Autistic Traits Differently Account for Context-Based Predictions of Physical and Social Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. General Design
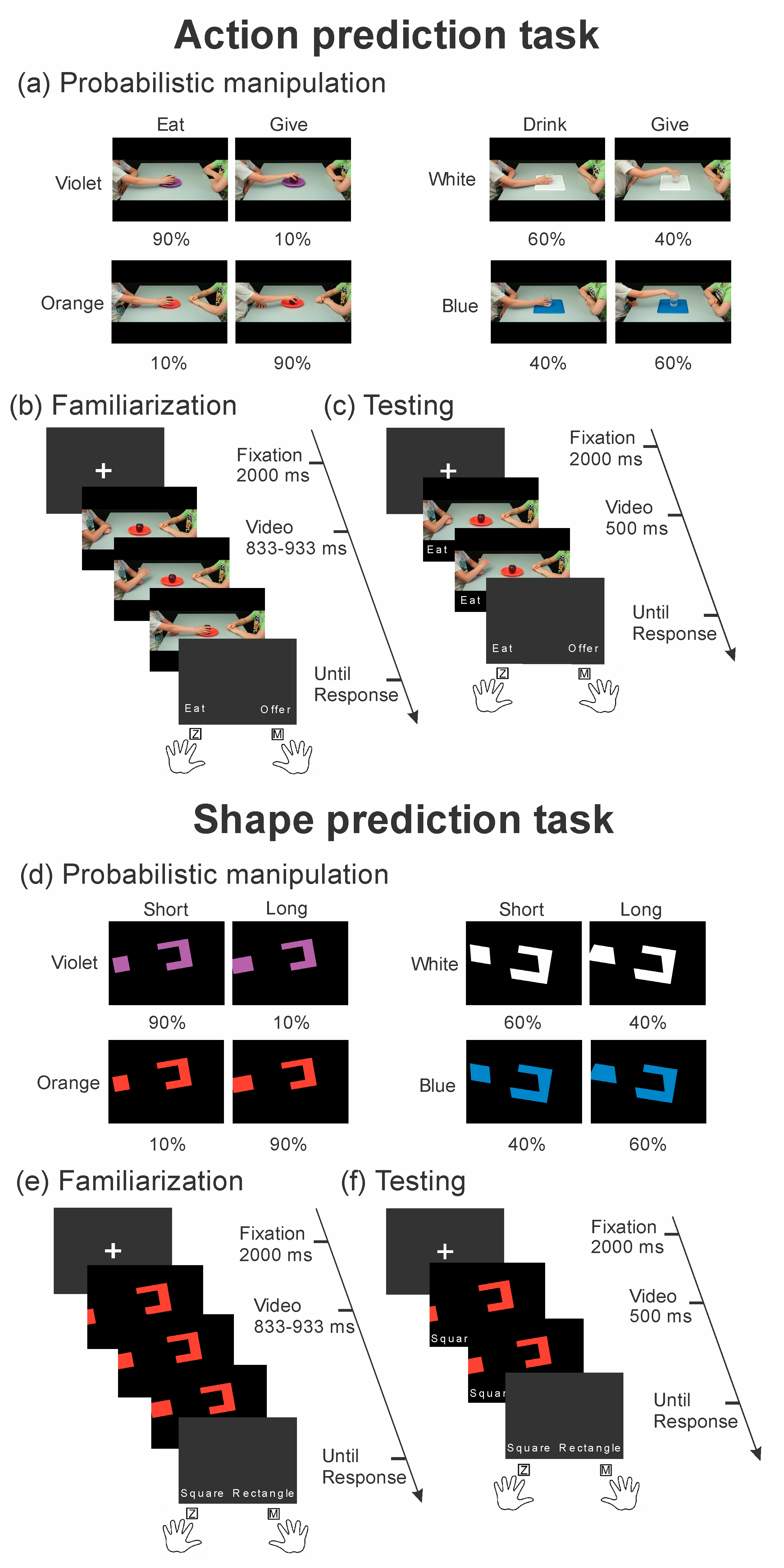
2.3. Stimuli and Tasks
Shape Prediction Tasks
2.4. Procedure and Trial Structure
2.5. Autistic Traits Measure
2.6. Data Handling
3. Results
3.1. AQ Scores
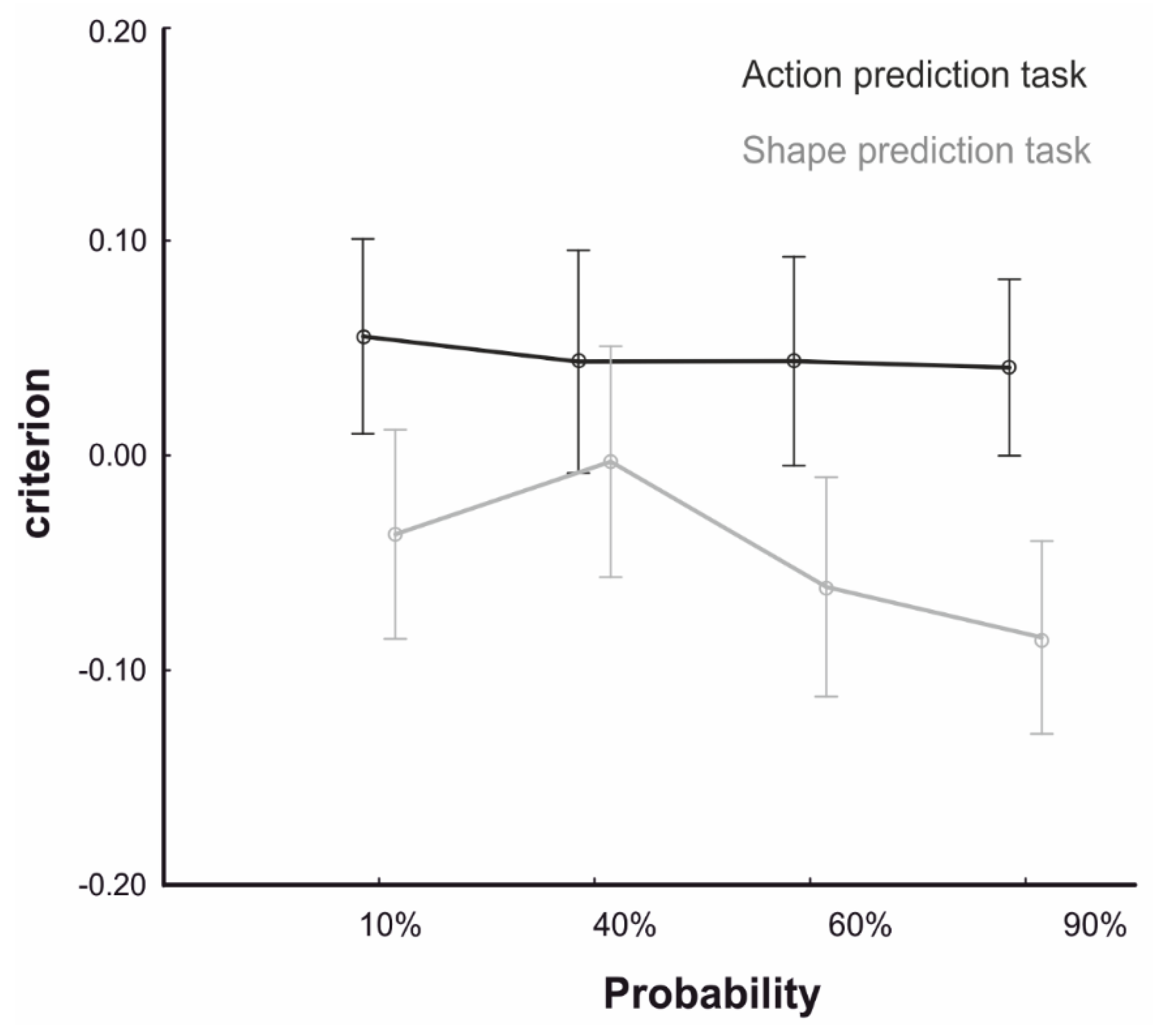
3.2. Behavioral Results: ANOVA
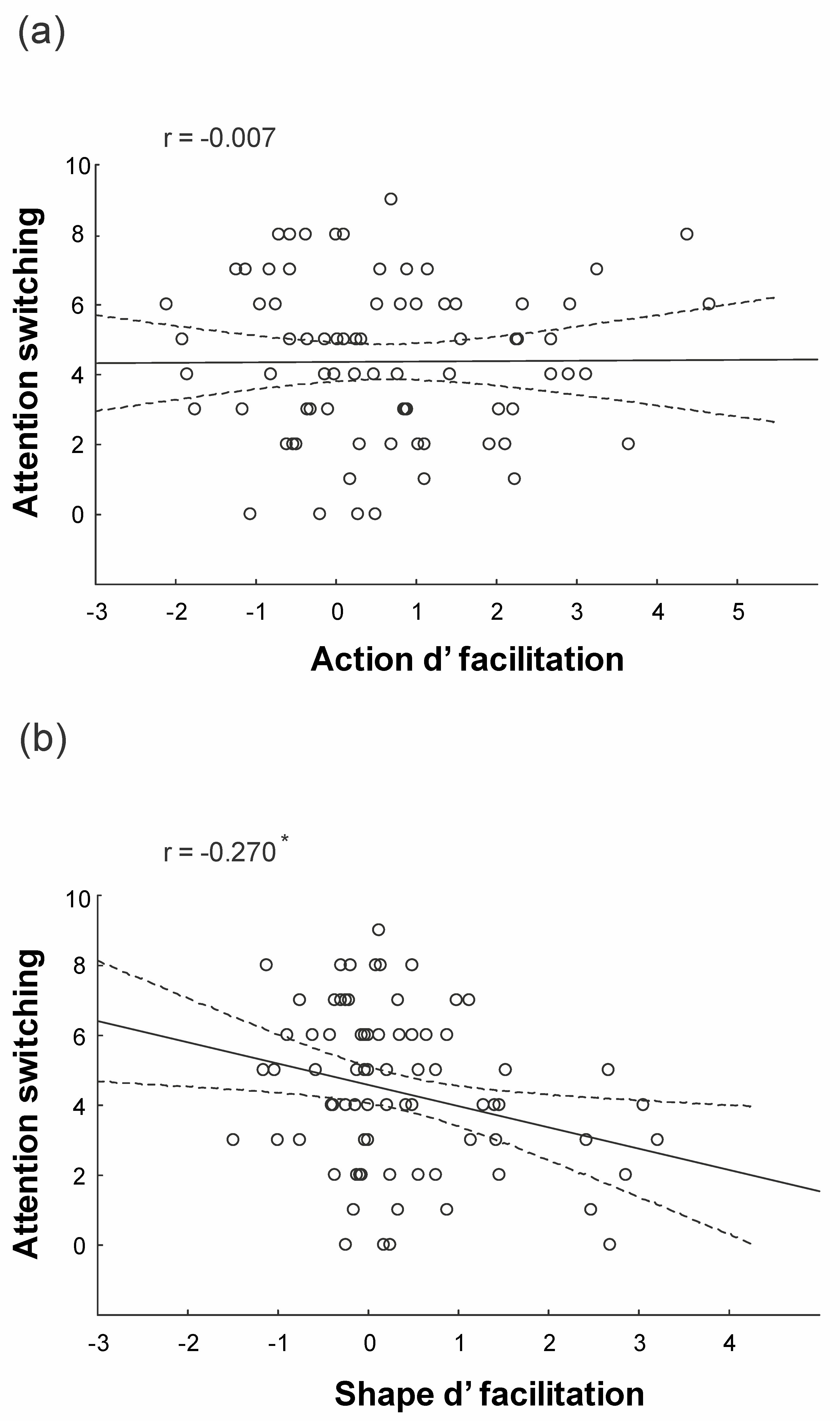
3.3. Behavioral Results: Regression Analysis
4. Discussion
4.1. Contextual Modulation of Social and Physical Event Prediction
4.2. Attention Switching Accounts for Context-Based Prediction of Physical Events
4.3. Autistic Traits Accounts for Context-Based Prediction of Actions
5. Conclusions and Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; Psychiatric Association: Washington, DC, USA; Burbank, CA, USA, 2013. [Google Scholar]
- Orsmond, G.I.; Shattuck, P.T.; Cooper, B.P.; Sterzing, P.R.; Anderson, K.A. Social participation among young adults with an autism spectrum disorder. J. Autism Dev. Disord. 2013, 43, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Hill, E.; Happé, F.; Frith, U. Revisiting the strange stories: Revealing mentalizing impairments in autism. Child Dev. 2009, 80, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Kjelgaard, M.M.; Gandhi, T.K.; Tsourides, K.; Cardinaux, A.L.; Pantazis, D.; Diamond, S.P. Held RM. Autism as a disorder of prediction. Proc. Natl. Acad. Sci. USA 2014, 111, 15220–15225. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, N.M.; Johnson, L.C.; Richards, T.; Mahurin, R.; Greenson, J.; Dawson, G.; Aylward, E. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am. J. Psychiatry 2009, 166, 467–475. [Google Scholar] [CrossRef]
- Gillott, A.; Furniss, F.; Walter, A. Anxiety in high-functioning children with autism. Autism 2001, 5, 277–286. [Google Scholar] [CrossRef]
- Amoruso, L.; Finisguerra, A. Low or high-level motor coding? The role of stimulus complexity. Front. Hum. Neurosci. 2019, 13, 332. [Google Scholar] [CrossRef]
- Finisguerra, A.; Amoruso, L.; Makris, S.; Urgesi, C. Dissociated representations of deceptive intentions and kinematic adaptations in the observer’s motor system. Cerebral Cortex 2018, 28, 33–47. [Google Scholar] [CrossRef]
- Iacoboni, M.; Molnar-Szakacs, I.; Gallese, V.; Buccino, G.; Mazziotta, J.C.; Rizzolatti, G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005, 3, e79. [Google Scholar] [CrossRef]
- Ansuini, C.; Giosa, L.; Turella, L.; Altoè, G.; Castiello, U. An object for an action, the same object for other actions: Effects on hand shaping. Exp. Brain Res. 2008, 185, 111–119. [Google Scholar] [CrossRef]
- Amoruso, L.; Finisguerra, A.; Urgesi, C. Contextualizing action observation in the predictive brain: Causal contributions of prefrontal and middle temporal areas. NeuroImage 2018, 177, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wurm, M.F.; Schubotz, R.I. 2016 What’s she doing in the kitchen? Context helps when actions are hard to recognize. Psychon. Bull Rev. 2016, 24, 503–509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amoruso, L.; Urgesi, C. Contextual modulation of motor resonance during the observation of everyday actions. NeuroImage 2016, 134, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Amoruso, L.; Finisguerra, A.; Urgesi, C. Tracking the time course of top-down contextual effects on motor responses during action comprehension. J. Neurosci. 2016, 36, 11590–11600. [Google Scholar] [CrossRef] [PubMed]
- Amoruso, L.; Finisguerra, A.; Urgesi, C. Autistic traits predict poor integration between top-down contextual expectations and movement kinematics during action observation. Sci. Rep. 2018, 8, 16208. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Land, E.H. The retinex theory of color vision. Sci. Am. 1977, 237, 108–129. [Google Scholar] [CrossRef]
- Gilbert, C.D.; Sigman, M.; Crist, R.E. The neural basis of perceptual learning. Neuron 2001, 31, 681–697. [Google Scholar] [CrossRef]
- Amoruso, L.; Narzisi, A.; Pinzino, M.; Finisguerra, A.; Billeci, L.; Calderoni, S.; Urgesi, C. Contextual priors do not modulate action prediction in children with autism. Proc. R. Soc. Lond. 2019, 286, 20191319. [Google Scholar] [CrossRef]
- Van de Cruys, S.; Evers, K.; Van der Hallen, R.; Van Eylen, L.; Boets, B.; de-Wit, L.; Wagemans, J. Precise minds in uncertain worlds: Predictive coding in autism. Psychol. Rev. 2014, 121, 649. [Google Scholar] [CrossRef]
- Lawson, R.P.; Rees, G.; Friston, K.J. An aberrant precision account of autism. Front. Hum. Neuroscience 2014, 8, 302. [Google Scholar]
- Hamilton, A.F.D.C. Research review: Goals, intentions and mental states: Challenges for theories of autism. J. Child Psychol. Psychiatry 2009, 50, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M.; Dapretto, M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 2006, 7, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Zalla, T.; Labruyere, N.; Georgieff, N. Goal-directed action representation in autism. J. Autism Dev. Disord. 2006, 36, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Fabbri-Destro, M.; Boria, S.; Pieraccini, C.; Monti, A.; Cossu, G.; Rizzolatti, G. Impairment of actions chains in autism and its possible role in intention understanding. Proc. Natl. Acad. Sci. USA 2007, 104, 17825–17830. [Google Scholar] [CrossRef]
- Chambon, V.; Farrer, C.; Pacherie, E.; Jacquet, P.O.; Leboyer, M.; Zalla, T. Reduced sensitivity to social priors during action prediction in adults with autism spectrum disorders. Cognition 2017, 160, 17–26. [Google Scholar] [CrossRef]
- Gomot, M.; Wicker, B. A challenging, unpredictable world for people with autism spectrum disorder. Int. J. Psychophysiol. 2012, 83, 240–247. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Cohen, D.J.; Paul, R. An evaluation of DSM-III criteria for infantile autism. J. Am. Acad. Child Psy. 1986, 25, 190–197. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef]
- Constantino, J.; Todd, R. Autistic traits in the general population—A twin study. Arch. Gen. Psychiatry 2003, 60, 524–530. [Google Scholar] [CrossRef]
- Posserud, M.B.; Lundervold, A.J.; Gillberg, C. Autistic features in a total population of 7–9-year-old children assessed by the ASSQ (Autism Spectrum Screening Questionnaire). J. Child Psychol. Psychiatry 2006, 47, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ruzich, E.; Allison, C.; Smith, P.; Watson, P.; Auyeung, B.; Ring, H.; Baron-Cohen, S. Measuring autistic traits in the general population: A systematic review of the Autism-Spectrum Quotient (AQ) in a nonclinical population sample of 6900 typical adult males and females. Mol. Autism 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Miu, A.C.; Pană, S.E.; Avram, J. Emotional face processing in neurotypicals with autistic traits: Implications for the broad autism phenotype. Psychiatry Res. 2012, 198, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.J.; Paton, B.; Kirkovski, M.; Enticott, P.G.; Hohwy, J. Context sensitivity in action decreases along the autism spectrum: A predictive processing perspective. Proc. R. Soc. B-Biol. Sci. 2015, 282, 20141557. [Google Scholar] [CrossRef]
- Amoruso, L.; Finisguerra, A.; Urgesi, C. Spatial frequency tuning of motor responses reveals differential contribution of dorsal and ventral systems to action comprehension. Proc. Natl. Acad. Sci. USA 2020, 117, 13151–13161. [Google Scholar] [CrossRef]
- Happé, F.; Ronald, A.; Plomin, R. Time to give up on a single explanation for autism. Nat. Neurosci. 2006, 9, 1218–1220. [Google Scholar] [CrossRef]
- Schubotz, R.I.; Von Cramon, D.Y. Sequences of abstract nonbiological stimuli share ventral premotor cortex with action observation and imagery. J. Neurosci. 2004, 24, 5467–5474. [Google Scholar] [CrossRef]
- Paracampo, R.; Montemurro, M.; de Vega, M.; Avenanti, A. Primary motor cortex crucial for action prediction: A. tDCS study. Cortex 2018, 109, 287–302. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. The effect size. Stat. Power Anal. Behav. Sci. 1988, 2, 77–83. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Summerfield, C.; Koechlin, E. A neural representation of prior information during perceptual inference. Neuron 2008, 59, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Ruta, L.; Mazzone, D.; Mazzone, L.; Wheelwright, S.; Baron-Cohen, S. The Autism-Spectrum Quotient—Italian version: A cross-cultural confirmation of the broader autism phenotype. J. Autism Dev. Disord. 2012, 42, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Conson, M.; Senese, V.P.; Baiano, C.; Zappullo, I.; Warrier, V.; Salzano, S.; Baron-Cohen, S. The effects of autistic traits and academic degree on visuospatial abilities. Cogn. Process. 2020, 21, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods 1999, 31, 137–149. [Google Scholar] [CrossRef]
- Shin, Y.K.; Proctor, R.W.; Capaldi, E.J. A review of contemporary ideomotor theory. Psychol. Bull. 2010, 136, 943. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.J.; Clark, A. Getting ahead: Forward models and their place in cognitive architecture. Trends Cogn. Sci. 2014, 18, 451–456. [Google Scholar] [CrossRef]
- Waszak, F.; Cardoso-Leite, P.; Hughes, G. Action effect anticipation: Neurophysiological basis and functional consequences. Neurosc. Biobehav. Rev. 2012, 36, 943–959. [Google Scholar] [CrossRef]
- Cardoso-Leite, P.; Mamassian, P.; Schütz-Bosbach, S.; Waszak, F. A new look at sensory attenuation: Action-effect anticipation affects sensitivity, not response bias. Psychol. Sci. 2010, 21, 1740–1745. [Google Scholar] [CrossRef]
- Blakemore, S.J.; Wolpert, D.M.; Frith, C.D. Central cancellation of self-produced tickle sensation. Nat. Neurosci. 1998, 1, 635–640. [Google Scholar] [CrossRef]
- Koul, A.; Soriano, M.; Tversky, B.; Becchio, C.; Cavallo, A. The kinematics that you do not expect: Integrating prior information and kinematics to understand intentions. Cognition 2019, 182, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.M.; Friston, K.J.; Frith, C.D. Predictive coding: An account of the mirror neuron system. Cogn. Process 2007, 8, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.M. More than one pathway to action understanding. Trends Cogn. Sci. 2011, 15, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Bar, M. Visual objects in context. Nat. Rev. Neurosci. 2004, 5, 617–629. [Google Scholar] [CrossRef]
- Ego, C.; Bonhomme, L.; de Xivry, J.J.O.; Da Fonseca, D.; Lefèvre, P.; Masson, G.S.; Deruelle, C. Behavioral characterization of prediction and internal models in adolescents with autistic spectrum disorders. Neuropsychologia 2016, 91, 335–345. [Google Scholar] [CrossRef]
- Tewolde, F.G.; Bishop, D.V.; Manning, C. Visual motion prediction and verbal false memory performance in autistic children. Autism Res. 2018, 11, 509–518. [Google Scholar] [CrossRef]
- Balsters, J.H.; Apps, M.A.; Bolis, D.; Lehner, R.; Gallagher, L.; Wenderoth, N. Disrupted prediction errors index social deficits in autism spectrum disorder. Brain 2017, 140, 235–246. [Google Scholar] [CrossRef]
- Goris, J.; Braem, S.; Nijhof, A.D.; Rigoni, D.; Deschrijver, E.; Van de Cruys, S.; Wiersema, J.R.; Brass, M. Sensory prediction errors are less modulated by global context in autism spectrum disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 667–674. [Google Scholar] [CrossRef]
- Turi, M.; Burr, D.C.; Igliozzi, R.; Aagten-Murphy, D.; Muratori, F.; Pellicano, E. Children with autism spectrum disorder show reduced adaptation to number. Proc. Natl. Acad. Sci. USA 2015, 112, 7868–7872. [Google Scholar] [CrossRef]
- Braukmann, R.; Ward, E.; Hessels, R.S.; Bekkering, H.; Buitelaar, J.K.; Hunnius, S. Action prediction in 10-month-old infants at high and low familial risk for Autism Spectrum Disorder. Res. Autism Spectrum Disord. 2018, 49, 34–46. [Google Scholar] [CrossRef]
- Karvelis, P.; Seitz, A.R.; Lawrie, S.M.; Seriès, P. Autistic traits, but not schizotypy, predict increased weighting of sensory information in Bayesian visual integration. ELife 2018, 7, e34115. [Google Scholar] [CrossRef] [PubMed]
- Skewes, J.C.; Jegindø, E.-M.; Gebauer, L. Perceptual inference and autistic traits. Autism 2015, 19, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Frith, U.; Happé, F. Autism: Beyond “theory of mind”. Cogn. Cogn. 1995, 13–30. [Google Scholar] [CrossRef]
- Mottron, L.; Burack, J.A. Enhanced perceptual functioning in the development of autism. In The Development of Autism: Perspectives from Theory and Research; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2001; pp. 131–148. [Google Scholar]
- Pellicano, E.; Burr, D.C. When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends Cogn. Sci. 2012, 16, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.P.; Aylward, J.; White, S.; Rees, G. A striking reduction of simple loudness adaptation in autism. Sci. Rep. 2015, 5, 16157. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.P.; Mathys, C.; Rees, G. Adults with autism overestimate the volatility of the sensory environment. Nat. Neurosci. 2017, 20, 1293–1299. [Google Scholar] [CrossRef]
- Manning, C.; Kilner, J.M.; Neil, L.; Karaminis, T.; Pellicano, E. Children on the autism spectrum update their behaviour in response to a volatile environment. Dev. Sci. 2017, 20, e12435. [Google Scholar] [CrossRef]
- Simmons, D.R.; Robertson, A.; McKay, L.S.; Toal, E.; McAleer, P.; Pollick, F.E. Vision in autism spectrum disorders. Vis. Res. 2009, 49, 2705–2739. [Google Scholar] [CrossRef]
- Courchesne, E.; Townsend, J.; Akshoomoff, N.; Saitoh, O.; Yeung-Courchesne, R.; Lincoln, A.J.; James, H.E.; Haas, R.H.; Schreibman, L.; Lau, L. Impairment in shifting attention in Autistic and Cerebellar Patients’. Behav. Neurosci. 1994, 108, 848–865. [Google Scholar]
- Ring, H.; Baron-Cohen, S.; Wheelwright, S.; Williams, S.C.; Brammer, M.; Andrew, C.; Bullmore, E.T. Cerebral correlates of preserved cognitive skills in autism: A functional MRI study of embedded figures task performance. Brain 1999, 122, 1305–1315. [Google Scholar] [CrossRef]
- Schuwerk, T.; Sodian, B.; Paulus, M. Cognitive Mechanisms Underlying Action Prediction in Children and Adults with Autism Spectrum Condition. J. Autism Dev. Disord. 2016, 46, 3623–3639. [Google Scholar] [CrossRef]
- Spiker, D.; Lotspeich, L.J.; DiMiceli, S.; Myers, R.M.; Risch, N. Behavioral phenotypic variation in autism multiplex families: Evidence for a continuous severity gradient. Am. J. Med. Genet. 2002, 114, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.; Simmons, D. The Relationship between Sensory Sensitivity and Autistic Traits in the General Population. J. Autism Dev. Disord. 2013, 43, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Grinter, E.J.; Maybery, M.; Van Beek, P.L.; Pellicano, E.; Badcock, J.C.; Badcock, D.R. Global Visual Processing and Self-Rated Autistic-like Traits. J. Autism Dev. Disord. 2009, 39, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Smith, C.J.; Schmeidler, J.; Hollander, E.; Lawlor, B.A.; Fitzgerald, M.; Buxbaum, J.D.; Delaney, K.; Galvin, P. the Autism Genetic Research Exchange Consortium Symptom domains in autism and related conditions: Evidence for familiality. Am. J. Med. Genet. 2002, 114, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.S.; Geschwind, D.H. Advances in autism genetics: On the threshold of a new neurobiology. Nat. Rev. Genet. 2008, 9, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.W.; Youngstrom, E.A.; Sinclair, L.; Kubu, C.S.; Law, P.; Rezai, A.; Constantino, J.N.; Eng, C. Autism Spectrum Disorders as a Qualitatively Distinct Category From Typical Behavior in a Large, Clinically Ascertained Sample. Assessment 2010, 17, 308–320. [Google Scholar] [CrossRef]
- Elton, A.; Di Martino, A.; Hazlett, H.C.; Gao, W. Neural Connectivity Evidence for a Categorical-Dimensional Hybrid Model of Autism Spectrum Disorder. Boil. Psychiatry 2016, 80, 120–128. [Google Scholar] [CrossRef]
- Frazier, T.W.; Youngstrom, E.A.; Speer, L.; Embacher, R.; Law, P.; Constantino, J.; Findling, R.L.; Hardan, A.Y.; Eng, C. Validation of Proposed DSM-5 Criteria for Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 28–40. [Google Scholar] [CrossRef]

| AQ Subscale | Mean | St. Dev. | Range | Skewness | Kurtosis | Tolerance |
|---|---|---|---|---|---|---|
| Attention Switching | 4.3 | 2.2 | 0–9 | −0.08 | −0.75 | 0.507 |
| Attention to detail | 4.8 | 2.2 | 0–10 | 0.05 | −0.45 | 0.847 |
| Communication | 2.0 | 2.0 | 0–8 | 0.87 | −0.17 | 0.463 |
| Imagination | 2.7 | 1.8 | 0–7 | 0.35 | −0.65 | 0.750 |
| Social skills | 2.3 | 2.2 | 0–9 | 1.03 | 0.30 | 0.413 |
| Action Facilitation Index | |||
|---|---|---|---|
| Coefficients | β | t | p-Level |
| Attention Switching | 0.051 | 0.316 | 0.752 |
| Attention to detail | −0.073 | −0.589 | 0.557 |
| Communication | −0.146 | −0.864 | 0.390 |
| Imagination | 0.219 | 1.648 | 0.103 |
| Social skills | −0.045 | −0.251 | 0.802 |
| Shape Facilitation Index | |||
| Coefficients | β | t | p-Level |
| Attention Switching | −0.375 | −2.392 | 0.019 |
| Attention to detail | 0.022 | 0.185 | 0.853 |
| Communication | 0.143 | 0.875 | 0.384 |
| Imagination | −0.077 | −0.597 | 0.552 |
| Social skills | 0.081 | 0.469 | 0.639 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, V.; Finisguerra, A.; Betti, S.; D’Argenio, G.; Urgesi, C. Autistic Traits Differently Account for Context-Based Predictions of Physical and Social Events. Brain Sci. 2020, 10, 418. https://doi.org/10.3390/brainsci10070418
Bianco V, Finisguerra A, Betti S, D’Argenio G, Urgesi C. Autistic Traits Differently Account for Context-Based Predictions of Physical and Social Events. Brain Sciences. 2020; 10(7):418. https://doi.org/10.3390/brainsci10070418
Chicago/Turabian StyleBianco, Valentina, Alessandra Finisguerra, Sonia Betti, Giulia D’Argenio, and Cosimo Urgesi. 2020. "Autistic Traits Differently Account for Context-Based Predictions of Physical and Social Events" Brain Sciences 10, no. 7: 418. https://doi.org/10.3390/brainsci10070418
APA StyleBianco, V., Finisguerra, A., Betti, S., D’Argenio, G., & Urgesi, C. (2020). Autistic Traits Differently Account for Context-Based Predictions of Physical and Social Events. Brain Sciences, 10(7), 418. https://doi.org/10.3390/brainsci10070418






