Functional Electrical Stimulation Controlled by Motor Imagery Brain-Computer Interface for Rehabilitation
Abstract
1. Introduction
2. Background
2.1. FES Rehabilitation for Stroke and TBI Patients
2.2. SMR-Based BCI-Controlled FES Systems
2.3. Limitations of Current SMR-Based BCIs for FES
3. Phase 1: Development and Evaluation of an SMR-Based BCI
3.1. Objectives and Hypotheses
3.2. Methods
3.2.1. Participants
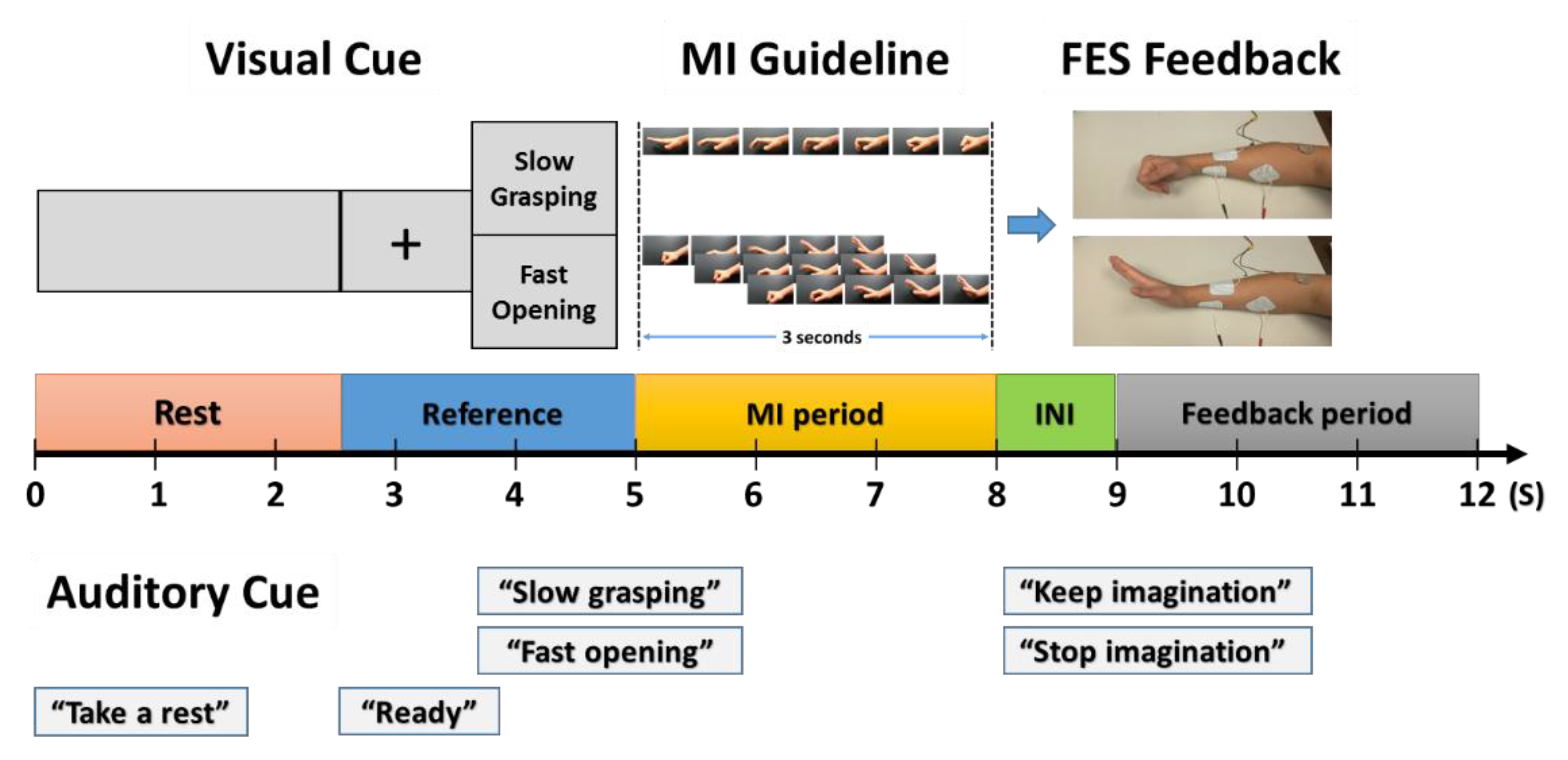
3.2.2. Visually Guided Instructions for MI Tasks
3.2.3. Procedure
3.2.4. Signal Acquisition and Processing
3.2.5. Independent and Dependent Variables
3.3. Results: Accuracy (%)
3.4. Discussion
4. Phase 2: Feasibility of the Proposed BCI-FES System
4.1. Objectives and Hypotheses
4.2. Methods
4.2.1. Participants
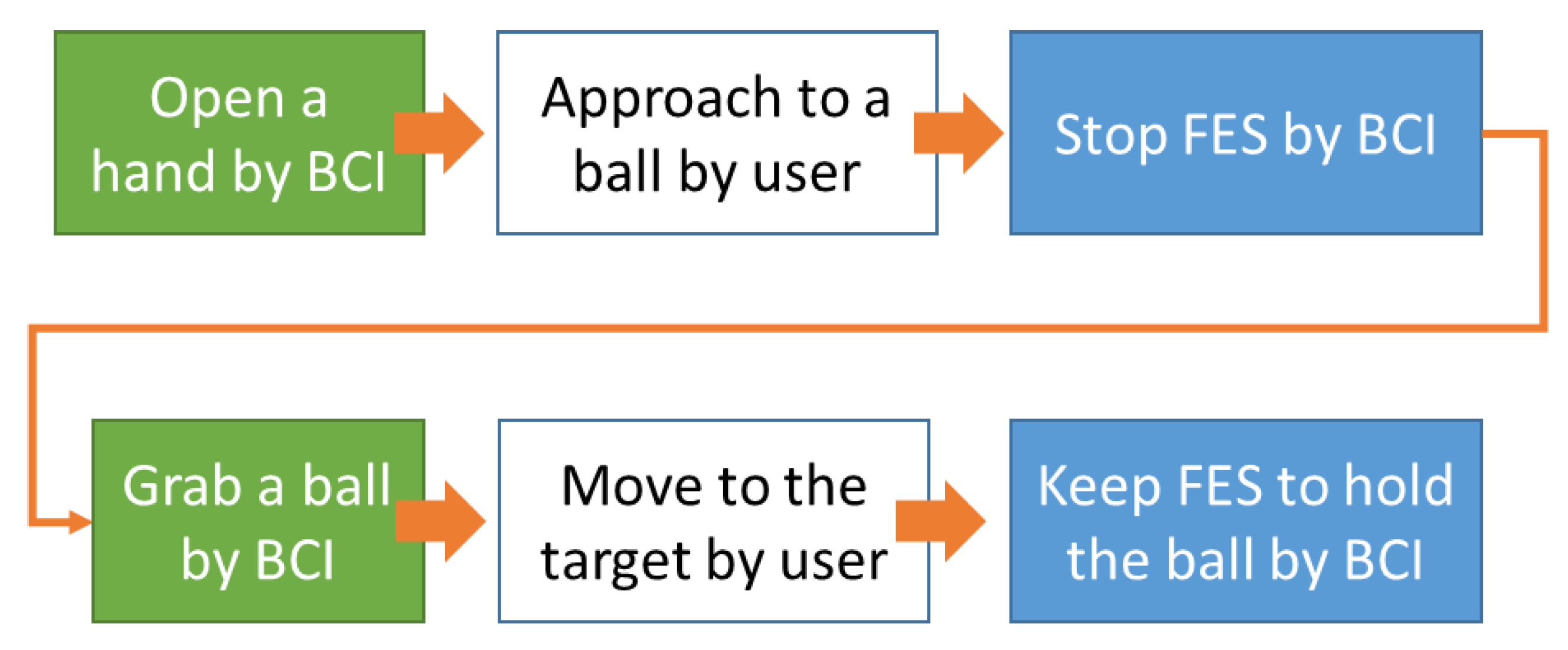
4.2.2. Experimental Task and Modes
4.2.3. Procedure
4.3. Signal Acquisition and Processing
4.4. Independent and Dependent Variables
4.5. Results
4.5.1. Task Performance
4.5.2. Workload
4.6. Discussion
4.6.1. The Feasibility of the Proposed BCI-FES System
4.6.2. FES Existence Type
4.6.3. Learning Type
4.6.4. Subjective Assessment
5. General Discussion
5.1. SMR-Based BCI Systems for a 2-Class MI Task in a Single Hand
5.2. Semi-Asynchronous Mode
6. Conclusion and Future Research
6.1. Contributions to BCI-FES Research
6.2. Research Implications in Human Factor and Ergonomics (HF/E)
6.3. Research Limitation and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Delden, A.E.Q.; Peper, C.E.; Kwakkel, G.; Beek, P.J. A systematic review of bilateral upper limb training devices for poststroke rehabilitation. Stroke Res. Treat. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Hesse, S.; Oliviero, A.; Paolucci, S. New technologies for stroke rehabilitation. Stroke Res. Stroke Res. Treat. 2013, 2013, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Peckham, P.H.; Knutson, J.S. Functional electrical stimulation for neuromuscular applications. Annu. Rev. Biomed. Eng. 2005, 7, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Sujith, O.K. Functional electrical stimulation in neurological disorders. Eur. J. Neurol. 2008, 15, 437–444. [Google Scholar] [CrossRef]
- Johnson, L.A.; Fuglevand, A.J. Mimicking muscle activity with electrical stimulation. J. Neural Eng. 2011, 8, 16009. [Google Scholar] [CrossRef]
- Popovic, M.R.; Curt, A.; Keller, T.; Dietz, V. Functional electrical stimulation for grasping and walking: Indications and limitations. Spinal Cord 2001, 39, 403–412. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Daly, J.J.; Wolpaw, J.R. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Müller, G.R.; Pfurtscheller, J.; Gerner, H.J.; Rupp, R. “Thought”—Control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci. Lett. 2003, 351, 33–36. [Google Scholar] [CrossRef]
- McGie, S.C.; Zariffa, J.J.; Popovic, M.R.; Nagai, M.K. Short-term neuroplastic effects of brain-controlled and muscle-controlled electrical stimulation. Neuromodulation 2015, 18, 233–240. [Google Scholar] [CrossRef]
- Rohm, M.; Muller-Putz, G.R.; Kreilinger, A.; Von Ascheberg, A.; Rupp, R. A hybrid-Brain Computer Interface for control of a reaching and grasping neuroprosthesis. Biomed. Tech. 2010, 55 (Suppl. S1), 1–4. [Google Scholar]
- Hara, Y. Neurorehabilitation with new functional electrical stimulation for hemiparetic upper extremity in stroke patients. J. Nippon. Med Sch. 2008, 75, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Despres, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 2015, 133. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, D.; Thurman, D.; Gwinn-Hardy, K.; Mohamed, M.; Chaudhuri, A.; Zalutsky, R. How common are the “common” neurologic disorders? Neurology 2007, 68, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Teasell, R.; Graham, R.; Salter, K. Rehabilitation of Severe Stroke. Evid. Based Rev. Stroke Rehabil. Module 2013, 23, 1–32. [Google Scholar]
- Miller, E.L.; Murray, L.; Richards, L.; Zorowitz, R.D.; Bakas, T.; Clark, P.; Billinger, S.A. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the American heart association. Stroke 2010, 41, 2402–2448. [Google Scholar] [CrossRef]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Duncan, P.W.; Zorowitz, R.D.; Bates, B.; Choi, J.Y.; Glasberg, J.J.; Graham, G.D.; Katz, R.C.; Lamberty, K.; Reker, D. Management of Adult Stroke Rehabilitation Care. Stroke 2005, 36, e100–e143. [Google Scholar] [CrossRef]
- Matsumoto, S.; Shimodozono, M.; Etoh, S.; Shimozono, Y.; Tanaka, N.; Kawahira, K. Beneficial effects of footbaths in controlling spasticity after stroke. Int. J. Biometeorol. 2010, 54, 465–473. [Google Scholar] [CrossRef]
- Kawashima, N.; Popovic, M.R.; Zivanovic, V. Effect of Intensive Functional Electrical Stimulation Therapy on Upper-Limb Motor Recovery after Stroke: Case Study of a Patient with Chronic Stroke. Physiother. Can. 2013, 65, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Quandt, F.; Hummel, F.C. The influence of functional electrical stimulation on hand motor recovery in stroke patients: A review. Exp. Transl. Stroke Med. 2014, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.L.; Popovic, M.R. Functional electrical stimulation. IEEE Control Syst. 2008, 28, 40–50. [Google Scholar]
- Levin, M.F.; Hui-Chan, C.W. Relief of hemiparetic spasticity by TENS is associated with improvement in reflex and voluntary motor functions. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1992, 85, 131–142. [Google Scholar] [CrossRef]
- Sabut, S.K.; Sikdar, C.; Kumar, R.; Mahadevappa, M. Functional electrical stimulation of dorsiflexor muscle: Effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. Neurorehabilitation 2011, 29, 393–400. [Google Scholar] [CrossRef]
- Zhang, D.; Guan, T.H.; Widjaja, F.; Ang, W.T. Functional electrical stimulation in rehabilitation engineering: A Survey. In i-CREATe ’07: Proceedings of the 1st International Convention on Rehabilitation Engineering & Assistive Technology in Conjunction with 1st Tan Tock Seng Hospital Neurorehabilitation Meeting; ACM Press: New York, NY, USA, 2007; pp. 221–226. [Google Scholar]
- Kottink, A.I.R.; Oostendorp, L.J.; Buurke, J.H.; Nene, A.V.; Hermens, H.J.; Ijzerman, M.J. The Orthotic Effect of Functional Electrical Stimulation on the Improvement of Walking in Stroke Patients with a Dropped Foot: A Systematic Review. Artif. Organs 2004, 28, 577–586. [Google Scholar] [CrossRef]
- Jackson, A.; Mavoori, J.; Fetz, E.E. Long-term motor cortex plasticity induced by an electronic neural implant. Nature 2006, 444, 56–60. [Google Scholar] [CrossRef]
- Teasell, R.; Bayona, N.A.; Bitensky, J. Plasticity and Reorganization of the Brain Post Stroke. Top. Stroke Rehabil. 2005, 12, 11–26. [Google Scholar] [CrossRef]
- Papachristos, A. Functional Electrical Stimulation in Paraplegia. In Topics in Paraplegia; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Young, B.M.; Nigogosyan, Z.; Nair, V.A.; Walton, L.M.; Song, J.; Tyler, M.E.; Edwards, D.F.; Caldera, K.; Sattin, J.A.; Williams, J.C.; et al. Case report: Post-stroke interventional BCI rehabilitation in an individual with preexisting sensorineural disability. Front. Neuroeng. 2014, 7, 18. [Google Scholar] [CrossRef]
- Chae, J.; Sheffler, L.R.; Knutson, J.S. Neuromuscular Electrical Stimulation for Motor Restoration in Hemiplegia. Top. Stroke Rehabil. 2008, 15, 412–426. [Google Scholar] [CrossRef]
- Lawrence, M. Transcutaneous Electrode Technology for Neuroprostheses. Ph.D. Thesis, Federal Technical University, Zurich, Switzerland, 2009. [Google Scholar]
- Mangold, S.; Keller, T.; Curt, A.; Dietz, V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord 2004, 43, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Neuroplasticity: Changes in grey matter induced by training. Nature 2004, 427, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Lourenção, M.I.P.; Battistella, L.R.; De Brito, C.M.M.; Tsukimoto, G.R.; Miyazaki, M.H. Effect of biofeedback accompanying occupational therapy and functional electrical stimulation in hemiplegic patients. Int. J. Rehabil. Res. 2008, 31, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.-M.; Van Erp, J.B.F. A tactile P300 brain-computer interface. Front. Mol. Neurosci. 2010, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.N.; Wolpaw, J.R. Clinical Applications of Brain-Computer Interfaces: Current State and Future Prospects. IEEE Rev. Biomed. Eng. 2009, 2, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Käthner, I.; Ruf, C.A.; Pasqualotto, E.; Braun, C.; Birbaumer, N.; Halder, S. A portable auditory P300 brain–computer interface with directional cues. Clin. Neurophysiol. 2013, 124, 327–338. [Google Scholar] [CrossRef]
- Barsi, G.I.; Popović, D.B.; Tarkka, I.M.; Sinkjær, T.; Grey, M.J. Cortical excitability changes following grasping exercise augmented with electrical stimulation. Exp. Brain Res. 2008, 191, 57–66. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Solis-Escalante, T.; Ortner, R.; Linortner, P.; Müller-Putz, G.R. Self-Paced Operation of an SSVEP-Based Orthosis with and Without an Imagery-Based “Brain Switch”: A Feasibility Study Towards a Hybrid BCI. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 409–414. [Google Scholar] [CrossRef]
- Holz, E.M.; Botrel, L.; Kübler, A. Independent home use of Brain Painting improves quality of life of two artists in the locked-in state diagnosed with amyotrophic lateral sclerosis. Brain Comput. Interfaces 2015, 2, 117–134. [Google Scholar] [CrossRef]
- Li, Y.; Nam, C.S. A Collaborative Brain-Computer Interface (BCI) for ALS Patients. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2015, 59, 716–720. [Google Scholar] [CrossRef]
- Nam, C.S.; Lee, J.; Bahn, S.; Li, Y.; Choi, I. Brain-Computer Interface Supported Collaborative Work. In Proceedings of the 5th International Brain-Computer Interface Meeting, Abingdon, UK, 3–7 June 2014. [Google Scholar]
- Nam, C.S.; Moore, M.; Choi, I.; Li, Y. Designing Better, Cost-Effective Brain–Computer Interfaces. Ergon. Des. Q. Hum. Factors Appl. 2015, 23, 13–19. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Khasnobish, A.; Ghosh, P.; Mazumder, A.; Tibarewala, D.N. A Review on Brain Imaging Techniques for BCI Applications. In Medical Imaging: Concepts, Methodologies, Tools and Applications; IGI Global: Hershey, PA, USA, 2015. [Google Scholar]
- Homer, M.L.; Nurmikko, A.V.; Donoghue, J.P.; Hochberg, L.R. Implants and Decoding for Intracortical Brain Computer Interfaces. Annu. Rev. Biomed. Eng. 2013, 15, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Rabbi, A.; Azinfar, L.; Fazel-Rezai, R.; Asadpour, V. A Review of P300, SSVEP, and Hybrid P300 / SSVEP Brain- Computer Interface Systems. Brain Comput. Interfaces Syst. 2013, 2013, 1–8. [Google Scholar]
- Beisteiner, R.; Höllinger, P.; Lindinger, G.; Lang, W.; Berthoz, A. Mental representations of movements. Brain potentials associated with imagination of hand movements. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1995, 96, 183–193. [Google Scholar] [CrossRef]
- Müller, G.R.; Neuper, C.; Rupp, R.; Keinrath, C.; Gerner, H.J.; Pfurtscheller, G. Event-related beta EEG changes during wrist movements induced by functional electrical stimulation of forearm muscles in man. Neurosci. Lett. 2003, 340, 143–147. [Google Scholar] [CrossRef]
- Neuper, C.; Wörtz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar]
- Pfurtscheller, G. Graphical display and statistical evaluation of event-related desynchronization (ERD). Electroencephalogr. Clin. Neurophysiol. 1977, 43, 757–760. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Event-related synchronization (ERS): An electrophysiological correlate of cortical areas at rest. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 62–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Nam, C.S.; Zhou, G.; Jin, J.; Wang, X.; Cichocki, A. Temporally constrained sparse group spatial patterns for motor imagery BCI. IEEE trans. cyber. 2018, 49, 3322–3332. [Google Scholar] [CrossRef]
- Daly, J.J.; Cheng, R.; Rogers, J.; Litinas, K.; Hrovat, K.; Dohring, M. Feasibility of a New Application of Noninvasive Brain Computer Interface (BCI): A Case Study of Training for Recovery of Volitional Motor Control After Stroke. J. Neurol. Phys. Ther. 2009, 33, 203–211. [Google Scholar] [CrossRef]
- Takahashi, M.; Takeda, K.; Otaka, Y.; Osu, R.; Hanakawa, T.; Gouko, M.; Ito, K. Event related desynchronization-modulated functional electrical stimulation system for stroke rehabilitation: A feasibility study. J. Neuroeng. Rehabil. 2012, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Pomeroy, V.M.; Baron, J.C. Motor imagery: A backdoor to the motor system after stroke? Stroke 2006, 37, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.; Mulder, T. Motor imagery and stroke rehabilitation: A critical discussion. J. Rehabil. Med. 2007, 39, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Krusienski, D.J.; Grosse-Wentrup, M.; Galán, F.; Coyle, D.; Miller, K.J.; Forney, E.; Anderson, C.W. Critical issues in state-of-the-art brain–computer interface signal processing. J. Neural Eng. 2011, 8, 25002. [Google Scholar] [CrossRef] [PubMed]
- Lotte, F.; Congedo, M.; Lecuyer, A.; Lamarche, F.; Arnaldi, B. A review of classification algorithms for EEG-based brain–computer interfaces. J. Neural Eng. 2007, 4, R1–R13. [Google Scholar] [CrossRef]
- Vidaurre, C.; Blankertz, B. Towards a Cure for BCI Illiteracy. Brain Topogr. 2009, 23, 194–198. [Google Scholar] [CrossRef]
- Guger, C.; Edlinger, G.; Harkam, W.; Niedermayer, I.; Pfurtscheller, G. How many people are able to operate an eeg-based brain-computer interface (BCI)? IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 145–147. [Google Scholar] [CrossRef]
- Gatti, R.; Tettamanti, A.; Gough, P.M.; Riboldi, E.; Marinoni, L.; Buccino, G. Action observation versus motor imagery in learning a complex motor task: A short review of literature and a kinematics study. Neurosci. Lett. 2013, 540, 37–42. [Google Scholar] [CrossRef]
- Schuster, C.; Hilfiker, R.; Amft, O.; Scheidhauer, A.; Andrews, B.; Butler, J.A.; Kischka, U.; Ettlin, T. Best practice for motor imagery: A systematic literature review on motor imagery training elements in five different disciplines. BMC Med. 2011, 9, 75. [Google Scholar] [CrossRef]
- Choi, I.; Bond, K.; Krusienski, D.; Nam, C.S. Effects of Off-Site Attention on SSSEP Amplitude. In Proceedings of the 6th International Brain-Computer Interface Meeting, Pacific Grove, CA, USA, 30 May–3 June 2016. [Google Scholar]
- Gu, Y.; Dremstrup, K.; Farina, D. Single-trial discrimination of type and speed of wrist movements from EEG recordings. Clin. Neurophysiol. 2009, 120, 1596–1600. [Google Scholar] [CrossRef]
- Ahlstrom, V.; Kudrick, B. Human Factors Criteria for Displays: A human Factors Design Standard—Update of Chapter 5; FAA William J. Hughes Technical Centre: Atlantic City, NJ, USA, 2007. [Google Scholar]
- Popović, D.B. Advances in functional electrical stimulation (FES). J. Electromyo. Kinesi. 2014, 24, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Schalk, G.; McFarland, D.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J.R. BCI2000: A General-Purpose Brain-Computer Interface (BCI) System. IEEE Trans. Biomed. Eng. 2004, 51, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Nolan, H.; Whelan, R.; Reilly, R.B. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J. Neurosci. Methods 2010, 192, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Schalk, G.; Mellinger, J. A Practical Guide to Brain—Computer Interfacing with BCI2000: General-Purpose Software for Brain-Computer Interface Research, Data Acquisition, Stimulus Presentation, and Brain Monitoring; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Pfurtscheller, G.; Neuper, C. Future prospects of ERD / ERS in the context of brain-computer interface (BCI) developments. Prog. Brain Res. 2006, 159, 433–437. [Google Scholar]
- Golub, M.D.; Chase, S.M.; Batista, A.P.; Yu, B.M. Brain–computer interfaces for dissecting cognitive processes underlying sensorimotor control. Curr. Opin. Neurobiol. 2016, 37, 53–58. [Google Scholar] [CrossRef]
- Nam, C.S.; Nijholt, A.; Lotte, F. Brain–Computer Interfaces Handbook: Technological and Theoretical Advances; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Pfurtscheller, G.; Lopes, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Novi, Q.; Guan, C.; Dat, T.H.; Xue, P. Sub-band Common Spatial Pattern (SBCSP) for Brain-Computer Interface. In Proceedings of the 3rd International IEEE/EMBS Conference on Neural Engineering, Kohala Coast, HI, USA, 2–5 May 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 204–207. [Google Scholar]
- Bashashati, A.; Fatourechi, M.; Ward, R.K.; Birch, G.E.E. A survey of signal processing algorithms in brain–computer interfaces based on electrical brain signals. J. Neural Eng. 2007, 4, R32–R57. [Google Scholar] [CrossRef]
- Polikar, R. Ensemble based systems in decision making. IEEE Circuits Syst. Mag. 2006, 6, 21–45. [Google Scholar] [CrossRef]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. IJCAI 1995, 14, 1137–1145. [Google Scholar]
- Pfurtscheller, G.; Neuper, C. Motor imagery and direct brain-computer communication. Proc. IEEE 2001, 89, 1123–1134. [Google Scholar] [CrossRef]
- Tonet, O.; Tecchio, F.; Sepulveda, F.; Citi, L.; Rossini, P.M.; Marinelli, M.; Tombini, M.; Laschi, C.; Dario, P. Critical Review and Future Perspectives of Non-Invasive Brain-Machine Interfaces. European Space Agency, the Advanced Concepts Team, Ariadna Final Report (05-6402). 2006. Available online: https://www.esa.int/gsp/ACT/doc/ARI/ARI%20Study%20Report/ACT-RPT-BIO-ARI-056402-Non_invasive_brain-machine_interfaces_-_Pisa_S%27Anna.pdf (accessed on 20 June 2020).
- Ortner, R.; Allison, B.Z.; Korisek, G.; Gaggl, H.; Pfurtscheller, G. An SSVEP BCI to Control a Hand Orthosis for Persons with Tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain Computer Interfaces, a Review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, C.; Sannelli, C.; Müller, K.-R.; Blankertz, B. Machine-Learning-Based Coadaptive Calibration for Brain-Computer Interfaces. Neural Comput. 2011, 23, 791–816. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; McFarland, D.J. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 17849–17854. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Li, G.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted Muscle Reinnervation for Real-time Myoelectric Control of Multifunction Artificial Arms. JAMA 2009, 301, 619–628. [Google Scholar] [CrossRef]
- Wodlinger, B.; Downey, J.E.; Tyler, E.C.; Schwartz, A.B.; Boninger, M.L.; Collinger, J.L. Ten-dimensional anthropomorphic arm control in a human brain−machine interface: Difficulties, solutions, and limitations. J. Neural Eng. 2014, 12, 16011. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Ramoser, H.; McFarland, D.; Pfurtscheller, G. EEG-based communication: Improved accuracy by response verification. IEEE Trans. Rehabil. Eng. 1998, 6, 326–333. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. Adv. Psychol. 1988, 52, 139–183. [Google Scholar]
- Perelmouter, J.; Birbaumer, N. A binary spelling interface with random errors. IEEE Trans. Rehabil. Eng. 2000, 8, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Roset, S.A.; Gant, K.; Prasad, A.; Sanchez, J.C. An adaptive brain actuated system for augmenting rehabilitation. Front. Neurosci. 2014, 8, 415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fazel-Rezai, R.; Allison, B.Z.; Guger, C.; Sellers, E.W.; Kleih, S.C.; Kübler, A. P300 brain computer interface: Current challenges and emerging trends. Front. Neuroeng. 2012, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Rohm, M.; Schneiders, M.; Müller, C.; Kreilinger, A.; Kaiser, V.; Müller-Putz, G.R.; Rupp, R.R. Hybrid brain–computer interfaces and hybrid neuroprostheses for restoration of upper limb functions in individuals with high-level spinal cord injury. Artif. Intell. Med. 2013, 59, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Vuagnat, H.; Chantraine, A. Shoulder pain in hemiplegia revisited: Contribution of functional electrical stimulation and other therapies. J. Rehabil. Med. 2003, 35, 49–56. [Google Scholar] [CrossRef]
- Blokland, Y.; Spyrou, L.; Thijssen, D.; Eijsvogels, T.M.; Colier, W.; Floor-Westerdijk, M.; Vlek, R.; Bruhn, J.; Farquhar, J. Combined EEG-fNIRS Decoding of Motor Attempt and Imagery for Brain Switch Control: An Offline Study in Patients With Tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 22, 222–229. [Google Scholar] [CrossRef]
- Huang, D.; Qian, K.; Fei, D.-Y.; Jia, W.; Chen, X.; Bai, O. Electroencephalography (EEG)-Based Brain–Computer Interface (BCI): A 2-D Virtual Wheelchair Control Based on Event-Related Desynchronization/Synchronization and State Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 379–388. [Google Scholar] [CrossRef]
- Yu, T.; Li, Y.; Long, J.; Gu, Z. Surfing the internet with a BCI mouse. J. Neural Eng. 2012, 9, 36012. [Google Scholar] [CrossRef]
- Yu, T.; Li, Y.; Long, J.; Li, F. A Hybrid Brain-Computer Interface-Based Mail Client. Comput. Math. Methods Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.; Lee, B. Effects of Action Observational Training Plus Brain-Computer Interface-Based Functional Electrical Stimulation on Paretic Arm Motor Recovery in Patient with Stroke: A Randomized Controlled Trial. Occup. Ther. Int. 2015, 23, 39–47. [Google Scholar] [CrossRef]
- Looned, R.; Webb, J.; Xiao, Z.G.; Menon, C. Assisting drinking with an affordable BCI-controlled wearable robot and electrical stimulation: A preliminary investigation. J. Neuroeng. Rehabil. 2014, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Toriyama, H.; Ushiba, J.; Ushiyama, J. Subjective Vividness of Kinesthetic Motor Imagery Is Associated with the Similarity in Magnitude of Sensorimotor Event-Related Desynchronization Between Motor Execution and Motor Imagery. Front. Hum. Neurosci. 2018, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Kuhn, A. Electrodes for transcutaneous (surface) electrical stimulation. J. Autom. Control. 2008, 18, 35–45. [Google Scholar] [CrossRef]

| SMR Period | ACT Period | FES Period | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LDA | SVM | Ensemble | LDA | SVM | Ensemble | LDA | SVM | Ensemble | |
| S01 | 56.29 | 56.31 | 59.17 | 64.04 | 65.45 | 69.51 | 46.29 | 50.52 | 48.03 |
| S02 | 74.31 | 73.97 | 76.03 | 70.23 | 67.99 | 73.94 | 53.71 | 63.18 | 58.71 |
| S03 | 74.28 | 74.68 | 76.44 | 69.30 | 68.07 | 70.26 | 60.00 | 66.67 | 62.50 |
| S04 | 84.06 | 81.92 | 83.62 | 66.40 | 67.50 | 70.15 | 55.05 | 54.38 | 53.55 |
| S05 | 67.90 | 64.92 | 67.50 | 58.75 | 63.07 | 64.24 | 54.85 | 60.23 | 55.83 |
| S06 | 67.23 | 65.51 | 68.04 | 70.27 | 73.64 | 76.59 | 53.33 | 62.73 | 61.74 |
| S07 | 75.48 | 78.23 | 79.11 | 66.52 | 70.27 | 73.91 | 58.64 | 59.85 | 60.91 |
| S08 | 75.00 | 74.50 | 78.00 | 70.20 | 70.02 | 71.43 | 73.00 | 72.18 | 74.00 |
| Mean | 71.82 | 71.26 | 73.49 | 66.96 | 68.25 | 71.25 | 56.86 | 61.22 | 59.41 |
| SD | 7.61 | 7.81 | 7.42 | 3.78 | 2.99 | 3.49 | 7.20 | 6.33 | 7.13 |
| Grasping | Opening | No-FES | Keep | Stop | Yes-FES | Grand | |
|---|---|---|---|---|---|---|---|
| Average | Average | Average | |||||
| S01 | 71.7 | 70.8 | 71.3 | 31.7 | 68.3 | 50.0 | 60.6 |
| S02 | 71.7 | 60.8 | 66.3 | 26.7 | 70.0 | 48.3 | 57.3 |
| S03 | 38.3 | 29.2 | 33.8 | 40.0 | 84.2 | 62.1 | 47.9 |
| S04 | 42.5 | 93.3 | 67.9 | 13.3 | 95.0 | 54.2 | 61.0 |
| S05 | 78.3 | 50.0 | 64.2 | 90.0 | 71.7 | 80.8 | 72.5 |
| S06 | 73.3 | 72.5 | 72.9 | 52.5 | 59.2 | 55.8 | 64.4 |
| S07 | 45.0 | 55.0 | 50.0 | 68.3 | 22.5 | 45.4 | 47.7 |
| S08 | 65.0 | 90.0 | 77.5 | 51.7 | 40.0 | 45.8 | 61.7 |
| SD | 15.0 | 19.8 | 13.4 | 22.9 | 21.8 | 11.0 | 19.9 |
| Average | 55.7 | 65.2 | 63.0 | 46.8 | 63.9 | 55.3 |
| Before Learning | After Learning | ||||||
|---|---|---|---|---|---|---|---|
| No-FES | Yes-FES | Average | No-FES | Yes-FES | Average | Grand | |
| S01 | 71.7 | 49.2 | 60.4 | 70.8 | 50.0 | 60.4 | 60.4 |
| S02 | 57.5 | 39.2 | 48.3 | 75.0 | 48.3 | 61.7 | 55.0 |
| S03 | 44.2 | 62.5 | 53.3 | 23.3 | 62.1 | 42.7 | 48.0 |
| S04 | 55.8 | 49.2 | 52.5 | 80.0 | 54.2 | 67.1 | 59.8 |
| S05 | 58.3 | 72.5 | 65.4 | 70.0 | 80.8 | 75.4 | 70.4 |
| S06 | 73.3 | 50.0 | 61.7 | 72.5 | 55.8 | 64.2 | 62.9 |
| S07 | 40.0 | 43.3 | 41.7 | 60.0 | 45.4 | 52.7 | 47.2 |
| S08 | 46.7 | 61.7 | 54.2 | 45.0 | 65.0 | 55.0 | 54.6 |
| SD | 11.38 | 10.41 | 7.19 | 17.80 | 10.73 | 9.26 | 7.25 |
| Average | 55.94 | 53.44 | 54.69 | 62.08 | 57.71 | 59.90 | |
| Grasping | Opening | No-FES | Keep | Stop | Yes-FES | Grand | |
|---|---|---|---|---|---|---|---|
| Average | Average | Average | |||||
| S01 | 60.0 | 55.0 | 57.5 | 50.0 | 45.0 | 47.5 | 52.5 |
| S02 | 65.0 | 55.0 | 60.0 | 35.0 | 55.0 | 45.0 | 52.5 |
| S03 | 30.0 | 35.0 | 32.5 | 50.0 | 60.0 | 55.0 | 43.8 |
| S04 | 40.0 | 85.0 | 62.5 | 15.0 | 80.0 | 47.5 | 55.0 |
| S05 | 70.0 | 45.0 | 57.5 | 90.0 | 45.0 | 67.5 | 62.5 |
| S06 | 70.0 | 60.0 | 65.0 | 60.0 | 55.0 | 57.5 | 61.3 |
| S07 | 35.0 | 50.0 | 42.5 | 75.0 | 10.0 | 42.5 | 42.5 |
| S08 | 65.0 | 70.0 | 67.5 | 65.0 | 10.0 | 37.5 | 52.5 |
| SD | 15.5 | 14.3 | 11.2 | 21.8 | 22.6 | 8.9 | 6.7 |
| Average | 54.4 | 56.9 | 55.6 | 55.0 | 45.0 | 50.0 |
| Before Learning | After Learning | ||||||
|---|---|---|---|---|---|---|---|
| No-FES | Yes-FES | Average | No-FES | Yes-FES | Average | Grand | |
| S01 | 55.0 | 45.0 | 50.0 | 60.0 | 50.0 | 55.0 | 55.0 |
| S02 | 50.0 | 30.0 | 40.0 | 70.0 | 60.0 | 65.0 | 65.0 |
| S03 | 30.0 | 55.0 | 42.5 | 35.0 | 55.0 | 45.0 | 45.0 |
| S04 | 55.0 | 40.0 | 47.5 | 70.0 | 55.0 | 62.5 | 62.5 |
| S05 | 50.0 | 55.0 | 52.5 | 65.0 | 80.0 | 72.5 | 72.5 |
| S06 | 60.0 | 55.0 | 57.5 | 70.0 | 60.0 | 65.0 | 65.0 |
| S07 | 30.0 | 40.0 | 35.0 | 55.0 | 45.0 | 50.0 | 50.0 |
| S08 | 65.0 | 40.0 | 52.5 | 70.0 | 35.0 | 52.5 | 52.5 |
| SD | 12.1 | 8.7 | 7.0 | 11.4 | 12.2 | 8.7 | 8.7 |
| Average | 49.4 | 45.0 | 47.2 | 61.9 | 55.0 | 58.4 | 58.4 |
| Before Learning | After Learning | Grand | |||||
|---|---|---|---|---|---|---|---|
| No-FES | Yes-FES | Average | No-FES | Yes-FES | Average | ||
| S01 | 13.12 | 12.71 | 12.91 | 12.87 | 13.03 | 12.95 | 12.93 |
| S02 | 11.81 | 14.13 | 12.97 | 12.24 | 13.47 | 12.86 | 12.91 |
| S03 | 10.80 | 12.39 | 11.60 | 14.03 | 9.53 | 11.78 | 11.69 |
| S04 | 11.68 | 15.91 | 13.79 | 14.96 | 14.03 | 14.50 | 14.14 |
| S05 | 11.88 | 10.69 | 11.29 | 10.47 | 12.95 | 11.71 | 11.50 |
| S06 | 13.29 | 10.10 | 11.70 | 13.29 | 13.20 | 13.25 | 12.47 |
| S07 | 10.52 | 10.10 | 10.31 | 9.40 | 12.03 | 10.71 | 10.51 |
| S08 | 13.47 | 10.52 | 12.00 | 11.10 | 14.03 | 12.56 | 12.28 |
| Average | 12.07 | 12.07 | 12.07 | 12.29 | 12.78 | 12.54 | 12.30 |
| Estimate | SD | t Ratio | Pr. > |t| | |
|---|---|---|---|---|
| Mental Demand | 3.5 | 1.4516 | 2.41 | 0.0467* |
| Temporal Demand | 3.125 | 1.574773 | 1.98 | 0.0876 |
| Performance | −4.25 | 1.485044 | −2.86 | 0.0243* |
| Effort | 0.25 | 1.346291 | 0.19 | 0.858 |
| Frustration | 6.25 | 1.644797 | 3.8 | 0.0067* |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, I.; Kwon, G.H.; Lee, S.; Nam, C.S. Functional Electrical Stimulation Controlled by Motor Imagery Brain-Computer Interface for Rehabilitation. Brain Sci. 2020, 10, 512. https://doi.org/10.3390/brainsci10080512
Choi I, Kwon GH, Lee S, Nam CS. Functional Electrical Stimulation Controlled by Motor Imagery Brain-Computer Interface for Rehabilitation. Brain Sciences. 2020; 10(8):512. https://doi.org/10.3390/brainsci10080512
Chicago/Turabian StyleChoi, Inchul, Gyu Hyun Kwon, Sangwon Lee, and Chang S. Nam. 2020. "Functional Electrical Stimulation Controlled by Motor Imagery Brain-Computer Interface for Rehabilitation" Brain Sciences 10, no. 8: 512. https://doi.org/10.3390/brainsci10080512
APA StyleChoi, I., Kwon, G. H., Lee, S., & Nam, C. S. (2020). Functional Electrical Stimulation Controlled by Motor Imagery Brain-Computer Interface for Rehabilitation. Brain Sciences, 10(8), 512. https://doi.org/10.3390/brainsci10080512





