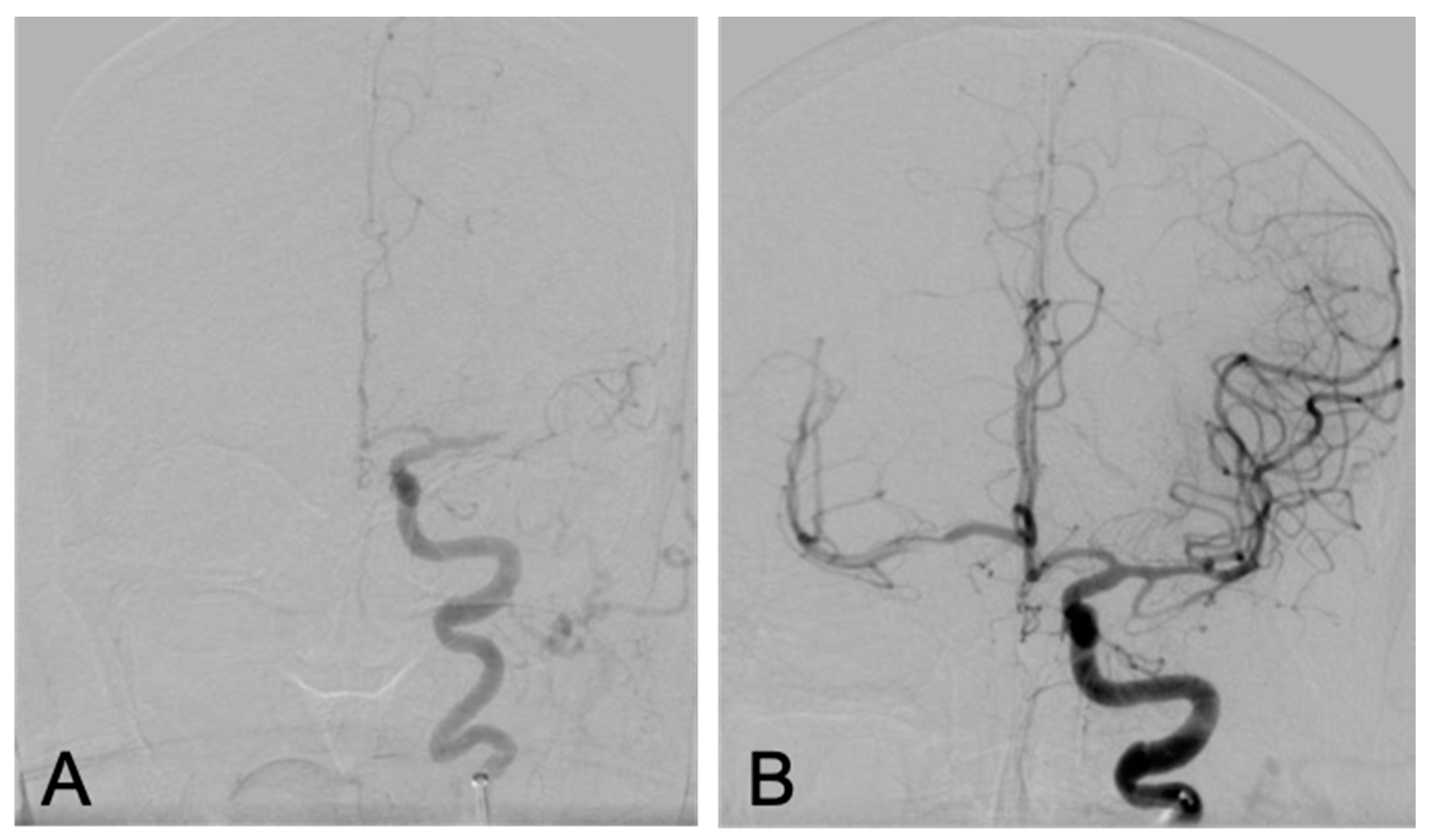
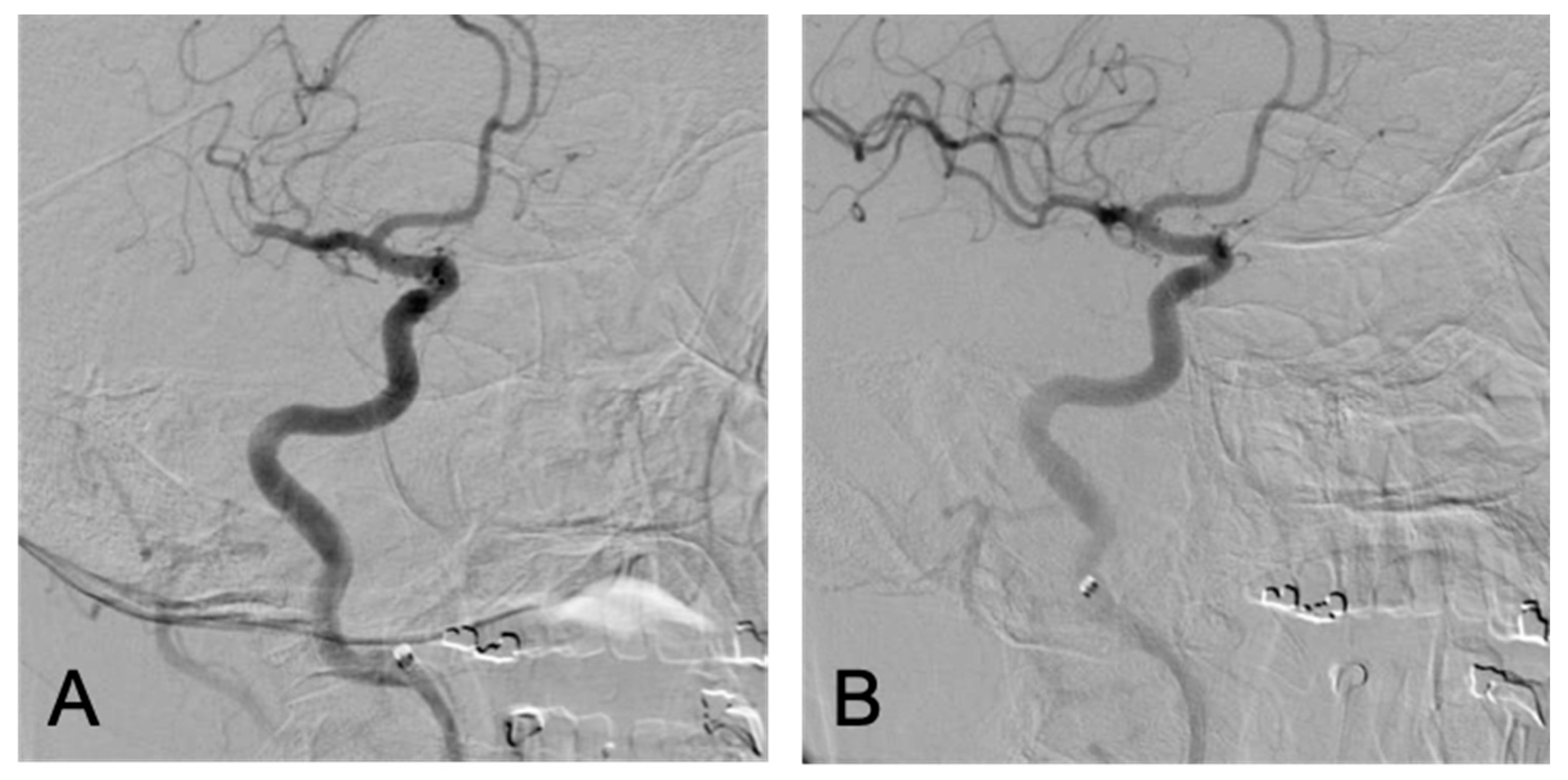
2. The Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marinho, F.C.d.A.; Teresa, Y.T. Hypercoagulability and lung cancer. J. Bras. Pneumol. 2008, 34, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Trousseau, A. Clinique Médicale de l’Hôtel-Dieu de Paris; Baillière: Paris, France, 1865; Volume 2. [Google Scholar]
- Nguyen, T.; Lisa, M.D. Stroke in cancer patients. Curr. Neurol. Neurosci. Rep. 2006, 6, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e99. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.G.; Spence, J.D. Antiplatelet therapy in ischemic stroke and transient ischemic attack: An overview of major trials and meta-analyses. Stroke 2019, 50, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chung, J.; Ahn, M.-J.; Kim, S.; Seok, J.M.; Jang, H.M.; Kim, G.-M.; Chung, C.-S.; Lee, K.H.; Bang, O.Y. Hypercoagulability and Mortality of Patients with Stroke and Active Cancer: The OASIS-CANCER Study. J. Stroke 2017, 19, 77–87. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.-W.; Cho, Y.H.; Oh, M.J.; Seo, W.-K.; Kim, G.-M.; Ahn, M.-J. Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke: The OASIS-Cancer Study. Stroke 2019, 50, 2944–2947. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.-W.; Lee, M.J.; Seo, W.-K.; Kim, G.-M.; Ahn, M.-J. OASIS-Cancer Study Investigators Cancer-Related Stroke: An Emerging Subtype of Ischemic Stroke with Unique Pathomechanisms. J. Stroke 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Świtońska, M.; Słomka, A.; Korbal, P.; Piekus, N.; Sinkiewicz, W.; Sokal, P.; Żekanowska, E. Association of Neutrophil-to-Lymphocyte Ratio and Lymphocyte-to-Monocyte Ratio with Treatment Modalities of Acute Ischaemic Stroke: A Pilot Study. Medicina 2019, 55, 342. [Google Scholar] [CrossRef]
- Pirson, F.A.V.; Van Oostenbrugge, R.J.; Van Zwam, W.H.; Remmers, M.J.; Dippel, D.W.; Van Es, A.C.; Wijngaard, I.R.V.D.; Schonewille, W.J.; Staals, J. Repeated endovascular thrombectomy in patients with acute ischemic stroke: Results from a nationwide multicenter database. Stroke 2020, 51, 526–532. [Google Scholar] [CrossRef]
- Neilson, E.L.; Rogers, L.R.; Sundararajan, S. Evaluation and Treatment of a Patient with Recurrent Stroke in the Setting of Active Malignancy. Stroke 2019, 50, e9–e11. [Google Scholar] [CrossRef]
- Dardiotis, E.; Aloizou, A.-M.; Markoula, S.; Siokas, V.; Tsarouhas, K.; Tzanakakis, G.; Libra, M.; Kyritsis, A.P.; Brotis, A.G.; Aschner, M.; et al. Cancer-associated stroke: Pathophysiology, detection and management (Review). Int. J. Oncol. 2019, 54, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.Y.; Peterson, E.A. Treatment of cancer-associated thrombosis. Blood J. Am. Soc. Hematol. 2013, 122, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-B.; Hsu, J.-Y. Anticoagulants for cancer-associated ischemic stroke. Tzu Chi Med. J. 2019, 31, 144–148. [Google Scholar] [CrossRef]
- Navi, B.B.; Iadecola, C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann. Neurol. 2018, 83, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Mosarla, R.C.; Vaduganathan, M.; Qamar, A.; Moslehi, J.; Piazza, G.; Giugliano, R.P. Anticoagulation Strategies in Patients With Cancer. J. Am. Coll. Cardiol. 2019, 73, 1336–1349. [Google Scholar] [CrossRef]
- Raskob, G.; Van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.J.; Lee, M.J.; Ryoo, S.; Yoon, C.H.; Kim, G.-M.; Chung, C.-S.; Lee, K.H.; Bang, O.Y.; Kim, S.J. Comparison of Enoxaparin and Warfarin for Secondary Prevention of Cancer-Associated Stroke. J. Oncol. 2015, 2015, 502089. [Google Scholar] [CrossRef]
- Khorana, A.A.; McCrae, K.R.; Milentijevic, D.; Fortier, J.; Nelson, W.W.; Laliberté, F.; Crivera, C.; Lefebvre, P.; Yannicelli, D.; Schein, J. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res. Pract. Thromb. Haemost. 2017, 1, 14–22. [Google Scholar] [CrossRef]
- Lopes, R.D.; Alexander, J.H.; Al-Khatib, S.M.; Ansell, J.; Diaz, R.; Easton, J.D.; Gersh, B.J.; Granger, C.B.; Hanna, M.; Horowitz, J.; et al. Apixaban for Reduction In Stroke and Other ThromboemboLic Events in Atrial Fibrillation (ARISTOTLE) trial: Design and rationale. Am. Heart J. 2010, 159, 331–339. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, Á.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Navi, B.B.; Marshall, R.S.; Bobrow, D.; Singer, S.; Stone, J.B.; DeSancho, M.T.; DeAngelis, L.M. Enoxaparin vs Aspirin in Patients With Cancer and Ischemic Stroke: The TEACH Pilot Randomized Clinical Trial. JAMA Neurol. 2018, 75, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, M.; Bastianello, S.; Von Kummer, R.; Del Zoppo, G.J.; Larrue, V.; Lesaffre, E.; Ringleb, A.P.; Lorenzano, S.; Manelfe, C.; Bozzao, L. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999, 30, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Gupta, A.; Merkler, A.E.; Navi, B.B.; Mandava, P.; Iadecola, C.; Sheth, K.N.; Hanley, D.F.; Ziai, W.C.; Kamel, H. Restarting Anticoagulant Therapy After Intracranial Hemorrhage: A Systematic Review and Meta-Analysis. Stroke 2017, 48, 1594–1600. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicilioni, K.; Cristiano, B.; Jacobson, J.P.; Hoss, D.; Lund, M.; Cheung, S.; Dye, J. Multiple Thrombectomies in the Same Patient within One Month: Case Report of a Patient with Trousseau Syndrome and Acute Ischemic Stroke. Brain Sci. 2020, 10, 590. https://doi.org/10.3390/brainsci10090590
Cicilioni K, Cristiano B, Jacobson JP, Hoss D, Lund M, Cheung S, Dye J. Multiple Thrombectomies in the Same Patient within One Month: Case Report of a Patient with Trousseau Syndrome and Acute Ischemic Stroke. Brain Sciences. 2020; 10(9):590. https://doi.org/10.3390/brainsci10090590
Chicago/Turabian StyleCicilioni, Kurt, Brian Cristiano, J. Paul Jacobson, Daniel Hoss, Matthew Lund, Shauna Cheung, and Justin Dye. 2020. "Multiple Thrombectomies in the Same Patient within One Month: Case Report of a Patient with Trousseau Syndrome and Acute Ischemic Stroke" Brain Sciences 10, no. 9: 590. https://doi.org/10.3390/brainsci10090590
APA StyleCicilioni, K., Cristiano, B., Jacobson, J. P., Hoss, D., Lund, M., Cheung, S., & Dye, J. (2020). Multiple Thrombectomies in the Same Patient within One Month: Case Report of a Patient with Trousseau Syndrome and Acute Ischemic Stroke. Brain Sciences, 10(9), 590. https://doi.org/10.3390/brainsci10090590



