Age-Related Changes in Attentional Refocusing during Simulated Driving
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Materials and Procedures
2.2.1. Driving Simulator
2.2.2. EEG Acquisition
3. Data Analysis
3.1. Driving Simulator Task RTs and Accuracy
3.2. EEG Analysis
3.2.1. Preprocessing
3.2.2. EEG Sensor Level Analysis
3.2.3. Exploratory EEG Source Analysis
3.2.4. Statistical Analysis
4. Results
4.1. Driving Simulator Task
Indicator RTs
4.2. EEG
4.2.1. Sensor-Level TFRs
4.2.2. Single-Task vs. Sequential-Task Conditions
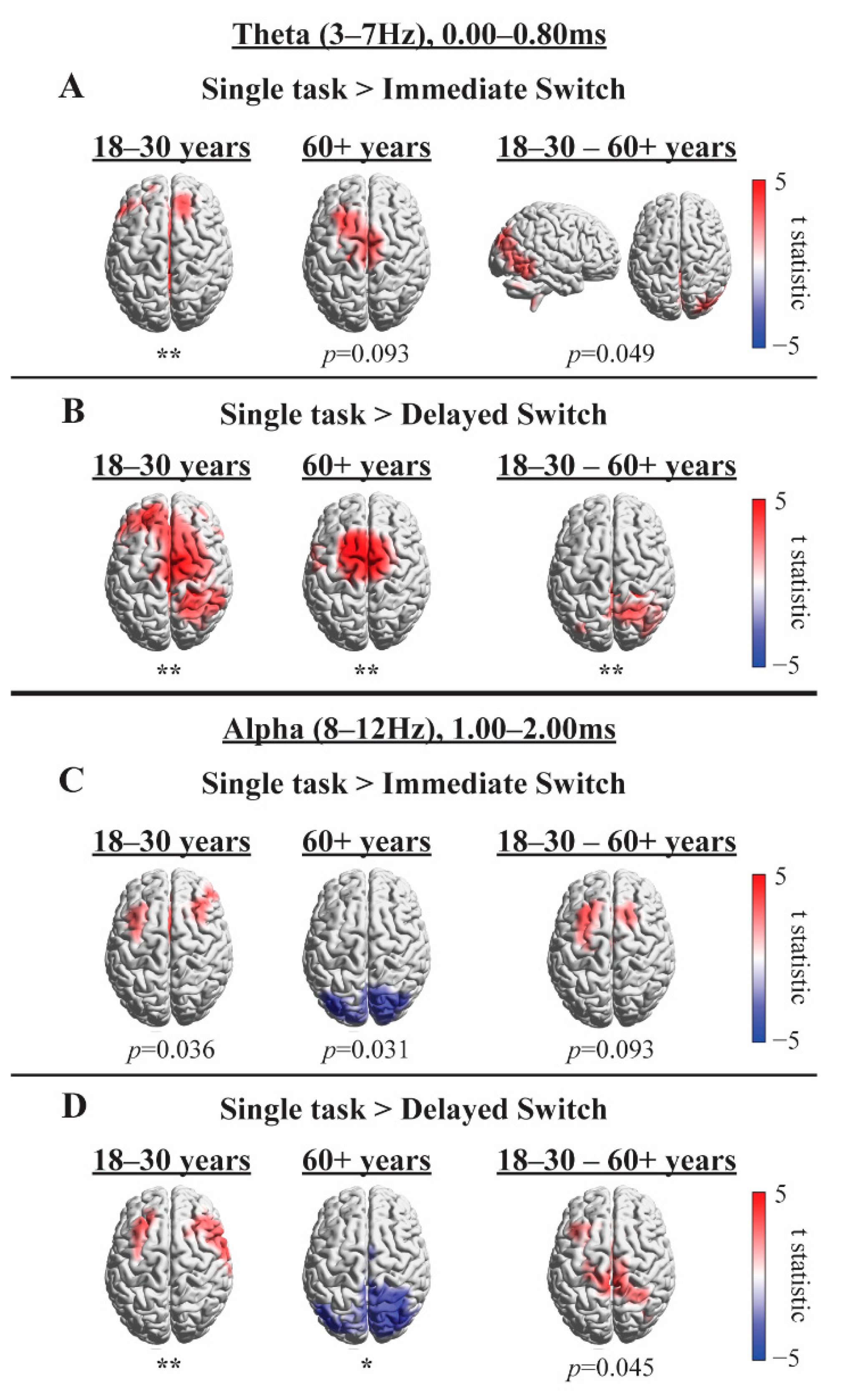
4.2.3. Exploratory Source Analysis
5. Discussion
5.1. Response Times
5.2. EEG
5.3. Limitations and Future Work
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arai, A.; Arai, Y. Self-assessed driving behaviors associated with age among middle-aged and older adults in Japan. Arch. Gerontol. Geriatr. 2015, 60, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Brake, J.; Edwards, S.; Blythe, P.; Fairchild, R. The application of in-vehicle systems for elderly drivers. Eur. Transp. Res. Rev. 2010, 2, 165–174. [Google Scholar] [CrossRef]
- Hakamies-Blomqvist, L.E. Fatal accidents of older drivers. Accid. Anal. Prev. 1993, 25, 19–27. [Google Scholar] [CrossRef]
- McGwin, J.G.; Brown, D.B. Characteristics of traffic crashes among young, middle-aged, and older drivers. Accid. Anal. Prev. 1999, 31, 181–198. [Google Scholar] [CrossRef]
- Musselwhite, C.B.A.; Haddad, H. Exploring older drivers’ perceptions of driving. Eur. J. Ageing 2010, 7, 181–188. [Google Scholar] [CrossRef]
- Parasuraman, R.; Nestor, P.G. Attention and Driving Skills in Aging and Alzheimer’s Disease. J. Hu. Factors Ergon. Soc. 1991, 33, 539–557. [Google Scholar] [CrossRef]
- Callaghan, E.; Holland, C.; Kessler, K. Age-related changes in the ability to switch between temporal and spatial attention. Front. Aging Neurosci. 2017, 9, 28. [Google Scholar] [CrossRef]
- Bennett, I.J.; Motes, M.A.; Rao, N.K.; Rypma, B. White matter tract integrity predicts visual search performance in young and older adults. Neurobiol. Aging 2012, 33. [Google Scholar] [CrossRef]
- Foster, J.K.; Behrmann, M.; Stuss, D.T. Aging and visual search: Generalized cognitive slowing or selective deficit in attention? Aging Cogn. 1995, 2, 279–299. [Google Scholar] [CrossRef]
- Humphrey, D.G.; Kramer, A.F. Age differences in visual search for feature, conjunction, and triple-conjunction targets. Psychol. Aging 1997, 12, 704–717. [Google Scholar] [CrossRef]
- Nagamatsu, L.S.; Munkacsy, M.; Liu-Ambrose, T.; Handy, T.C. Altered visual-spatial attention to task-irrelevant information is associated with falls risk in older adults. Neuropsychologia 2013, 51, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Plude, D.J.; Doussard-Roosevelt, J.A. Aging, selective attention and feature integration. Psychol. Aging 1989, 4, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Hsieh, S. The Limits of Attention for Visual Perception and Action in Aging. Aging Neuropsychol. Cogn. 2009, 16, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Lahar, C.J.; Isaak, M.I.; McArthur, A.D. Age Differences in the Magnitude of the Attentional Blink. Aging Neuropsychol. Cogn. 2001, 8, 149–159. [Google Scholar] [CrossRef]
- Maciokas, J.B.; Crognale, M.A. Cognitive and attentional changes with age: Evidence from attentional blink deficits. Exp. Aging Res. 2003, 29, 137–153. [Google Scholar] [CrossRef]
- Choi, H.; Kasko, J.; Feng, J. An Attention Assessment for Informing Older Drivers’ Crash Risks in Various Hazardous Situations. Gerontologist 2019, 59, 112–123. [Google Scholar] [CrossRef]
- Wasylyshyn, C.; Verhaeghen, P.; Sliwinski, M.J. Aging and task switching: A meta-analysis. Psychol. Aging 2011, 26, 15–20. [Google Scholar] [CrossRef]
- Torrens-Burton, A.; Hanley, C.J.; Wood, R.; Basoudan, N.; Norris, J.E.; Richards, E.; Tales, A. Lacking Pace but Not Precision: Age-Related Information Processing Changes in Response to a Dynamic Attentional Control Task. Brain Sci. 2020, 10, 390. [Google Scholar] [CrossRef]
- Salthouse, T.A. Speed of behavior and its implications for cognition. In Handbook of the Psychology of Aging, 2nd ed.; Van Nostrand Reinhold Co.: New York, NY, USA, 1985; pp. 400–426. [Google Scholar]
- Birren, J.E. Translations in gerontology: From lab to life: Psychophysiology and speed of response. Am. Psychol. 1974, 29, 808–815. [Google Scholar] [CrossRef]
- Campagne, A.; Pebayle, T.; Muzet, A. Correlation between driving errors and vigilance level: Influence of the driver’s age. Physiol. Behav. 2004, 80, 515–524. [Google Scholar] [CrossRef]
- Lowden, A.; Anund, A.; Kecklund, G.; Peters, B.; Akerstedt, T. Wakefulness in young and elderly subjects driving at night in a car simulator. Accid. Anal. Prev. 2009, 41, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Ross, V.; Vossen, A.Y.; Smulders, F.T.Y.; Ruiter, R.A.C.; Brijs, T.; Brijs, K.; Wets, G.; Jongen, E.M.M. Measuring working memory load effects on electrophysiological markers of attention orienting during a simulated drive. Ergonomics 2018, 61, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Nguyen, T.; Jang, H.; Kim, J.G.; Jun, S.C. Exploring Neuro-Physiological Correlates of Drivers’ Mental Fatigue Caused by Sleep Deprivation Using Simultaneous EEG, ECG, and fNIRS Data. Front. Human Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Jung, T.P. Monitoring alert and drowsy states by modeling EEG source nonstationarity. J. Neural Eng. 2017, 14. [Google Scholar] [CrossRef]
- Perrier, J.; Jongen, S.; Vuurman, E.; Bocca, M.L.; Ramaekers, J.G.; Vermeeren, A. Driving performance and EEG fluctuations during on-the-road driving following sleep deprivation. Biol. Psychol. 2016, 121, 1–11. [Google Scholar] [CrossRef]
- Huang, R.S.; Jung, T.P.; Makeig, S. Tonic Changes in EEG Power Spectra during Simulated Driving. In International Conference on Foundations of Augmented Cognition; Springer: Berlin/Heidelberg, Germany, 2009; pp. 394–403. [Google Scholar]
- Vossen, A.Y.; Ross, V.; Jongen, E.M.M.; Ruiter, R.A.C.; Smulders, F.T.Y. Effect of working memory load on electrophysiological markers of visuospatial orienting in a spatial cueing task simulating a traffic situation. Psychophysiology 2016, 53, 237–251. [Google Scholar] [CrossRef]
- Lin, C.T.; Chen, S.A.; Chiu, T.T.; Lin, H.Z.; Ko, L.W. Spatial and temporal EEG dynamics of dual-task driving performance. J. Neuroeng. Rehabil. 2011, 8. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Yamagishi, N.; Callan, D.E.; Goda, N.; Anderson, S.J.; Yoshida, Y.; Kawato, M. Attentional modulation of oscillatory activity in human visual cortex. Neuroimage 2003, 20, 98–113. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Aslan, A.; Staudigl, T.; Klimesch, W.; Herrmann, C.S.; Bäuml, K.-H. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage 2007, 37, 1465–1473. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Klimesch, W.; Sauseng, P.; Gruber, W.; Doppelmayr, M.; Freunberger, R.; Pecherstorfer, T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005, 375, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Stadler, W.; Schabus, M.; Doppelmayr, M.; Hanslmayr, S.; Gruber, W.R.; Birbaumer, N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur. J. Neurosci. 2005, 22, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Thut, G.; Nietzel, A.; Brandt, S.A.; Pascual-Leone, A. alpha-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 2006, 26, 9494–9502. [Google Scholar] [CrossRef] [PubMed]
- Capotosto, P.; Babiloni, C.; Romani, G.L.; Corbetta, M. Frontoparietal Cortex Controls Spatial Attention through Modulation of Anticipatory Alpha Rhythms. J. Neurosci. 2009, 29, 5863–5872. [Google Scholar] [CrossRef]
- Min, B.-K.; Park, H.-J. Task-related modulation of anterior theta and posterior alpha EEG reflects top-down preparation. BMC Neurosci. 2010, 11, 1–8. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Cohen, M.X.; Allen, J.J.B. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamica during action monitoring. J. Neurosci. 2009, 29, 98–105. [Google Scholar] [CrossRef]
- Demiralp, T.; Başar, E. Theta rhythmicities following expected visual and auditory targets. Int. J. Psychophysiol. 1992, 13, 147–160. [Google Scholar] [CrossRef]
- Green, J.J.; Conder, J.A.; McDonald, J.J. Lateralized frontal activity elicited by attention-directing visual and auditory cues. Psychophysiology 2008, 45, 579–587. [Google Scholar] [CrossRef]
- Wiesman, A.I.; Wilson, T.W. The impact of age and sex on the oscillatory dynamics of visuospatial processing. Neuroimage 2019, 185, 513–520. [Google Scholar] [CrossRef]
- Cummins, T.D.R.; Finnigan, S. Theta power is reduced in healthy cognitive aging. Int. J. Psychophysiol. 2007, 66, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, S.; O′Connell, R.G.; Cummins, T.D.R.; Broughton, M.; Robertson, I.H. ERP measures indicate both attention and working memory encoding decrements in aging. Psychophysiology 2011, 48, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.L.; Kober, S.E.; Witte, M.; Neuper, C.; Wood, G. Age-related effects on verbal and visuospatial memory are mediated by theta and alpha II rhythms. Int. J. Psychophysiol. 2016, 99, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Van de Vijver, I.; Cohen, M.X.; Ridderinkhof, K.R. Aging affects medial but not anterior frontal learning-related theta oscillations. Neurobiol. Aging 2014, 35, 692–704. [Google Scholar] [CrossRef]
- Li, L.; Zhao, D.D. Age-Related Inter-Region EEG Coupling Changes During the Control of Bottom-Up and Top-Down Attention. Front. Aging Neurosci. 2015, 7. [Google Scholar] [CrossRef]
- Fu, S.M.; Greenwood, P.M.; Parasuraman, R. Brain mechanisms of involuntary visuospatial attention: An event-related potential study. Human Brain Mapp. 2005, 25, 378–390. [Google Scholar] [CrossRef]
- Gross, J.; Schmitz, F.; Schnitzler, I.; Kessler, K.; Shapiro, K.; Hommel, B.; Schnitzler, A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 13050–13055. [Google Scholar] [CrossRef]
- Coull, J.T.; Nobre, A.C. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 1998, 18, 7426–7435. [Google Scholar] [CrossRef]
- Madden, D.J.; Spaniol, J.; Whiting, W.L.; Bucur, B.; Provenzale, J.M.; Cabeza, R.; White, L.E.; Huettel, S.A. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiol. Aging 2007, 28, 459–476. [Google Scholar] [CrossRef]
- Shapiro, K.; Hillstrom, A.P.; Husain, M. Control of visuotemporal attention by inferior parietal and superior temporal cortex. Curr. Biol. 2002, 12, 1320–1325. [Google Scholar] [CrossRef]
- Fabiani, M.; Low, K.A.; Wee, E.; Sable, J.J.; Gratton, G. Reduced Suppression or Labile Memory? Mechanisms of Inefficient Filtering of Irrelevant Information in Older Adults. J. Cogn. Neurosci. 2006, 18, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Dennis, N.A.; Daselaar, S.M.; Fleck, M.S.; Cabeza, R. Qué PASA? The Posterior–Anterior Shift in Aging. Cereb. Cortex 2008, 18, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Noone, P. Addenbrooke’s Cognitive Examination-III. Occup. Med. 2015, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wu, T.J. Fatigued driver’s driving behavior and cognitive task performance: Effects of road environments and road environment changes. Saf. Sci. 2009, 47, 1083–1089. [Google Scholar] [CrossRef]
- Jaeger, T.F.; Burchill, Z.; Bushong, W. Strong Evidence for Expectation Adaptation during Language Understanding, not a Replication Failure. A Reply to Harrington Stack, James, and Watson (2018). Available online: https://osf.io/4vxyp/ (accessed on 15 February 2019).
- Hale, S.; Myerson, J.; Smith, G.A.; Poon, L.W. Age, variability, and speed: Between-subjects diversity. Psychol. Aging 1988, 3, 407. [Google Scholar] [CrossRef]
- Morse, C.K. Does variability increase with age? An archival study of cognitive measures. Psychol. Aging 1993, 8, 156. [Google Scholar] [CrossRef]
- Budescu, D.V. The power of the F test in normal populations with heterogeneous variances. Educ. Psychol. Meas. 1982, 42, 409–416. [Google Scholar] [CrossRef]
- Budescu, D.V.; Appelbaum, M.I. Variance stabilizing transformations and the power of the F test. J. Educ. Behav. Stat. 1981, 6, 55–74. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W. Statistical Methods; Iowa State University: Ames, IA, USA, 1967; p. 593. [Google Scholar]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 9. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: Exact, zero error localization. arXiv 2007, arXiv:0710.3341. [Google Scholar]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef]
- Aoki, Y.; Ishii, R.; Pascual-Marqui, R.D.; Canuet, L.; Ikeda, S.; Hata, M.; Imajo, K.; Matsuzaki, H.; Musha, T.; Asada, T.; et al. Detection of EEG-resting state independent networks by eLORETA-ICA method. Front. Human Neurosci. 2015, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG-and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Karniski, W.; Blair, R.C.; Snider, A.D. An exact statistical method for comparing topographic maps, with any number of subjects and electrodes. Brain Topogr. 1994, 6, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bögels, S.; Barr, D.J.; Garrod, S.; Kessler, K. Conversational Interaction in the Scanner: Mentalizing during Language Processing as Revealed by MEG. Cereb. Cortex 2014. [Google Scholar] [CrossRef]
- Wang, H.; Callaghan, E.; Gooding-Williams, G.; McAllister, C.; Kessler, K. Rhythm makes the world go round: An MEG-TMS study on the role of right TPJ theta oscillations in embodied perspective taking. Cortex 2016, 75, 68–81. [Google Scholar] [CrossRef]
- Fieldtrip. How Can I Test An Interaction Effect Using Cluster-Based Permutation Tests? Available online: http://www.fieldtriptoolbox.org/faq/how_can_i_test_an_interaction_effect_using_cluster-based_permutation_tests (accessed on 8 December 2019).
- Gola, M.; Kaminski, J.; Brzezicka, A.; Wrobel, A. Beta band oscillations as a correlate of alertness—Changes in aging. Int. J. Psychophysiol. 2012, 85, 62–67. [Google Scholar] [CrossRef]
- Gola, M.; Magnuski, M.; Szumska, I.; Wrobel, A. EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly subjects. Int. J. Psychophysiol. 2013, 89, 334–341. [Google Scholar] [CrossRef]
- Donoghue, J.P.; Sanes, J.N.; Hatsopoulos, N.G.; Gaál, G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J. Neurophysiol. 1998, 79, 159–173. [Google Scholar] [CrossRef]
- Farmer, S.F. Rhythmicity, synchronization and binding in human and primate motor systems. J. Physiol. 1998, 509, 3–14. [Google Scholar] [CrossRef]
- Gross, J.; Schmitz, F.; Schnitzler, I.; Kessler, K.; Shapiro, K.; Hommel, B.; Schnitzler, A. Anticipatory control of long-range phase synchronization. Eur. J. Neurosci. 2006, 24, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Williams, D.; Kupsch, A.; Limousin, P.; Hariz, M.; Schneider, G.H.; Yarrow, K.; Brown, P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain 2004, 127, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.C.; Miniussi, C.; Nobre, A.C. Orienting attention in time. Front. Biosci. 2001, 6, 660–671. [Google Scholar] [CrossRef]
- Woodrow, H. The measurement of attention. Psychol. Monogr. 1914, 17, 1–158. [Google Scholar] [CrossRef]
- Correa, A.; Nobre, A.C. Spatial and temporal acuity of visual perception can be enhanced selectively by attentional set. Exp. Brain Res. 2008, 189, 339–344. [Google Scholar] [CrossRef]
- Posner, M.I.; Cohen, Y. Components of visual orienting. Atten. Perform. X Control Lang. Process. 1984, 32, 531–556. [Google Scholar]
- Posner, M.I.; Rafal, R.D.; Choate, L.S.; Vaughan, J. Inhibition of return: Neural basis and function. Cogn. Neuropsychol. 1985, 2, 211–228. [Google Scholar] [CrossRef]
- Neider, M.B.; Kramer, A.F. Older adults capitalize on contextual information to guide search. Exp. Aging Res. 2011, 37, 539–571. [Google Scholar] [CrossRef]
- McLaughlin, P.M.; Murtha, S.J.E. The Effects of Age and Exogenous Support on Visual Search Performance. Exp. Aging Res. 2010, 36, 325–345. [Google Scholar] [CrossRef]
- Watson, D.G.; Maylor, E.A. Aging and visual marking: Selective deficits for moving stimuli. Psychol. Aging 2002, 17, 321–339. [Google Scholar] [CrossRef]
- Gamboz, N.; Zamarian, S.; Cavallero, C. Age-Related Differences in the Attention Network Test (ANT). Exp. Aging Res. 2010, 36, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Deiber, M.-P.; Ibanez, V.; Missonnier, P.; Rodriguez, C.; Giannakopoulos, P. Age-associated modulations of cerebral oscillatory patterns related to attention control. Neuroimage 2013, 82, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Zanto, T.P.; Pan, P.; Liu, H.; Bollinger, J.; Nobre, A.C.; Gazzaley, A. Age-Related Changes in Orienting Attention in Time. J. Neurosci. 2011, 31, 12461–12470. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Ferdinand, N.K.; Kray, J.; Falkenstein, M. Understanding sources of adult age differences in task switching: Evidence from behavioral and ERP studies. Neurosci. Biobehav. Rev. 2018, 92, 255–275. [Google Scholar] [CrossRef]
- Callaghan, E.; Holland, C.; Kessler, K. Flexible allocation of attention in time or space across the life span: Theta and alpha oscillatory signatures of age-related decline and compensation as revealed by MEG. Biorxiv 2018, 461020. [Google Scholar] [CrossRef]
- Andrews, E.C.; Westerman, S.J. Age differences in simulated driving performance: Compensatory processes. Accid. Anal. Prev. 2012, 45, 660–668. [Google Scholar] [CrossRef]
- Jefferies, L.; Roggeveen, A.; Enns, J.; Bennett, P.; Sekuler, A.; Di Lollo, V. On the time course of attentional focusing in older adults. Psychol. Res. 2015, 79, 28–41. [Google Scholar] [CrossRef]
- Summala, H.; Lamble, D.; Laakso, M. Driving experience and perception of the lead car’s braking when looking at in-car targets. Accid. Anal. Prev. 1998, 30, 401–407. [Google Scholar] [CrossRef]
- Bao, S.; Boyle, L.N. Age-related differences in visual scanning at median-divided highway intersections in rural areas. Accid. Anal. Prev. 2009, 41, 146–152. [Google Scholar] [CrossRef]
- Lopez, M.E.; Aurtenetxe, S.; Pereda, E.; Cuesta, P.; Castellanos, N.P.; Bruna, R.; Niso, G.; Maestu, F.; Bajo, R. Cognitive reserve is associated with the functional organization of the brain in healthy aging: A MEG study. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef]
- Anstey, K.; Christensen, H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology 2000, 46, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Fratiglioni, L.; Paillard-Borg, S.; Winblad, B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004, 3, 343–353. [Google Scholar] [CrossRef]
- Roldán-Tapia, L.; García, J.; Cánovas, R.; León, I. Cognitive Reserve, Age, and Their Relation to Attentional and Executive Functions. Appl. Neuropsychol.: Adult 2012, 19, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Marottoli, R.A.; Leon, C.F.M.; Glass, T.A.; Williams, C.S.; Cooney, L.M.; Berkman, L.F.; Tinetti, M.E. Driving cessation and increased depressive symptoms: Prospective evidence from the New Haven EPESE. J. Am. Geriatr. Soc. 1997, 45, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Ragland, D.R.; Satariano, W.A.; MacLeod, K.E. Driving cessation and increased depressive symptoms. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Windsor, T.D.; Anstey, K.J.; Butterworth, P.; Luszcz, M.A.; Andrews, G.R. The role of perceived control in explaining depressive symptoms associated with driving cessation in a longitudinal study. Gerontologist 2007, 47, 215–223. [Google Scholar] [CrossRef]
- Paulo, A.C.; Sampaio, A.; Santos, N.C.; Costa, P.S.; Cunha, P.; Zihl, J.; Cerqueira, J.; Palha, J.A.; Sousa, N. Patterns of Cognitive Performance in Healthy Ageing in Northern Portugal: A Cross-Sectional Analysis. PLoS ONE 2011, 6, e24553. [Google Scholar] [CrossRef]
- De Felice, S.; Holland, C.A. Intra-Individual Variability Across Fluid Cognition Can Reveal Qualitatively Different Cognitive Styles of the Aging Brain. Front. Psychol. 2018, 9. [Google Scholar] [CrossRef]
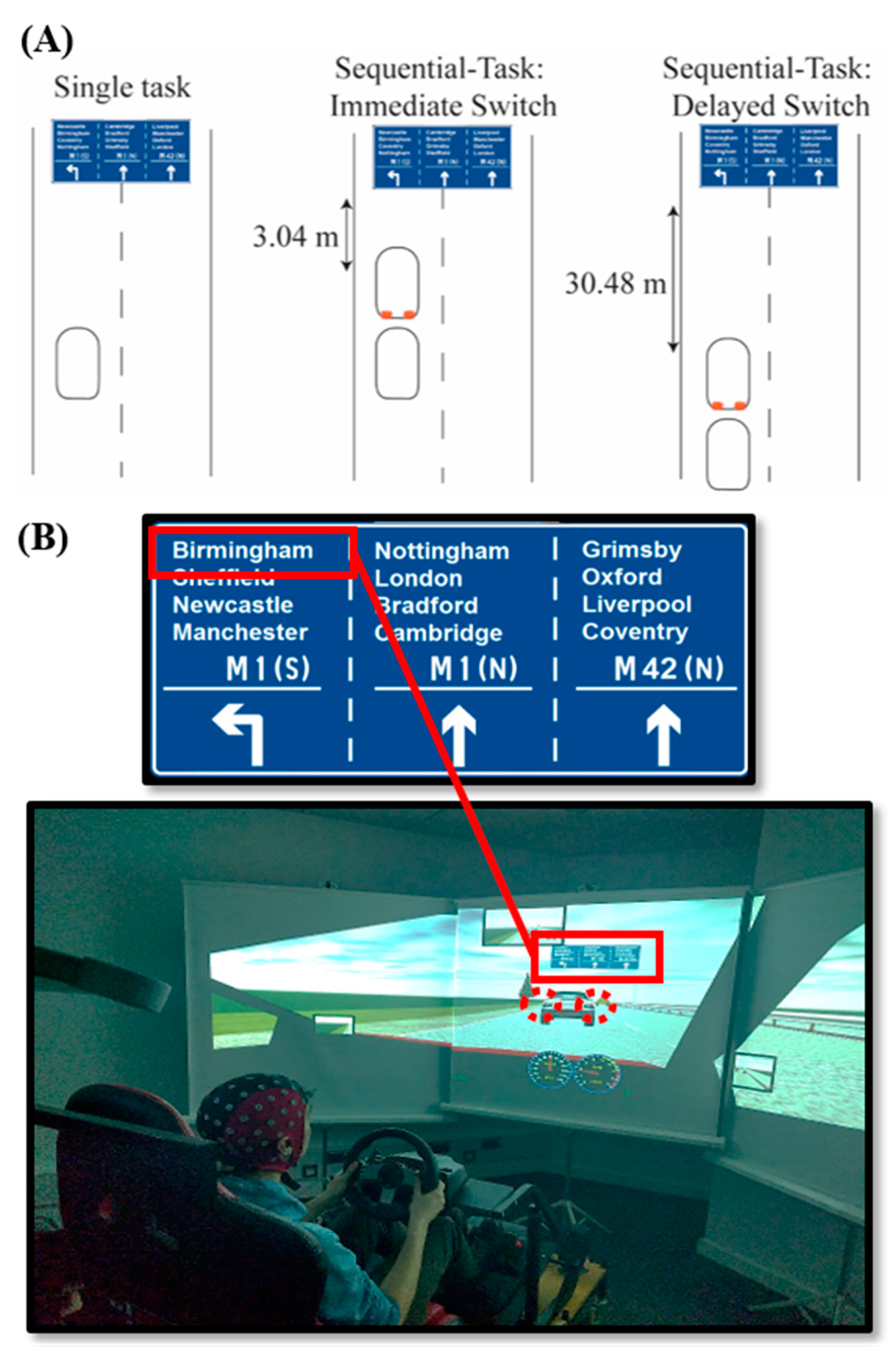
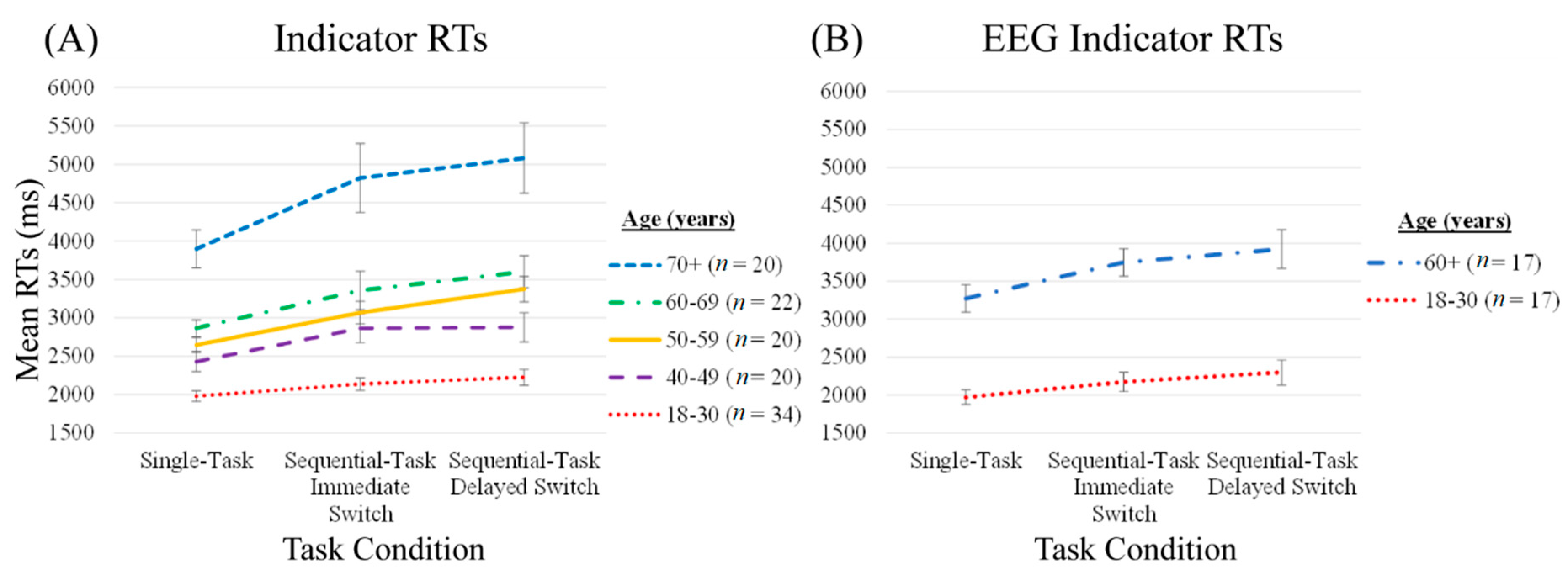
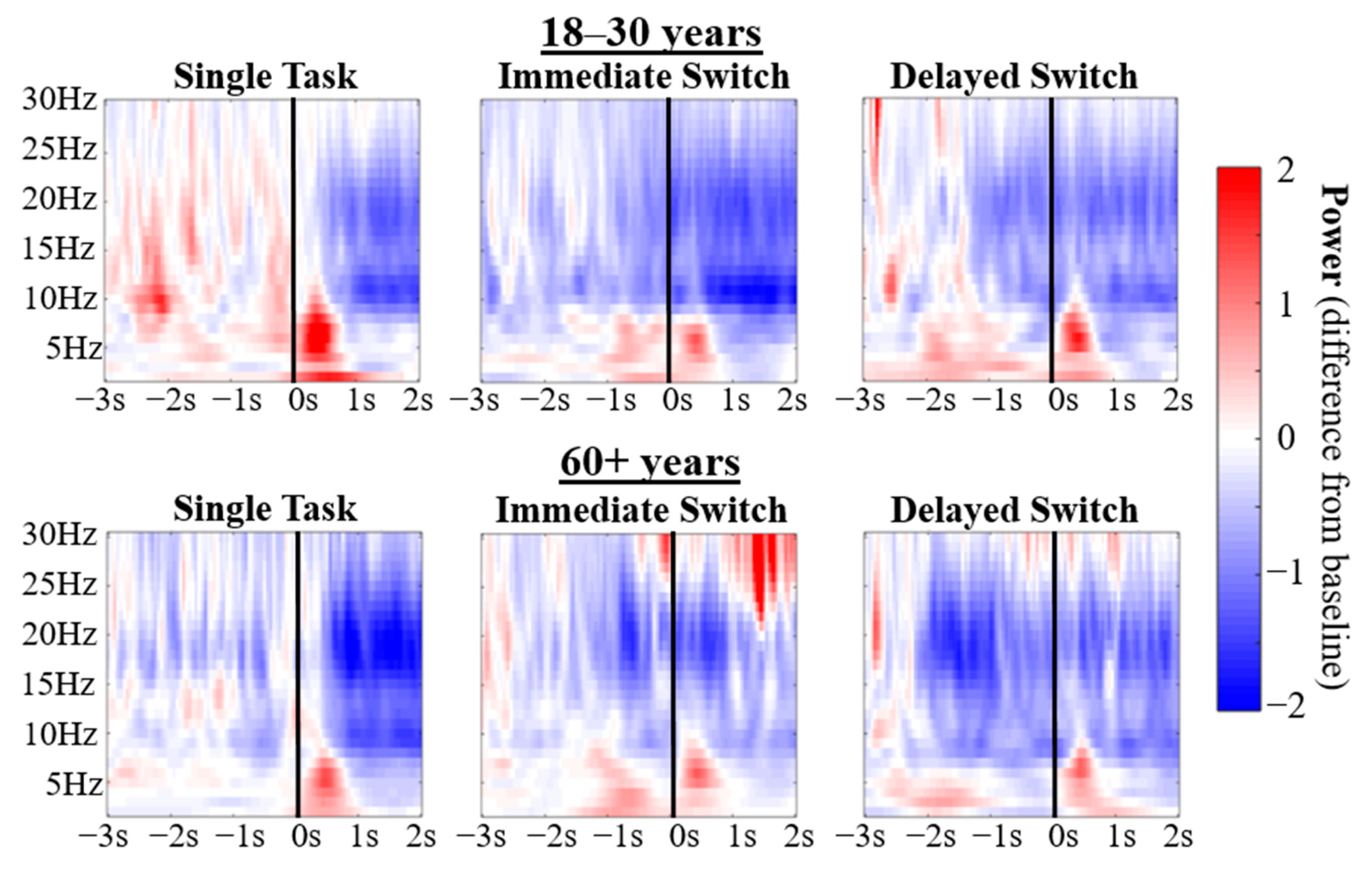
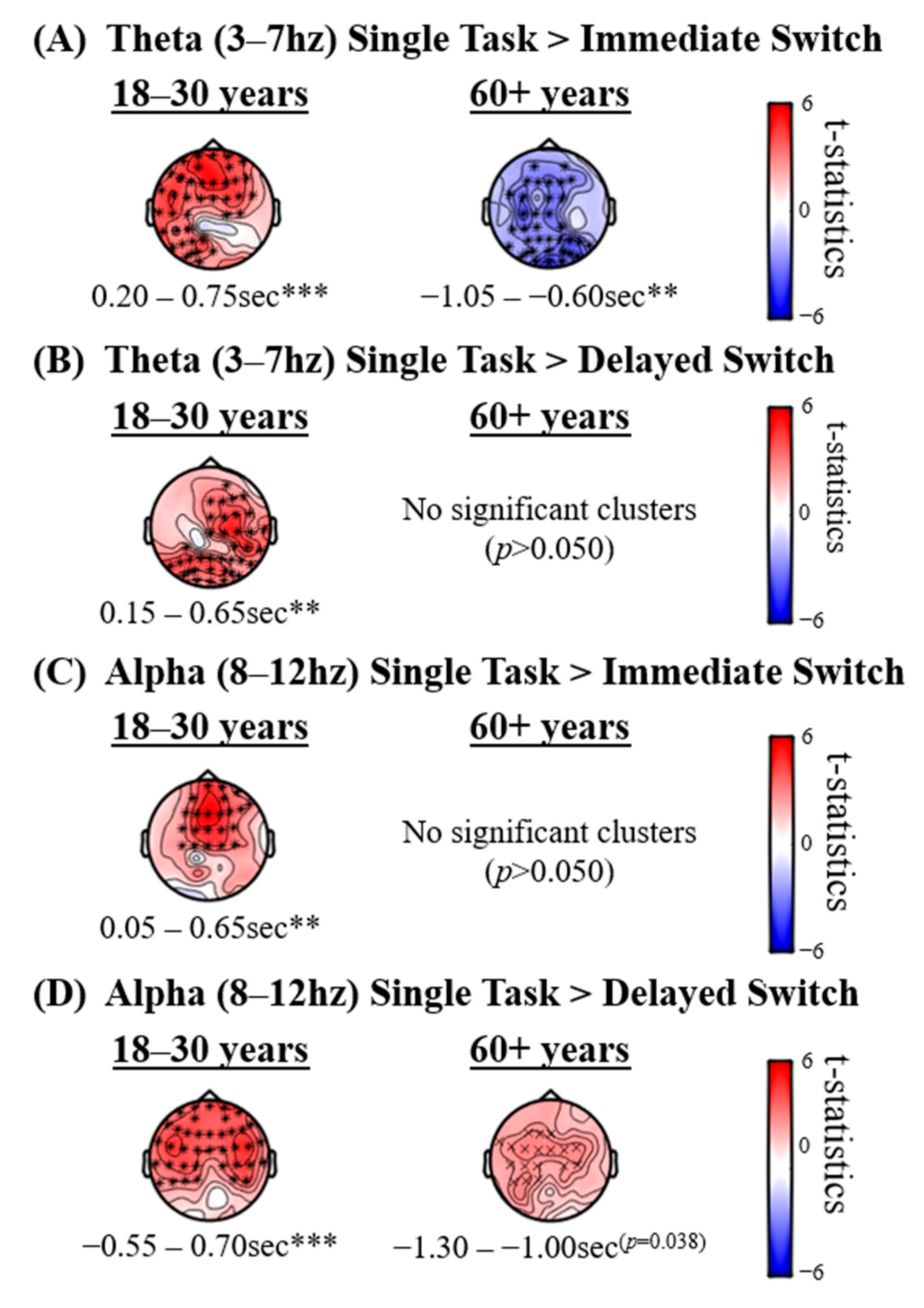
| Age Group (Years) | EEG Group | |||||||
|---|---|---|---|---|---|---|---|---|
| 18–30 (n = 34) | 40–49 (n = 20) | 50–59 (n = 20) | 60–69 (n = 22) | 70–91 (n = 20) | 18–30 (n = 17) | 60+ (n = 17) | ||
| Age (years) | Mean | 21.21 | 43.65 | 54.75 | 64.77 | 75.35 | 22.88 | 70.12 |
| SD | 3.36 | 2.93 | 2.40 | 2.86 | 4.40 | 4.04 | 5.20 | |
| Sex | Male | 10 | 8 | 6 | 14 | 9 | 4 | 10 |
| Female | 24 | 12 | 14 | 8 | 11 | 13 | 7 | |
| Handedness | Right | 30 | 17 | 18 | 20 | 20 | 15 | 16 |
| Left | 4 | 2 | 2 | 2 | 0 | 2 | 1 | |
| ACE-3 | Mean | N/A | N/A | 96.60 | 96.18 | 95.21 | N/A | 95.29 |
| SD | N/A | N/A | 2.50 | 2.44 | 2.49 | N/A | 2.64 | |
| Age Group (Years) | EEG subgroup | |||||||
|---|---|---|---|---|---|---|---|---|
| 18–30 (n = 34) | 40–49 (n = 20) | 50–59 (n = 20) | 60–69 (n = 22) | 70–91 (n = 20) | 18–30 (n = 17) | 60+ (n = 17) | ||
| Immediate S-T Costs | Mean | 8.62 | 17.68 | 15.21 | 18.71 | 22.76 | 10.38 | 16.98 |
| SD | 16.50 | 21.34 | 11.56 | 29.34 | 24.35 | 15.55 | 15.89 | |
| Delayed S-T Costs | Mean | 12.67 | 18.59 | 26.81 | 27.58 | 30.50 | 15.97 | 21.45 |
| SD | 20.30 | 20.31 | 14.31 | 24.28 | 32.37 | 23.72 | 21.17 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huizeling, E.; Wang, H.; Holland, C.; Kessler, K. Age-Related Changes in Attentional Refocusing during Simulated Driving. Brain Sci. 2020, 10, 530. https://doi.org/10.3390/brainsci10080530
Huizeling E, Wang H, Holland C, Kessler K. Age-Related Changes in Attentional Refocusing during Simulated Driving. Brain Sciences. 2020; 10(8):530. https://doi.org/10.3390/brainsci10080530
Chicago/Turabian StyleHuizeling, Eleanor, Hongfang Wang, Carol Holland, and Klaus Kessler. 2020. "Age-Related Changes in Attentional Refocusing during Simulated Driving" Brain Sciences 10, no. 8: 530. https://doi.org/10.3390/brainsci10080530
APA StyleHuizeling, E., Wang, H., Holland, C., & Kessler, K. (2020). Age-Related Changes in Attentional Refocusing during Simulated Driving. Brain Sciences, 10(8), 530. https://doi.org/10.3390/brainsci10080530






