Delayed Ischemic Neurological Deficit after Uneventful Elective Clipping of Unruptured Intracranial Aneurysms
Abstract
1. Introduction
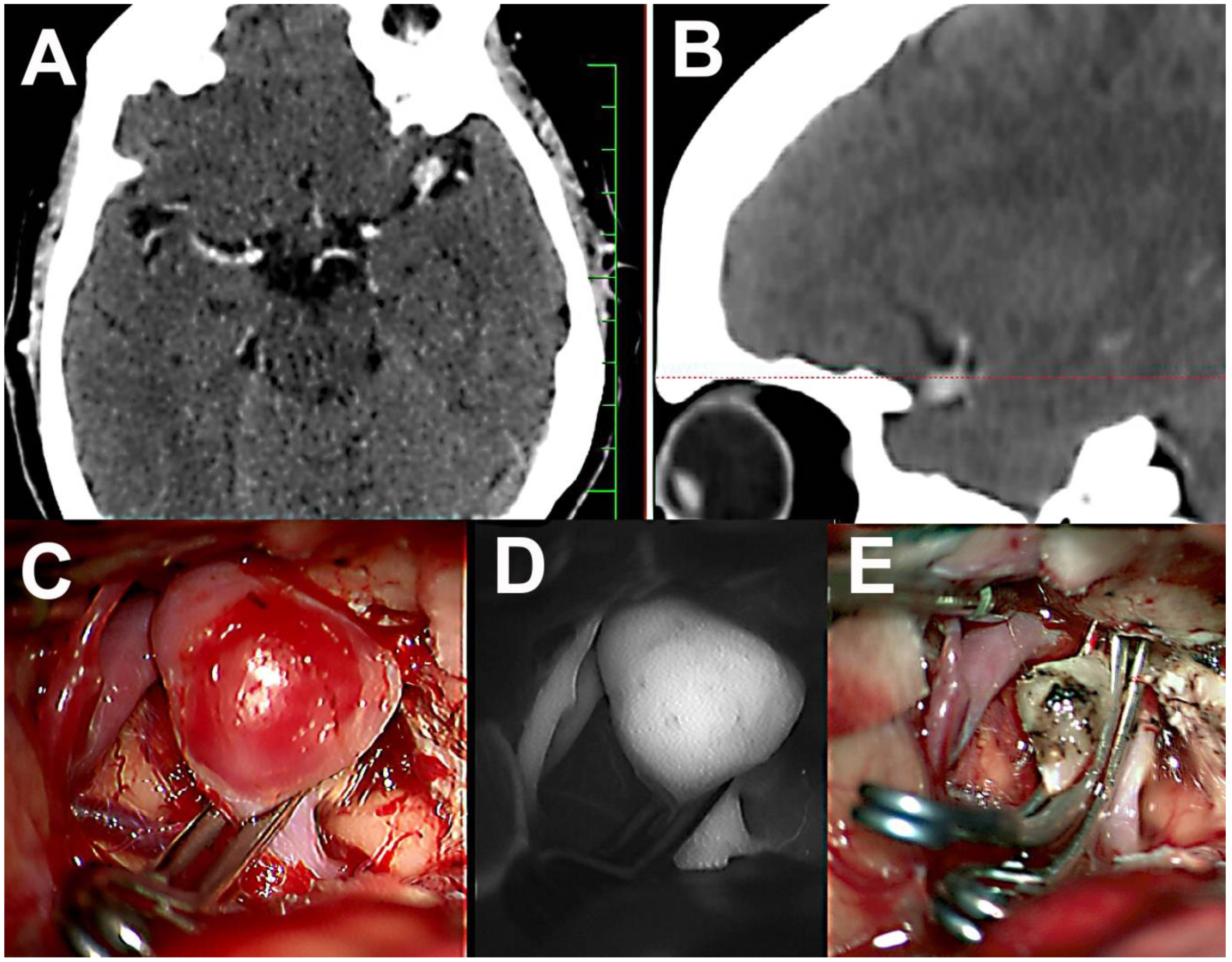
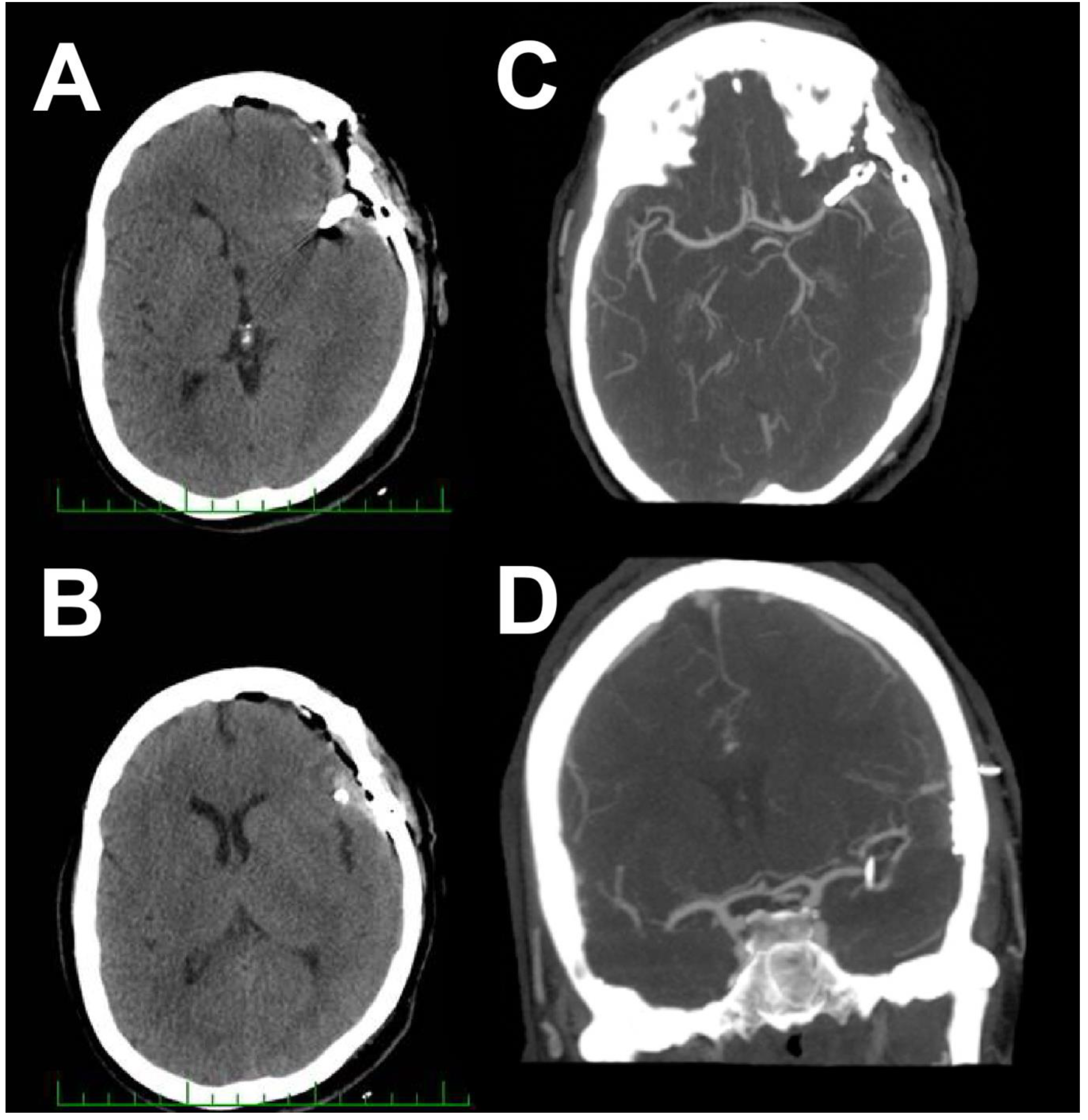
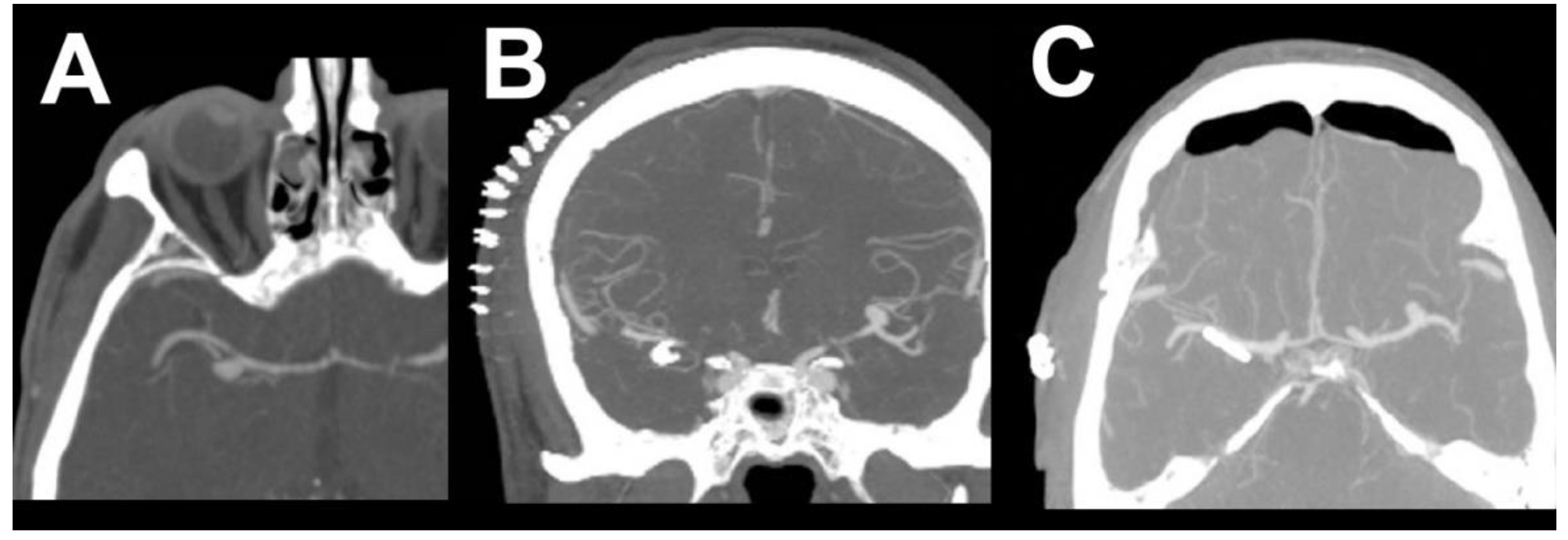
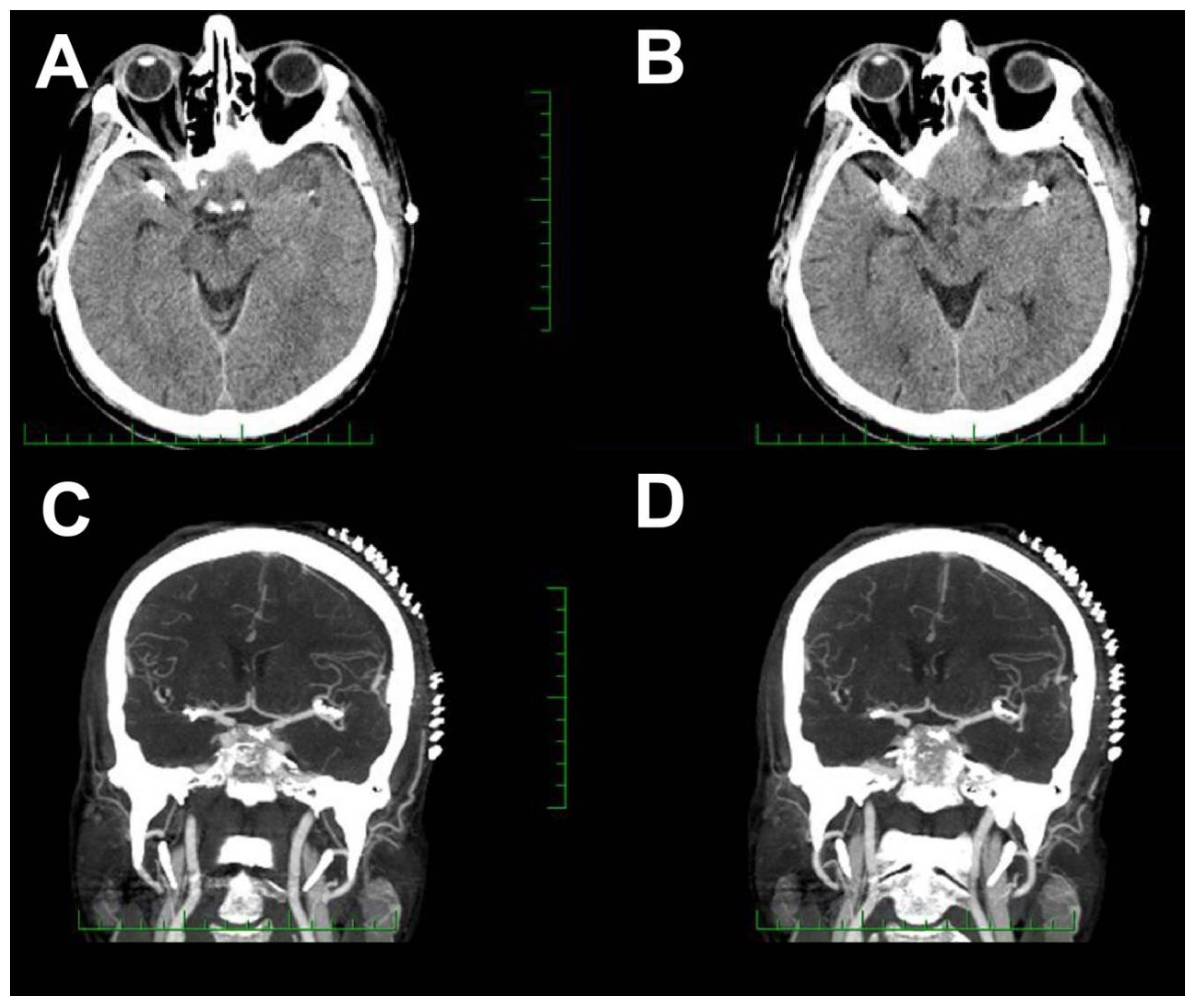
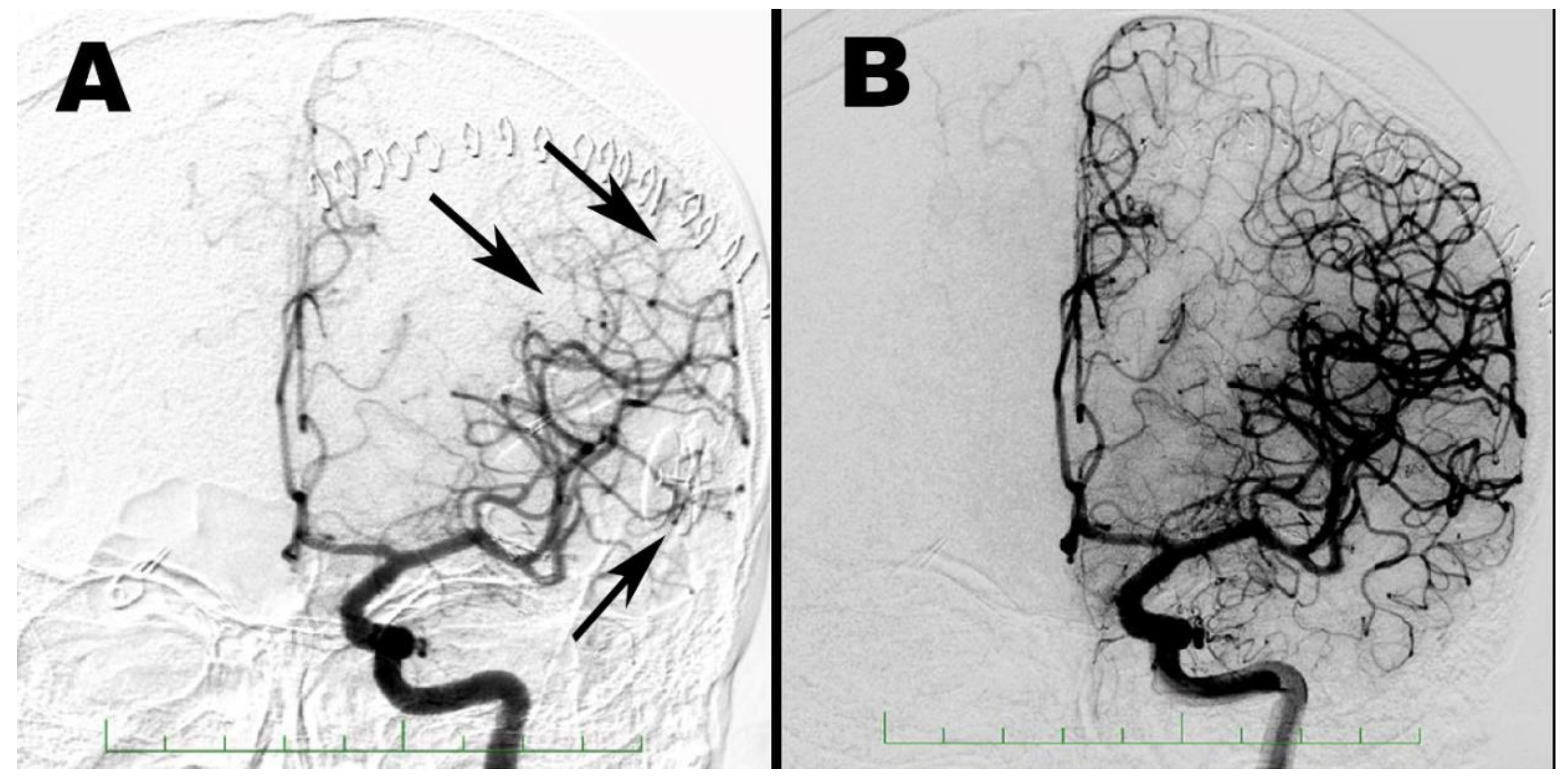
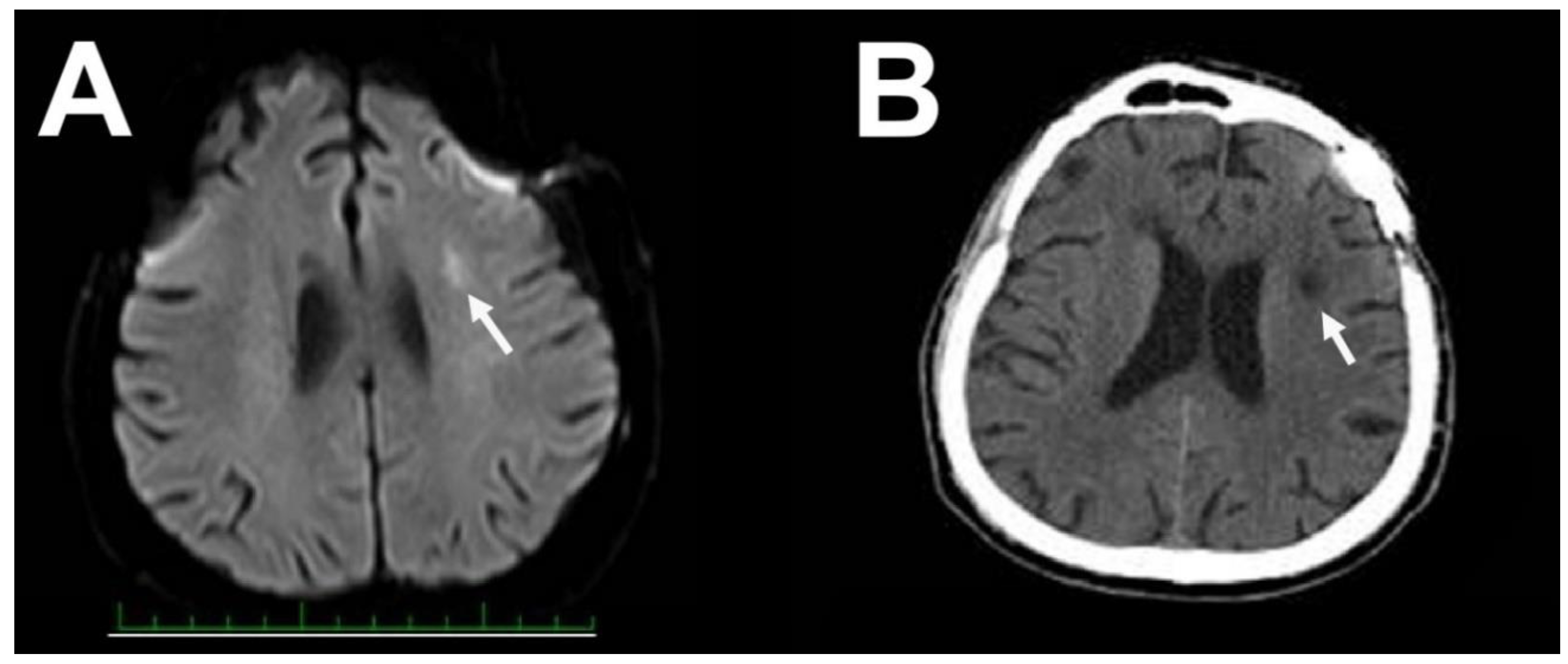
2. Case Report 1
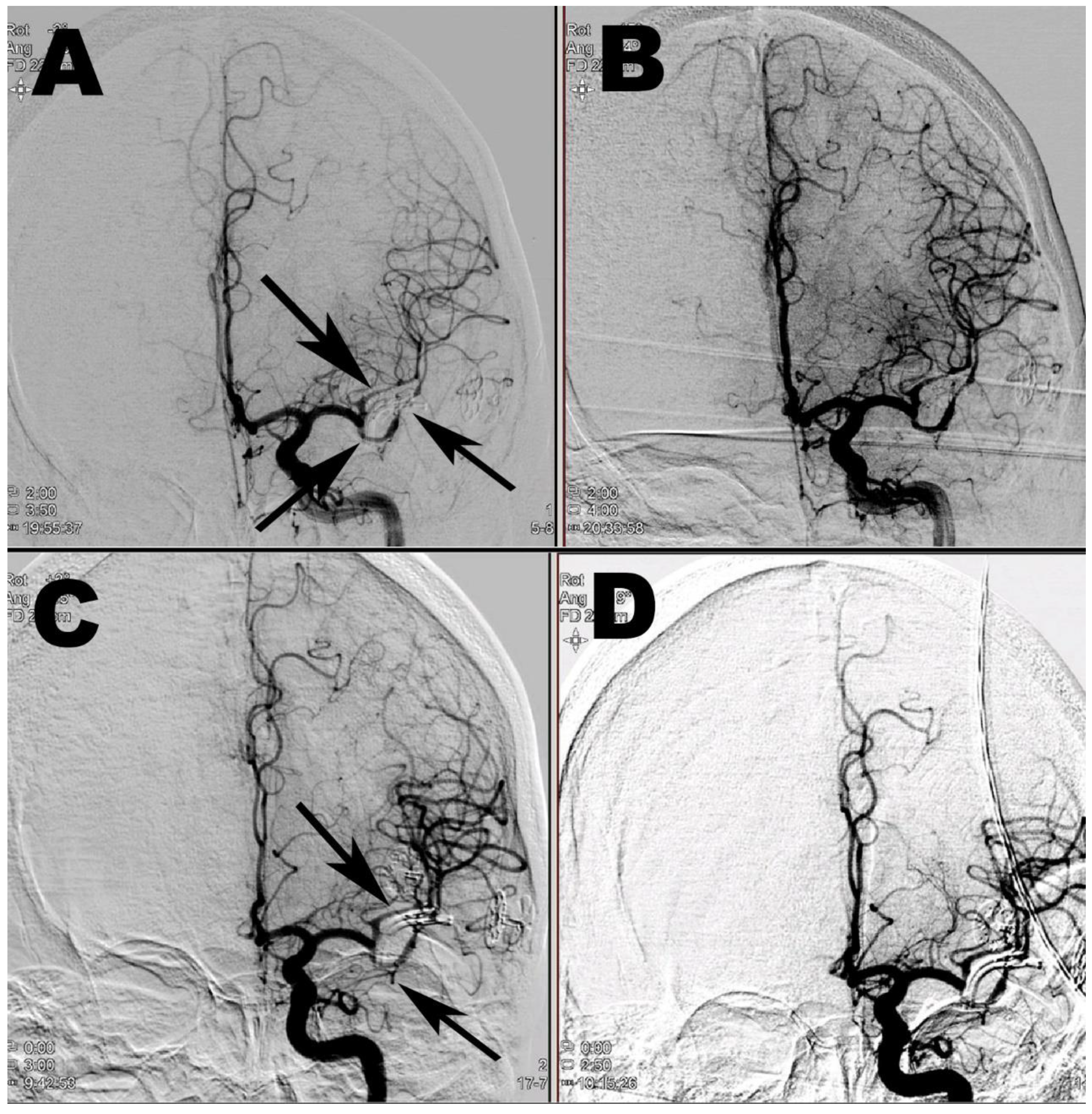
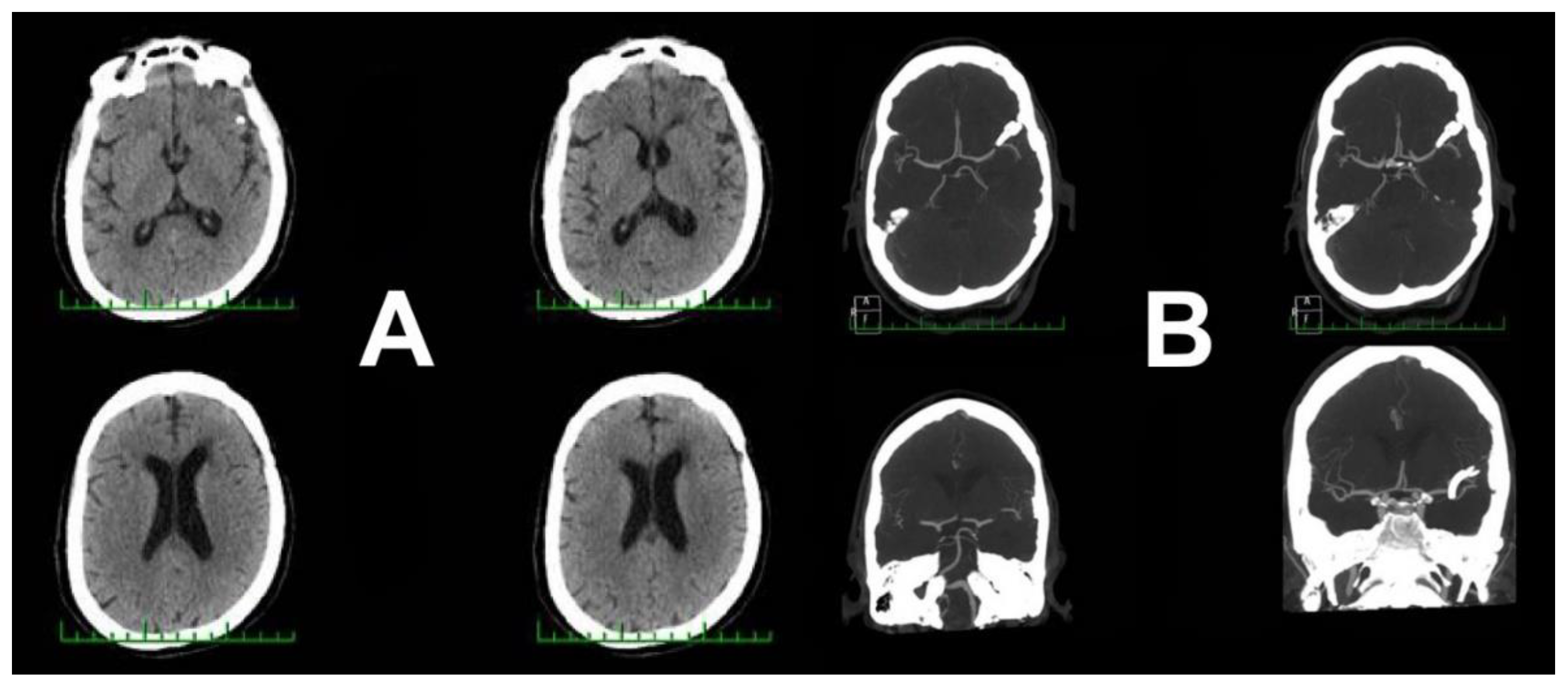
3. Case Report 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.M. Cerebral vasospasm. In Youman’s Neurological Surgery; HR, W., Ed.; WB Saunders: Philadelphia, PA, USA, 2003; pp. 1839–1864. [Google Scholar]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, M.; Takemae, T.; Tazawa, T.; Kawase, T.; Matsuzaki, T. Value of computed tomography in the prediction of cerebral vasospasm after aneurysm rupture. Neurosurgery 1980, 7, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Hejcl, A.; Cihlar, F.; Smolka, V.; Vachata, P.; Bartos, R.; Prochazka, J.; Cihlar, J.; Sames, M. Chemical angioplasty with spasmolytics for vasospasm after subarachnoid hemorrhage. Acta Neurochir. (Wien) 2017, 159, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Fein, J.M. Unruptured aneurysms and cerebral spasm. In Cerebral Arterial Spasm: Proceedings of the Second International Workshop; Wilkins, R.H., Ed.; Springer International Publishing: Cham, Switzerland, 1980; pp. 499–504. [Google Scholar]
- Peerles, S.J. Postoperative cerebral vasospasm without SAH. In Cerebral Arterial Vasospasm: Proceedings of the Second International Workshop; Wilkins, R.H., Ed.; Wiliam and Wilkins: Baltimore, MD, USA, 1980; pp. 496–498. [Google Scholar]
- Raynor, R.B.; Messer, H.D. Severe vasospasm with an unruptured aneurysm: Case report. Neurosurgery 1980, 6, 92–95. [Google Scholar] [CrossRef]
- Simeone, F.; Peerless, S.J. Prolonged cerebral vasospasm without subarachnoid hemorrhage. In Subarachnoid Hemorhage and Cerebrovascular Spasm; JT, S.R., Ed.; Charles C Thomas: Spingfield, IL, USA, 1975; pp. 206–225. [Google Scholar]
- Friedman, P.; Gass, H.H.; Magidson, M. Vasospasm with an unruptured and unoperated aneurysm. Surg. Neurol. 1983, 19, 21–25. [Google Scholar] [CrossRef]
- Bloomfield, S.M.; Sonntag, V.K. Delayed cerebral vasospasm after uncomplicated operation on an unruptured aneurysm: Case report. Neurosurgery 1985, 17, 792–796. [Google Scholar] [CrossRef]
- Campe, C.; Neumann, J.; Sandalcioglu, I.E.; Rashidi, A.; Luchtmann, M. Vasospasm and delayed cerebral ischemia after uneventful clipping of an unruptured intracranial aneurysm-A case report. BMC Neurol. 2019, 19, 226. [Google Scholar] [CrossRef]
- Ceraudo Marco, T.M.; Pasquale, A.; Alessandra, P.; Alessandro, D.; Gianluigi, Z.; Pietro, F. A case report of cerebral vasospasm following elective clipping of unruptured aneurysm: Pathogenetic and clinical features of a dangerous underestimated event. Surg. Case Rep. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Cuoco, J.A.; Guilliams, E.L.; Rogers, C.M.; Patel, B.M.; Marvin, E.A. Recurrent Cerebral Vasospasm and Delayed Cerebral Ischemia Weeks Subsequent to Elective Clipping of an Unruptured Middle Cerebral Artery Aneurysm. World Neurosurg. 2020, 141, 52–58. [Google Scholar] [CrossRef]
- Gutierrez, O.; Caldas, J.G.; Rabello, J.P. Unruptured aneurysm: Vasospasm after surgery and endovascular treatment. A case report. Interv. Neuroradiol. 2001, 7, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Kameda, M.; Yasuhara, T.; Date, I. A Case of Unexpected Symptomatic Vasospasm after Clipping Surgery for an Unruptured Intracranial Aneurysm. J. Stroke Cerebrovasc. Dis. 2016, 25, e25–e27. [Google Scholar] [CrossRef]
- Kitazawa, K.; Hongo, K.; Tanaka, Y.; Oikawa, S.; Kyoshima, K.; Kobayashi, S. Postoperative vasospasm of unruptured paraclinoid carotid aneurysms: Analysis of 30 cases. J. Clin. Neurosci. 2005, 12, 150–155. [Google Scholar] [CrossRef]
- Knight, J.A., 2nd; Bigder, M.G.; Mandel, M.; Li, Y.; Steinberg, G.K. Contralateral Vasospasm in an Uncomplicated Elective Anterior Communicating Artery Aneurysm Clipping. World Neurosurg. 2020, 138, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Paolini, S.; Kanaan, Y.; Wagenbach, A.; Fraser, K.; Lanzino, G. Cerebral vasospasm in patients with unruptured intracranial aneurysms. Acta Neurochir. (Wien) 2005, 147, 1181–1188, discussion 1188. [Google Scholar] [CrossRef] [PubMed]
- Tsyben, A.; Paldor, I.; Laidlaw, J. Cerebral vasospasm and delayed ischaemic deficit following elective aneurysm clipping. J. Clin. Neurosci. 2016, 34, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ahn, J.S.; Park, J.C.; Kwon, D.H.; Kwun, B.D. Clinical and Angiographical Delayed Cerebral Vasospasms After Uncomplicated Surgical Clipping of Unruptured Intracranial Aneurysms: Illustrated Review and Two Case Reports. Turk Neurosurg. 2015, 25, 662–665. [Google Scholar] [CrossRef]
- Kassell, N.F.; Torner, J.C.; Haley, E.C., Jr.; Jane, J.A.; Adams, H.P.; Kongable, G.L. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J. Neurosurg. 1990, 73, 18–36. [Google Scholar] [CrossRef]
- Lantigua Hector, O.-G.S.; Schmidt, J.M.; Kwon, L.; Neeraj, B.; Sachin, A.; Jan, C.; Connolly, E.S.; Mayer, S.A. Subarachnoid hemolrrhage: Who dies, and why? Crit. Care 2015, 19, 1–10. [Google Scholar] [CrossRef]
- Etminam Nima, V.M.D.I.; Macdonald, R.L. Angiographic vasospasm versus cerebral infarction as outcome measures after aneurysmal subarachnoid hemorrhage. Acta Neurochirugica Suppl. 2013, 115, 33–40. [Google Scholar] [CrossRef]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T. The role of the nitric oxide pathway in brain injury and its treatment--From bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wickman, G.; Lan, C.; Vollrath, B. Functional roles of the rho/rho kinase pathway and protein kinase C in the regulation of cerebrovascular constriction mediated by hemoglobin: Relevance to subarachnoid hemorrhage and vasospasm. Circ. Res. 2003, 92, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Hubschmann, O.R.; Kornhauser, D. Cerebral arterial spasm. J. Neurosurg. 1980, 53, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Morigi, M.; Donadelli, R.; Aiello, S.; Foppolo, M.; Todeschini, M.; Orisio, S.; Remuzzi, G.; Remuzzi, A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ. Res. 1995, 76, 536–543. [Google Scholar] [CrossRef]
- Sercombe, R.; Dinh, Y.R.; Gomis, P. Cerebrovascular inflammation following subarachnoid hemorrhage. Jpn. J. Pharmacol. 2002, 88, 227–249. [Google Scholar] [CrossRef]
- Schaller, C.; Klemm, E.; Haun, D.; Schramm, J.; Meyer, B. The transsylvian approach is “minimally invasive” but not “atraumatic”. Neurosurgery 2002, 51, 971–976, discussion 976–977. [Google Scholar] [CrossRef]
- Malinova, V.; Schatlo, B.; Voit, M.; Suntheim, P.; Rohde, V.; Mielke, D. The impact of temporary clipping during aneurysm surgery on the incidence of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2018, 129, 84–90. [Google Scholar] [CrossRef]
- Arienta, C.; Balbi, S.; Caroli, M.; Fumagalli, G. Depletion of calcitonin gene-Related peptide in perivascular nerves during acute phase of posthemorrhagic vasospasm in the rabbit. Brain Res. Bull. 1991, 27, 605–609. [Google Scholar] [CrossRef]
- Buki, A.; Horvath, Z.; Kallo, I.; Liposits, Z.; Lengvari, I.; Doczi, T.P. Peptidergic innervation of human cerebral blood vessels and saccular aneurysms. Acta Neuropathol. 1999, 98, 383–388. [Google Scholar] [CrossRef]
- Edvinsson, L.; Juul, R.; Jansen, I. Perivascular neuropeptides (NPY, VIP, CGRP and SP) in human brain vessels after subarachnoid haemorrhage. Acta Neurol. Scand. 1994, 90, 324–330. [Google Scholar] [CrossRef]
- Edvinsson, L.; Ekman, R.; Jansen, I.; McCulloch, J.; Mortensen, A.; Uddman, R. Reduced levels of calcitonin gene-related peptide-like immunoreactivity in human brain vessels after subarachnoid haemorrhage. Neurosci. Lett. 1991, 121, 151–154. [Google Scholar] [CrossRef]
- Juul, R.; Hara, H.; Gisvold, S.E.; Brubakk, A.O.; Fredriksen, T.A.; Waldemar, G.; Schmidt, J.F.; Ekman, R.; Edvinsson, L. Alterations in perivascular dilatory neuropeptides (CGRP, SP, VIP) in the external jugular vein and in the cerebrospinal fluid following subarachnoid haemorrhage in man. Acta Neurochir. (Wien) 1995, 132, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-Related peptide: Trigeminal origin and co-Existence with substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef]
- Wilkins, R.H. Hypothalamic dysfunction and intracranial arterial spasms. Surg. Neurol. 1975, 4, 472–480. [Google Scholar] [PubMed]
- Wilson, J.L.; Feild, J.R. The production of intracranial vascular spasm by hypothalamic extract. J. Neurosurg. 1974, 40, 473–479. [Google Scholar] [CrossRef]
| Article | Age/Sex (year) | AN Location | AN Size (mm) | Clinical Presentation | Onset (day) | Temporary Clip | Intraoperative Event | Post-Operative CT Pathology | Treatment of CVS | Recovery |
|---|---|---|---|---|---|---|---|---|---|---|
| Bloomfield 1985 [11] | 54/F | Right MCA | 7 | Hemiparesis | 9 | Unknown | Focal spasm | No | Hypervolaemia Steroids | Residual hemiparesis |
| Gutiérrez 2001 [15] | 54/F | Left ophthalmic ICA | 5 | Aphasia Hemiparesis Coma | 1 | No | No | No | Papaverin | Residual hemiparesis |
| Kitazawa 2005 [17] | 21/F | Left ophthalmic ICA | 4 | Aphasia, Gerstmann sy | 12 | Yes | No | No | Triple H Papaverin | Good recovery |
| Kitazawa 2005 [17] | 63/F | Left ophthalmic ICA | 5 | Aphasia Hemiparesis | 5 | Yes | No | Mild EDH | Triple H | Good recovery |
| Kitazawa 2005 [17] | 59/F | Left ophthalmic ICA | 5 | Aphasia Hemiparesis | 2 | No | No | No | Triple H | Good recovery |
| Paolini 2005 [19] | 47/F | Right MCA | 8 | Hemiparesis | 2 | Yes | No | Small ICH | Hypervolemia Antiplatelet | Full recovery |
| Yang 2011 [21] | 41/F | Right MCA | 5 | Aphasia Facial numbness | 28 | Yes | No | No | Hypervolaemia, antiplatelet, nicardipine | Residual aphasia |
| Yang 2012 [21] | 61/F | Left MCA | 6 | Aphasia Mental change | 10 | Yes | No | No | Hypervolaemia, antiplatelet, nicardipine | Residual aphasia |
| Tsyben 2016 [20] | 53/F | Left MCA | 5 | Aphasia Hemiparesis | 2 | No | No | No | Hypervolaemia, hypertension, verapamil | Residual hemiparesis |
| Tsyben 2016 [20] | 70/M | Left MCA AComA | - | Unconsciousness Aphasia Hemiparesis | 2 | Only for ACoA | No | No | Hypervolaemia, hypertension, verapamil, nimodipine | Full recovery |
| Hashimoto 2016 [16] | 62/F | Left PComA | 5 | Aphasia Disorientation Hemiplegia | 11 | No | No | No | Hypervolaemia, antiplatelet | Acalculia, paraphasia |
| Campe 2019 [12] | 69/F | Right MCA | - | Hemiparesis Confused Motor speech disorder | 12 | No | No | No | Antiplatelet, nimodipine | Full recovery |
| Ceraudo 2020 [13] | 59/F | Left MCA | 5 | Motor aphasia Hemiparesis | 6 | Yes | No | No | Nimodpine | Paraphasia, occasional speech arrest |
| Knight 2020 [18] | 68/M | AComA | 5 | Facial drop Dysarthria Aphasia | 5 | . | No | No | Nicardipine, hypertension | Short-term memory deficit |
| Cuoco 2020 [14] | 53/F | Left MCA | 6 | Hemiparesis Hemianopsia | 13,26 | Yes | No | No | Hypertension, verapamil, nimodipine | Hemiparesis, hemianopsia |
| Vachata 2020 | 65/F | Left MCA | 10 | Aphasia Hemiparesis Seizures | 5 | No | No | No | Hypertension nimodipine, milrinone | Full recovery |
| Vachata 2020 | 72/M | Left MCA | 7 | Aphasia Disorientation | 6 | No | No | No | Hypertension, milrinone, nimodipine | Fatigue, depression Deterioration |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vachata, P.; Lodin, J.; Hejčl, A.; Cihlář, F.; Sameš, M. Delayed Ischemic Neurological Deficit after Uneventful Elective Clipping of Unruptured Intracranial Aneurysms. Brain Sci. 2020, 10, 495. https://doi.org/10.3390/brainsci10080495
Vachata P, Lodin J, Hejčl A, Cihlář F, Sameš M. Delayed Ischemic Neurological Deficit after Uneventful Elective Clipping of Unruptured Intracranial Aneurysms. Brain Sciences. 2020; 10(8):495. https://doi.org/10.3390/brainsci10080495
Chicago/Turabian StyleVachata, Petr, Jan Lodin, Aleš Hejčl, Filip Cihlář, and Martin Sameš. 2020. "Delayed Ischemic Neurological Deficit after Uneventful Elective Clipping of Unruptured Intracranial Aneurysms" Brain Sciences 10, no. 8: 495. https://doi.org/10.3390/brainsci10080495
APA StyleVachata, P., Lodin, J., Hejčl, A., Cihlář, F., & Sameš, M. (2020). Delayed Ischemic Neurological Deficit after Uneventful Elective Clipping of Unruptured Intracranial Aneurysms. Brain Sciences, 10(8), 495. https://doi.org/10.3390/brainsci10080495





