Effects of Phosphatidylserine Source of Docosahexaenoic Acid on Cerebellar Development in Preterm Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Preterm Pig Model, and Tissue Collection
2.2. Immunohistochemistry
2.3. Imaging, Cell Counts, and Statistical Analysis
3. Results
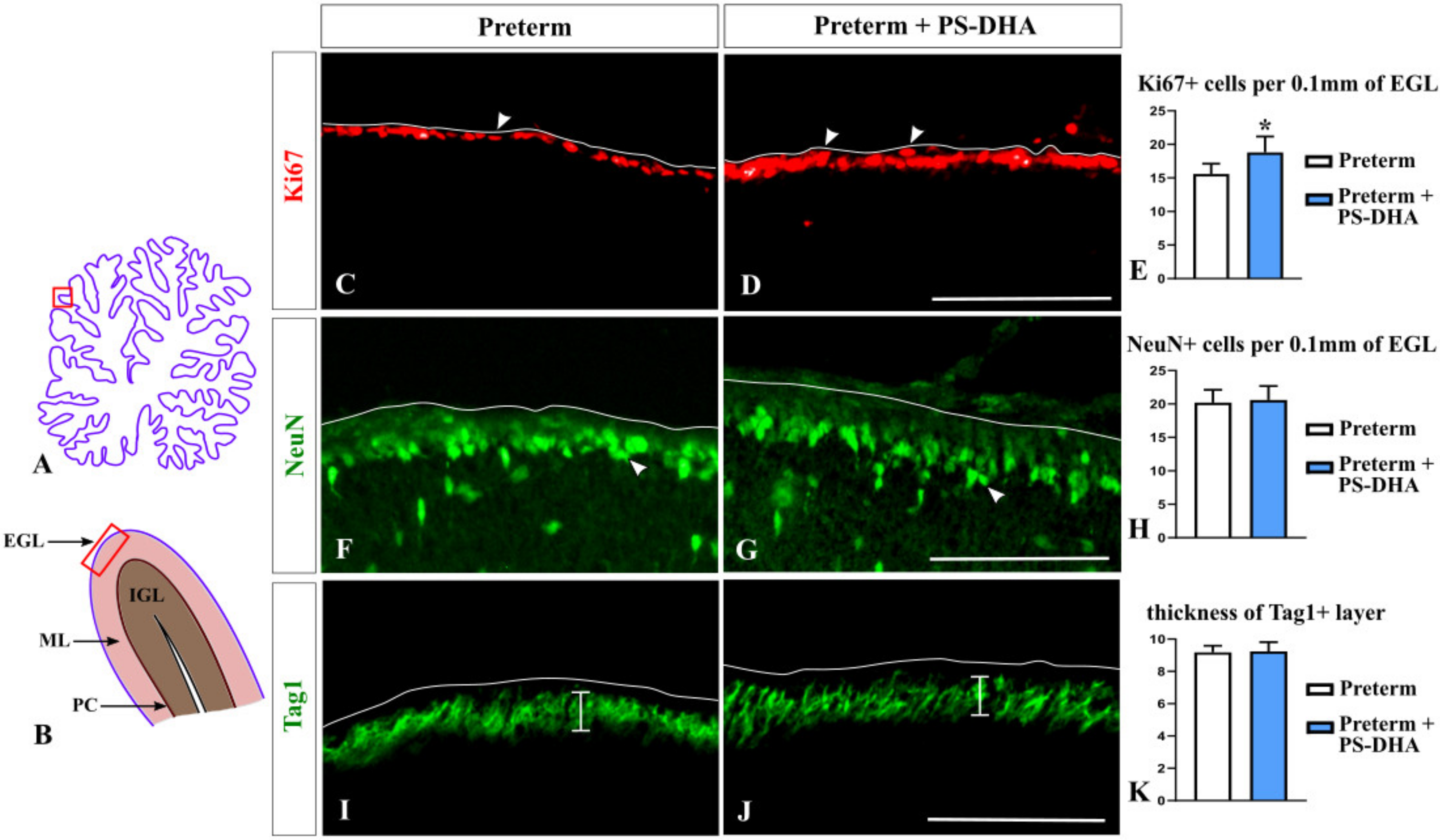
3.1. PS-DHA Promotes Proliferation of Cerebellar Granule Cell Precursors in Pigs Born Preterm
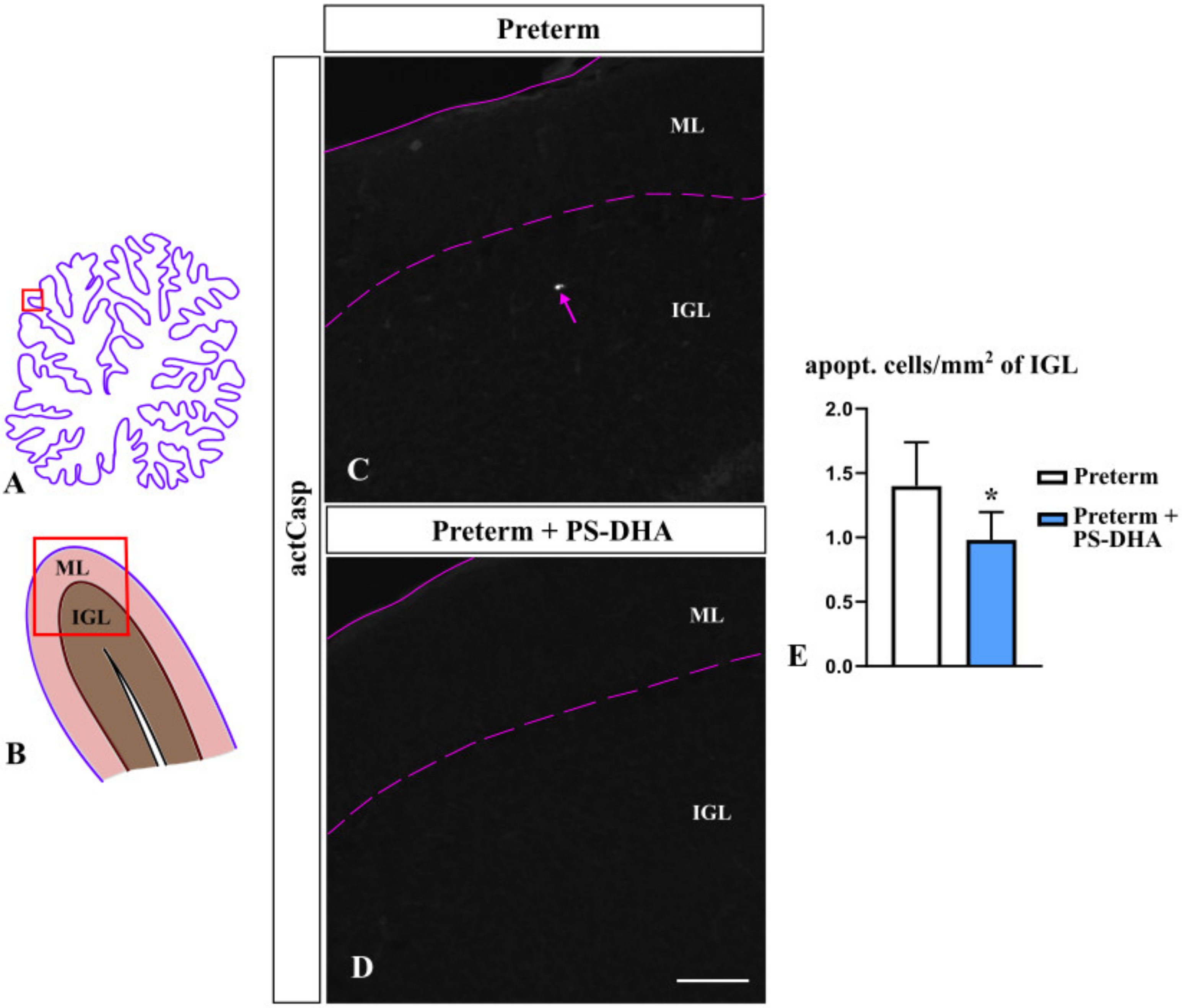
3.2. PS-DHA Promotes Cell Survival in the IGL of Pigs Born Preterm
3.3. Purkinje Cells Are Not Affected by PS-DHA Supplementation of Preterm Pigs
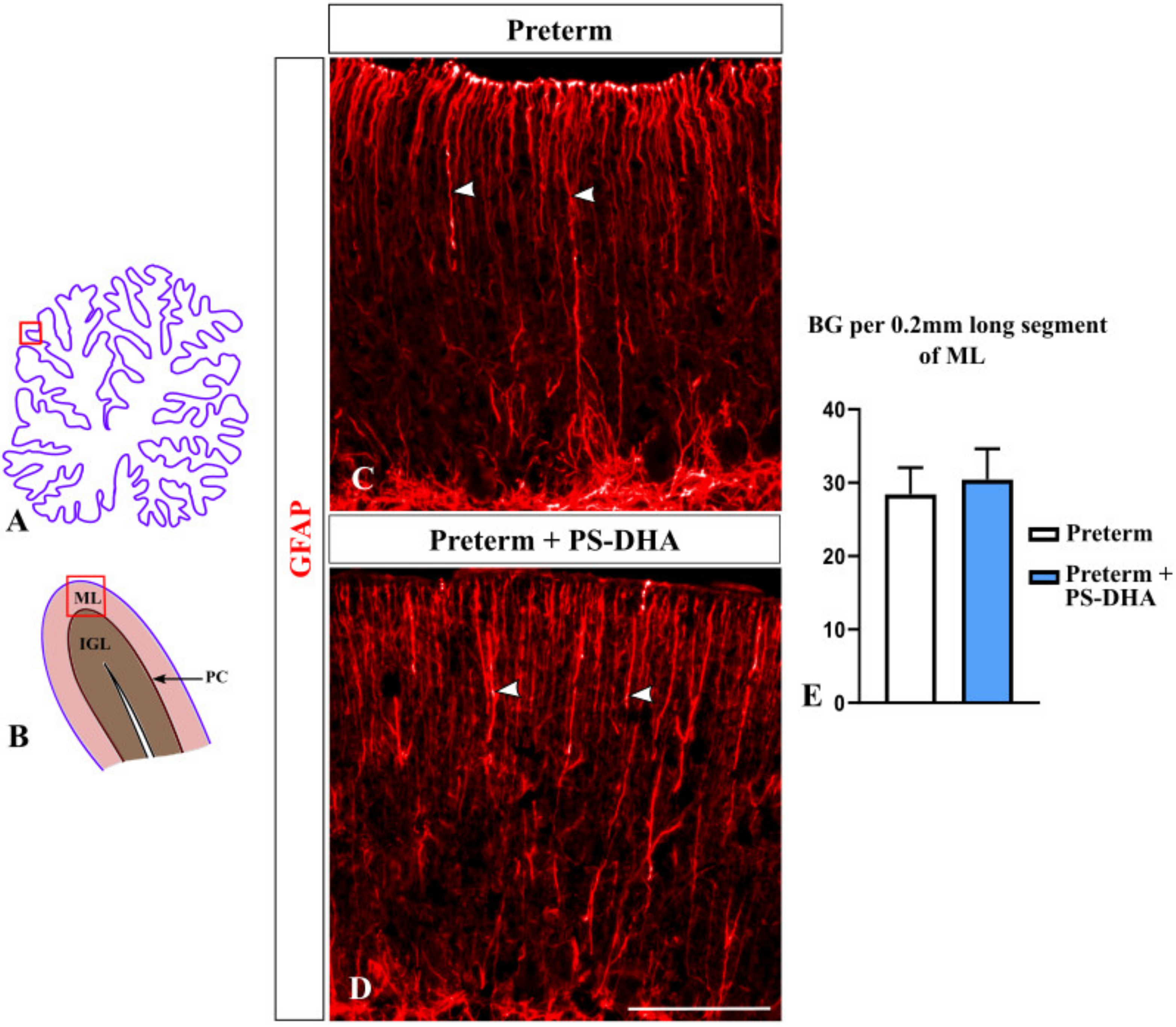
3.4. Bergmann Glia Are Not Affected by PS-DHA Supplementation of Preterm Pigs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Spittle, A.; Orton, J.; Anderson, P.; Boyd, R.; Doyle, L. Early Developmental Intervention Programmes Provided Post Hospital Discharge to Prevent Motor and Cognitive Impairment in Preterm Infants. Cochrane Database Syst. Rev. 2015, 11, CD005495. [Google Scholar] [CrossRef] [PubMed]
- Haldipur, P.; Bharti, U.; Alberti, C.; Sarkar, C.; Gulati, G.; Iyengar, S.; Gressens, P.; Mani, S. Preterm delivery disrupts the developmental program of the cerebellum. PLoS ONE 2011, 6, e23449. [Google Scholar] [CrossRef] [PubMed]
- Iskusnykh, I.; Buddington, R.; Chizhikov, V. Preterm birth disrupts cerebellar development by affecting granule cell proliferation program and Bergmann glia. Exp. Neurol. 2018, 306, 209–221. [Google Scholar] [CrossRef]
- Stoodley, C.; MacMore, J.; Makris, N.; Sherman, J.; Schmahmann, J. Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. Neuroimage Clin. 2016, 12, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Butts, T.; Green, M.; Wingate, R. Development of the cerebellum: Simple steps to make a ‘little brain’. Development 2014, 141, 4031–4041. [Google Scholar] [CrossRef]
- Basson, M.; Wingate, R. Congenital hypoplasia of the cerebellum: Developmental causes and behavioral consequences. Front. Neuroanat. 2013, 7, 29. [Google Scholar] [CrossRef]
- Haldipur, P.; Dang, D.; Millen, K. Embryology. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 154, pp. 29–44. [Google Scholar]
- Chizhikov, V.; Millen, K. Neurogenesis in the cerebellum. In Comprehensive Developmental Neuroscience; Rakic, P., Rubenstein, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Leto., K.; Arancillo, M.; Becker, E.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.; Dusart, I.; Haldipur, P.; Hatten, M.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef]
- Marzban, H.; Del Bigio, M.; Alizadeh, J.; Ghavami, S.; Zachariah, R.; Rastegar, M. Cellular commitment in the developing cerebellum. Front. Cell Neurosci. 2014, 8, 450. [Google Scholar]
- Wullimann, M.; Mueller, T.; Distel, M.; Babaryka, A.; Grothe, B.; Köster, R. The Long Adventurous Journey of Rhombic Lip Cells in Jawed Vertebrates: A Comparative Developmental Analysis. Front. Neuroanat. 2011, 5, 27. [Google Scholar] [CrossRef]
- Biran, V.; Verney, C.; Ferriero, D. Perinatal cerebellar injury in human and animal models. Neurol Res. Int. 2012, 858929. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Li, J. The molecular pathway regulating Bergmann glia and folia generation in the cerebellum. Cerebellum 2018, 17, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sathyanesan, A.; Kundu, S.; Abbah, J.; Gallo, V. Neonatal brain injury causes cerebellar learning deficits and Purkinje cell dysfunction. Nature Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.; Chizhikov, V.; Iskusnykh, I.; Sable, H.; Sable, J.; Holloway, Z.; Katzir, T.; van der Merwe, M.; Yakimkova, T.; Buddington, K.; et al. A Phosphatidylserine Source of Docosahexanoic Acid Improves Neurodevelopment and Survival of Preterm Pigs. Nutrients 2018, 10, 637. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, B.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Legette, L.; Ikonte, C.; Mitmesser, S. Choline and DHA in Maternal and Infant Nutrition: Synergistic Implications in Brain and Eye Health. Nutrients 2019, 11, 1125. [Google Scholar] [CrossRef]
- Smith, S.L.; Rouse, C.A. Docosahexaenoic Acid and the Preterm Infant. Matern. Health Neonatol. Perinatol. 2017, 3, 22. [Google Scholar] [CrossRef]
- Tam, E.; Chau, V.; Barkovich, A.; Ferriero, D.; Miller, S.; Rogers, E.; Grunau, R.; Synnes, A.; Xu, D.; Foong, J.; et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr. Res. 2016, 79, 723–730. [Google Scholar] [CrossRef]
- Sabel, K.; Strandvik, B.; Petzold, M.; Lundqvist-Persson, C. Motor, mental and behavioral developments in infancy are associated with fatty acid pattern in breast milk and plasma of premature infants. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 183–188. [Google Scholar] [CrossRef]
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvåg, A.; Rønnestad, A.; Grønn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145. [Google Scholar] [CrossRef]
- Vance, J.; Steenbergen, R. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 2005, 44, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, N.; Pelled, D. n-3 phosphatidylserine attenuated scopolamine-induced amnesia in middle-aged rats. Prog. Neuro Psychopharmacol. Biol Psychiatry 2009, 33, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Lacombe, R.; Metherel, A.; Masoodi, M.; Bazinet, R.P. DHA Esterified to Phosphatidylserine or Phosphatidylcholine is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Mol. Nutr. Food Res. 2019, 63, e1801224. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Umeshima, H.; Kengaku, M. Cerebellar Granule Cells Are Predominantly Generated by Terminal Symmetric Divisions of Granule Cell Precursors. Dev. Dyn. 2015, 244, 748–758. [Google Scholar] [CrossRef]
- Wen, J.; Yang, H.; Zhou, B.; Lou, H.; Duan, S. β-Catenin Is Critical for Cerebellar Foliation and Lamination. PLoS ONE 2013, 8, e64451. [Google Scholar] [CrossRef][Green Version]
- Van Hove, I.; Verslegers, M.; Buyens, T.; Delorme, N.; Lemmens, K.; Stroobants, S.; Gantois, I.; D’Hooge, R.; Moons, L. An Aberrant Cerebellar Development in Mice Lacking Matrix metalloproteinase-3. Mol. Neurobiol. 2012, 45, 17–29. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y. Signaling pathways in cerebellar granule cells development. Am. J. Stem Cells 2019, 8, 1–6. [Google Scholar]
- Miterko, L.; White, J.; Lin, T.; Brown, A.; O’Donovan, K.; Sillitoe, R. Persistent Motor Dysfunction Despite Homeostatic Rescue of Cerebellar Morphogenesis in the Car8 Waddles Mutant Mouse. Neural Dev. 2019, 14, 6. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Joyner, A.L. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 2007, 23, 549–577. [Google Scholar] [CrossRef]
- Fernandez, C.; Tatard, V.; Bertrand, N.; Dahmane, N. Differential modulation of Sonic-hedgehog-induced cerebellar granule cell precursor proliferation by the IGF signaling network. Dev. Neurosci. 2010, 32, 59–70. [Google Scholar] [CrossRef]
- Rio, C.; Rieff, H.; Qi, P.; Khurana, T.; Corfas, G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron 1997, 19, 39–50. [Google Scholar] [CrossRef]
- Ju, J.; Liu, Q.; Zhang, Y.; Liu, Y.; Jiang, M.; Zhang, L.; He, X.; Peng, C.; Zheng, T.; Lu, Q.; et al. Olig2 regulates Purkinje cell generation in the early developing mouse cerebellum. Sci. Rep. 2016, 6, 30711. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, T.C. Interactions between Purkinje neurones and Bergmann glia. Cerebellum 2006, 5, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Komine, O.; Nagaoka, M.; Watase, K.; Gutmann, D.; Tanigaki, K.; Honjo, T.; Radtke, F.; Saito, T.; Chiba, S.; Tanaka, K. The Monolayer Formation of Bergmann Glial Cells Is Regulated by Notch/RBP-J Signaling. Dev. Biol. 2007, 311, 238–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darmaun, D.; Lapillonne, A.; Simeoni, U.; Picaud, J.C.; Rozé, J.C.; Saliba, E.; Bocquet, A.; Chouraqui, J.; Dupont, C.; Feillet, F.; et al. Parenteral nutrition for preterm infants: Issues and strategy. Arch. Pediatr. 2018, 25, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Hafström, M.; Källén, K.; Serenius, F.; Maršál, K.; Rehn, E.; Drake, H.; Ådén, U.; Farooqi, A.; Thorngren-Jerneck, K.; Strömberg, B. Cerebral Palsy in Extremely Preterm Infants. Pediatrics 2018, 141, e20171433. [Google Scholar] [CrossRef]
- Lean, R.E.; Paul, R.A.; Smyser, T.A.; Smyser, C.D.; Rogers, C.E. Social Adversity and Cognitive, Language, and Motor Development of Very Preterm Children from 2 to 5 Years of Age. J. Pediatr. 2018, 203, 177–184. [Google Scholar] [CrossRef]
- Allotey, J.; Zamora, J.; Cheong-See, F.; Kalidindi, M.; Arroyo-Manzano, D.; Asztalos, E.; van der Post, J.; Mol, B.; Moore, D.; Moore, D.; et al. Cognitive, Motor, Behavioural and Academic Performances of Children Born Preterm: A Meta-Analysis and Systematic Review Involving 64 061 Children. BJOG 2018, 125, 16–25. [Google Scholar] [CrossRef]
- Srinivasan, L.; Allsop, J.; Counsell, S.; Boardman, J.; Edwards, A.; Rutherford, M. Smaller Cerebellar Volumes in Very Preterm Infants at Term-Equivalent Age are Associated with the Presence of Supratentorial Lesions. Am. J. Neuroradiol. 2006, 27, 573–579. [Google Scholar]
- Reeber, S.; Otis, T.; Sillitoe, R. New roles for the cerebellum in health and disease. Front. Syst. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef]
- Kopecky, B.; Decook, R.; Fritzsch, B. Mutational ataxia resulting from abnormal vestibular acquisition and processing is partially compensated for. Behav. Neurosci. 2012, 126, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.E.; Sillitoe, R.V. Interactions Between Purkinje Cells and Granule Cells Coordinate the Development of Functional Cerebellar Circuits. Neuroscience 2020. [Google Scholar] [CrossRef]
- Brossard-Racine, M.; du Plessis, A.; Limperopoulos, C. Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum 2015, 14, 151–164. [Google Scholar] [CrossRef]
- Wagner, M.; Kim, T.; Savall, J.; Schnitzer, M.; Luo, L. Cerebellar granule cells encode the expectation of reward. Nature 2017, 544, 96–100. [Google Scholar] [CrossRef]
- Roussel, M.; Hatten, M. Cerebellum development and medulloblastoma. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2011; Volume 94, pp. 235–282. [Google Scholar]
- Solecki, D.; Liu, X.; Tomoda, T.; Fang, Y.; Hatten, M. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron 2001, 31, 557–568. [Google Scholar] [CrossRef]
- Schmitz, T.; Scheuer, T.; Bührer, C. Development of the cerebellar white matter is impaired by postnatal hyperoxia and protected by minocycline. Mol. Cell Pediatr. 2014, 1, A6. [Google Scholar] [CrossRef][Green Version]
- Huun, M.; Garberg, H.; Escobar, J.; Chafer, C.; Vento, M.; Holme, I.; Saugstad, O.; Solberg, R. DHA reduces oxidative stress following hypoxia-ischemia in newborn piglets: A study of lipid peroxidation products in urine and plasma. J. Perinat. Med. 2018, 46, 209–217. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chizhikov, D.; Buddington, R.K.; Iskusnykh, I.Y. Effects of Phosphatidylserine Source of Docosahexaenoic Acid on Cerebellar Development in Preterm Pigs. Brain Sci. 2020, 10, 475. https://doi.org/10.3390/brainsci10080475
Chizhikov D, Buddington RK, Iskusnykh IY. Effects of Phosphatidylserine Source of Docosahexaenoic Acid on Cerebellar Development in Preterm Pigs. Brain Sciences. 2020; 10(8):475. https://doi.org/10.3390/brainsci10080475
Chicago/Turabian StyleChizhikov, Daniel, Randal K. Buddington, and Igor Y. Iskusnykh. 2020. "Effects of Phosphatidylserine Source of Docosahexaenoic Acid on Cerebellar Development in Preterm Pigs" Brain Sciences 10, no. 8: 475. https://doi.org/10.3390/brainsci10080475
APA StyleChizhikov, D., Buddington, R. K., & Iskusnykh, I. Y. (2020). Effects of Phosphatidylserine Source of Docosahexaenoic Acid on Cerebellar Development in Preterm Pigs. Brain Sciences, 10(8), 475. https://doi.org/10.3390/brainsci10080475





