Neurodevelopmental Consequences of Pediatric Cancer and Its Treatment: The Role of Sleep
Abstract
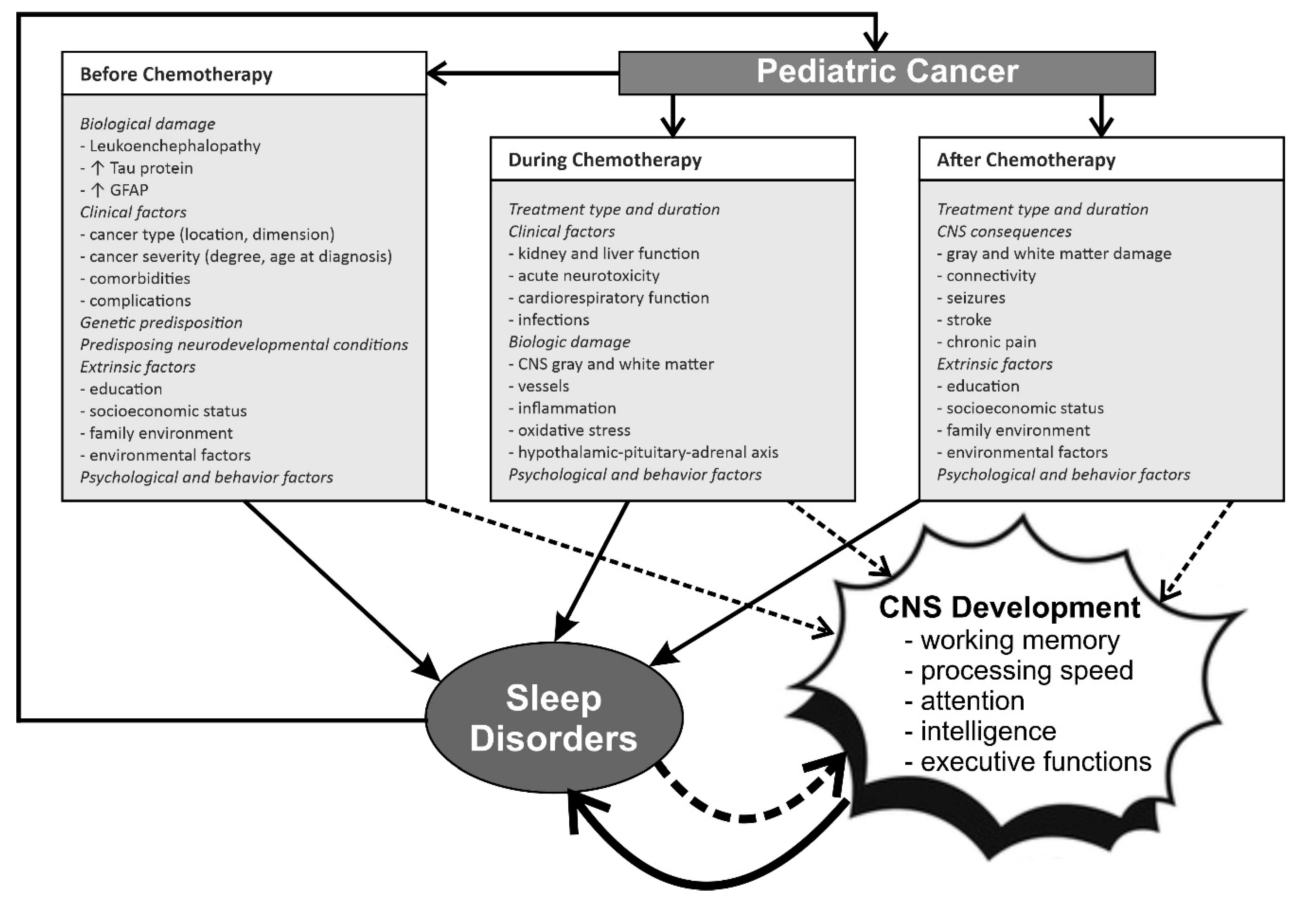
1. Introduction
1.1. Pediatric Cancer as a Cause of Neurodevelopmental Disorders
1.2. Pediatric Tumors Arising in Comorbidity with Neurodevelopmental Disorders
2. The Role of Sleep Disorders in Children with Cancer and Neurodevelopmental Disorders
2.1. Inflammation and Oxidative Stress
2.2. Neurogenesis and Synaptic Plasticity
2.3. The Role of Melatonin
2.4. Interaction between Sleep and Tau Protein
2.5. Brain Connectivity
3. The Effect of Sleep Disorder Treatment on Neurodevelopmental Disorders in Children with Cancer
3.1. Cognitive Behavioral Therapy
3.2. Melatonin
3.3. Other Treatment Options
3.4. Ventilatory Treatment of Respiratory Disorders in Sleep
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2013; National Cancer Institute: Bethesda, MD, USA, 2016. [Google Scholar]
- Walter, L.M.; Nixon, G.M.; Davey, M.J.; Downie, P.A.; Horne, R.S. Sleep and fatigue in pediatric oncology: A review of the literature. Sleep Med. Rev. 2015, 24, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Krull, K.R. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci. Biobehav. Rev. 2015, 53, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Sabin, N.D.; Reddick, W.E.; Bhojwani, D.; Liu, W.; Brinkman, T.M.; Glass, J.O.; Hwang, S.N.; Srivastava, D.; Pui, C.H.; et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: A longitudinal analysis. Lancet Haematol. 2016, 3, e456–e466. [Google Scholar] [CrossRef]
- Butler, R.W.; Haser, J.K. Neurocognitive effects of treatment for childhood cancer. Ment. Retard. Dev. Disabil. Res. Rev. 2006, 12, 184–191. [Google Scholar] [CrossRef]
- Ashford, J.; Schoffstall, C.; Reddick, W.E.; Leone, C.; Laningham, F.H.; Glass, J.O.; Pei, D.; Cheng, C.; Pui, C.H.; Conklin, H.M. Attention and working memory abilities in children treated for acute lymphoblastic leukemia. Cancer 2010, 116, 4638–4645. [Google Scholar] [CrossRef]
- Marusak, H.A.; Iadipaolo, A.S.; Harper, F.W.; Elrahal, F.; Taub, J.W.; Goldberg, E.; Rabinak, C.A. Neurodevelopmental consequences of pediatric cancer and its treatment: Applying an early adversity framework to understanding cognitive, behavioral, and emotional outcomes. Neuropsychol. Rev. 2018, 28, 123–175. [Google Scholar] [CrossRef]
- Duffner, P.K. Risk factors for cognitive decline in children treated for brain tumors. Eur. J. Paediatr. Neurol. Off. J. Eur. Paediatr. Neurol. Soc. 2010, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sleurs, C.; Deprez, S.; Emsell, L.; Lemiere, J.; Uyttebroeck, A. Chemotherapy-induced neurotoxicity in pediatric solid non-CNS tumor patients: An update on current state of research and recommended future directions. Crit. Rev. Oncol. Hematol. 2016, 103, 37–48. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, M.A.; van Mourik, R.; Schouten-van Meeteren, A.Y.; Grootenhuis, M.A.; Oosterlaan, J. Neurocognitive consequences of a paediatric brain tumour and its treatment: A meta-analysis. Dev. Med. Child Neurol. 2013, 55, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Kaleyias, J.; Manley, P.; Kothare, S.V. Sleep disorders in children with cancer. Semin. Pediatric Neurol. 2012, 19, 25–34. [Google Scholar] [CrossRef]
- Olson, K. Sleep-related disturbances among adolescents with cancer: A systematic review. Sleep Med. 2014, 15, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Brinkman, T.M.; Mulrooney, D.A.; Mzayek, Y.; Liu, W.; Banerjee, P.; Panoskaltsis-Mortari, A.; Srivastava, D.; Pui, C.H.; Robison, L.L.; et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2017, 123, 3410–3419. [Google Scholar] [CrossRef] [PubMed]
- Clanton, N.R.; Klosky, J.L.; Li, C.; Jain, N.; Srivastava, D.K.; Mulrooney, D.; Zeltzer, L.; Stovall, M.; Robison, L.L.; Krull, K.R. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2011, 117, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Rogers, V.E.; Zhu, S.; Ancoli-Israel, S.; Liu, L.; Mandrell, B.N.; Hinds, P.S. A pilot randomized controlled trial to improve sleep and fatigue in children with central nervous system tumors hospitalized for high-dose chemotherapy. Pediatric Blood Cancer 2019, 66, e27814. [Google Scholar] [CrossRef]
- van Kooten, J.; Maurice-Stam, H.; Schouten, A.Y.N.; van Vuurden, D.G.; Granzen, B.; Gidding, C.; de Ruiter, M.A.; van Litsenburg, R.R.L.; Grootenhuis, M.A. High occurrence of sleep problems in survivors of a childhood brain tumor with neurocognitive complaints: The association with psychosocial and behavioral executive functioning. Pediatric Blood Cancer 2019, 66, e27947. [Google Scholar] [CrossRef] [PubMed]
- Conklin, H.M.; Krull, K.R.; Reddick, W.E.; Pei, D.; Cheng, C.; Pui, C.H. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J. Natl. Cancer Inst. 2012, 104, 1386–1395. [Google Scholar] [CrossRef]
- Castellino, S.M.; Ullrich, N.J.; Whelen, M.J.; Lange, B.J. Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Price, J.; Kassam-Adams, N.; Alderfer, M.A.; Christofferson, J.; Kazak, A.E. Systematic Review: A Reevaluation and Update of the Integrative (Trajectory) Model of Pediatric Medical Traumatic Stress. J. Pediatric Psychol. 2016, 41, 86–97. [Google Scholar] [CrossRef]
- Turner, J.K.; Hutchinson, A.; Wilson, C. Correlates of post-traumatic growth following childhood and adolescent cancer: A systematic review and meta-analysis. Psycho-oncology 2018, 27, 1100–1109. [Google Scholar] [CrossRef]
- Dutil, C.; Walsh, J.J.; Featherstone, R.B.; Gunnell, K.E.; Tremblay, M.S.; Gruber, R.; Weiss, S.K.; Cote, K.A.; Sampson, M.; Chaput, J.P. Influence of sleep on developing brain functions and structures in children and adolescents: A systematic review. Sleep Med. Rev. 2018, 42, 184–201. [Google Scholar] [CrossRef]
- Robinson, J.L.; Erath, S.A.; Kana, R.K.; El-Sheikh, M. Neurophysiological differences in the adolescent brain following a single night of restricted sleep—A 7T fMRI study. Dev. Cogn. Neurosci. 2018, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Leung, L.H.; Kwong, D.L.; Chan, G.C.; Khong, P.L. Mapping radiation dose distribution on the fractional anisotropy map: Applications in the assessment of treatment-induced white matter injury. NeuroImage 2006, 31, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; Glass, J.O.; Helton, K.J.; Langston, J.W.; Li, C.S.; Pui, C.H. A quantitative MR imaging assessment of leukoencephalopathy in children treated for acute lymphoblastic leukemia without irradiation. Am. J. Neuroradiol. 2005, 26, 2371–2377. [Google Scholar] [PubMed]
- Reddick, W.E.; Glass, J.O.; Helton, K.J.; Langston, J.W.; Xiong, X.; Wu, S.; Pui, C.H. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. Am. J. Neuroradiol. 2005, 26, 1263–1269. [Google Scholar] [PubMed]
- Bornstein, M.H.; Scrimin, S.; Putnick, D.L.; Capello, F.; Haynes, O.M.; de Falco, S.; Carli, M.; Pillon, M. Neurodevelopmental functioning in very young children undergoing treatment for non-CNS cancers. J. Pediatric Psychol. 2012, 37, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Mullins, W.A.; O’Neil, S.H.; Wilson, K. Neuropsychological differences between survivors of supratentorial and infratentorial brain tumours. J. Intellect. Disabil. Res. 2011, 55, 30–40. [Google Scholar] [CrossRef]
- Ris, M.D.; Packer, R.; Goldwein, J.; Jones-Wallace, D.; Boyett, J.M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 3470–3476. [Google Scholar] [CrossRef]
- Riem, M.M.; Alink, L.R.; Out, D.; Van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J. Beating the brain about abuse: Empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev. Psychopathol. 2015, 27, 507–520. [Google Scholar] [CrossRef]
- Monje, M.; Dietrich, J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav. Brain Res. 2012, 227, 376–379. [Google Scholar] [CrossRef]
- Cole, P.D.; Finkelstein, Y.; Stevenson, K.E.; Blonquist, T.M.; Vijayanathan, V.; Silverman, L.B.; Neuberg, D.S.; Sallan, S.E.; Robaey, P.; Waber, D.P. Polymorphisms in Genes Related to Oxidative Stress Are Associated With Inferior Cognitive Function After Therapy for Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2205–2211. [Google Scholar] [CrossRef]
- Dietrich, J.; Prust, M.; Kaiser, J. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience 2015, 309, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Krull, K.R.; Hardy, K.K.; Kahalley, L.S.; Schuitema, I.; Kesler, S.R. Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Ogg, R.; Reddick, W.E.; Phillips, N.; Scoggins, M.; Glass, J.O.; Cheung, Y.T.; Pui, C.H.; Robison, L.L.; Hudson, M.M.; et al. Brain Network Connectivity and Executive Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Brain Connect. 2018, 8, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Fellah, S.; Cheung, Y.T.; Scoggins, M.A.; Zou, P.; Sabin, N.D.; Pui, C.H.; Robison, L.L.; Hudson, M.M.; Ogg, R.J.; Krull, K.R. Brain Activity Associated With Attention Deficits Following Chemotherapy for Childhood Acute Lymphoblastic Leukemia. J. Natl. Cancer Inst. 2019, 111, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Khan, R.B.; Liu, W.; Brinkman, T.M.; Edelmann, M.N.; Reddick, W.E.; Pei, D.; Panoskaltsis-Mortari, A.; Srivastava, D.; Cheng, C.; et al. Association of Cerebrospinal Fluid Biomarkers of Central Nervous System Injury With Neurocognitive and Brain Imaging Outcomes in Children Receiving Chemotherapy for Acute Lymphoblastic Leukemia. JAMA Oncol. 2018, 4, e180089. [Google Scholar] [CrossRef] [PubMed]
- Gapstur, R.; Gross, C.R.; Ness, K. Factors associated with sleep-wake disturbances in child and adult survivors of pediatric brain tumors: A review. Oncol. Nurs. Forum 2009, 36, 723–731. [Google Scholar] [CrossRef]
- Kesler, S.R.; Lacayo, N.J.; Jo, B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj. 2011, 25, 101–112. [Google Scholar] [CrossRef]
- Riggs, L.; Piscione, J.; Laughlin, S.; Cunningham, T.; Timmons, B.W.; Courneya, K.S.; Bartels, U.; Skocic, J.; de Medeiros, C.; Liu, F.; et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: A controlled clinical trial with crossover of training versus no training. Neuro-oncology 2017, 19, 440–450. [Google Scholar] [CrossRef]
- Baum, K.T.; Powell, S.K.; Jacobson, L.A.; Gragert, M.N.; Janzen, L.A.; Paltin, I.; Rey-Casserly, C.M.; Wilkening, G.N. Implementing guidelines: Proposed definitions of neuropsychology services in pediatric oncology. Pediatric Blood Cancer 2017, 64. [Google Scholar] [CrossRef]
- Kohman, R.A.; Rhodes, J.S. Neurogenesis, inflammation and behavior. Brain Behav. Immun. 2013, 27, 22–32. [Google Scholar] [CrossRef]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Wong-Siegel, J.R.; Johnson, K.J.; Gettinger, K.; Cousins, N.; McAmis, N.; Zamarione, A.; Druley, T.E. Congenital neurodevelopmental anomalies in pediatric and young adult cancer. Am. J. Med Genet. Part A 2017, 173, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.P. From neurodevelopment to neurodegeneration: The interaction of neurofibromin and valosin-containing protein/p97 in regulation of dendritic spine formation. J. Biomed. Sci. 2012, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Krull, K.R.; Bhojwani, D.; Conklin, H.M.; Pei, D.; Cheng, C.; Reddick, W.E.; Sandlund, J.T.; Pui, C.H. Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Krull, K.R.; Hockenberry, M.J.; Miketova, P.; Carey, M.; Moore, I.M. Chemotherapy-related changes in central nervous system phospholipids and neurocognitive function in childhood acute lymphoblastic leukemia. Leuk. Lymphoma 2013, 54, 535–540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shim, G.; Ricoul, M.; Hempel, W.M.; Azzam, E.I.; Sabatier, L. Crosstalk between telomere maintenance and radiation effects: A key player in the process of radiation-induced carcinogenesis. Mutat. Res. Rev. Mutat. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, M.A.; Oosterlaan, J.; Schouten-van Meeteren, A.Y.; Maurice-Stam, H.; van Vuurden, D.G.; Gidding, C.; Beek, L.R.; Granzen, B.; Caron, H.N.; Grootenhuis, M.A. Neurofeedback ineffective in paediatric brain tumour survivors: Results of a double-blind randomised placebo-controlled trial. Eur. J. Cancer 2016, 64, 62–73. [Google Scholar] [CrossRef]
- Trickett, J.; Heald, M.; Oliver, C.; Richards, C. A cross-syndrome cohort comparison of sleep disturbance in children with Smith-Magenis syndrome, Angelman syndrome, autism spectrum disorder and tuberous sclerosis complex. J. Neurodev. Disord. 2018, 10, 9. [Google Scholar] [CrossRef]
- Schubert, C.; Hong, S.; Natarajan, L.; Mills, P.J.; Dimsdale, J.E. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav. Immun. 2007, 21, 413–427. [Google Scholar] [CrossRef]
- Kesler, S.; Janelsins, M.; Koovakkattu, D.; Palesh, O.; Mustian, K.; Morrow, G.; Dhabhar, F.S. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav. Immun. 2013, 30, S109–S116. [Google Scholar] [CrossRef]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M.E. Microglia across the lifespan: From origin to function in brain development, plasticity and cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, I.; Akers, K.G.; Vergara, P.; Srinivasan, S.; Sakurai, T.; Sakaguchi, M. Memory consolidation during sleep and adult hippocampal neurogenesis. Neural Regen. Res. 2019, 14, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Yin, T.C.; Sung, P.H.; Chiang, J.Y.; Sun, C.K.; Yip, H.K. Melatonin enhances survival and preserves functional integrity of stem cells: A review. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Asif, N.; Iqbal, R.; Nazir, C.F. Human immune system during sleep. Am. J. Clin. Exp. Immunol. 2017, 6, 92–96. [Google Scholar]
- Walker, W.H., 2nd; Borniger, J.C. Molecular Mechanisms of Cancer-Induced Sleep Disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Seely, D.; Wu, P.; Fritz, H.; Kennedy, D.A.; Tsui, T.; Seely, A.J.; Mills, E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr. Cancer Ther. 2012, 11, 293–303. [Google Scholar] [CrossRef]
- Kurth, S.; Riedner, B.A.; Dean, D.C.; O’Muircheartaigh, J.; Huber, R.; Jenni, O.G.; Deoni, S.C.L.; LeBourgeois, M.K. Traveling Slow Oscillations During Sleep: A Marker of Brain Connectivity in Childhood. Sleep 2017, 40. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Pickett, L.A.; VanRyzin, J.W.; Kight, K.E. Surprising origins of sex differences in the brain. Horm. Behav. 2015, 76, 3–10. [Google Scholar] [CrossRef]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Merz, E.L.; Tomfohr-Madsen, L. Sleep Disruption in Pediatric Cancer Survivors: Conceptual Framework and Opportunities for Clinical Assessment and Behavioral Treatment. Am. J. Lifestyle Med. 2018, 12, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Levi, F.A. Systems Chronotherapeutics. Pharmacol. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.C.; Wang, M.; Mulrooney, D.A.; Srivastava, D.K.; Schwartz, L.A.; Edelstein, K.; Brinkman, T.M.; Zhou, E.S.; Howell, R.M.; Gibson, T.M.; et al. Sleep, emotional distress, and physical health in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Psycho-oncology 2019, 28, 903–912. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Jin, B.Z.; Ai, F.; Duan, C.H.; Lu, Y.Z.; Dong, T.F.; Fu, Q.L. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: A meta-analysis of randomized controlled trials. Cancer Chemother. Pharmacol. 2012, 69, 1213–1220. [Google Scholar] [CrossRef]
- Bruni, O.; Alonso-Alconada, D.; Besag, F.; Biran, V.; Braam, W.; Cortese, S.; Moavero, R.; Parisi, P.; Smits, M.; Van der Heijden, K.; et al. Current role of melatonin in pediatric neurology: Clinical recommendations. Eur. J. Paediatr. Neurol. Off. J. Eur. Paediatr. Neurol. Soc. 2015, 19, 122–133. [Google Scholar] [CrossRef]
- Bruni, O.; Angriman, M.; Melegari, M.G.; Ferri, R. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin. Pharmacother. 2019, 20, 2257–2271. [Google Scholar] [CrossRef]
- DelRosso, L.; Bruni, O. Treatment of pediatric restless legs syndrome. Adv. Pharmacol. 2019, 84, 237–253. [Google Scholar] [CrossRef]
- DelRosso, L.M.; Yi, T.; Chan, J.H.M.; Wrede, J.E.; Lockhart, C.T.; Ferri, R. Determinants of ferritin response to oral iron supplementation in children with sleep movement disorders. Sleep 2020, 43. [Google Scholar] [CrossRef] [PubMed]
- Angriman, M.; Cortese, S.; Bruni, O. Somatic and neuropsychiatric comorbidities in pediatric restless legs syndrome: A systematic review of the literature. Sleep Med. Rev. 2017, 34, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of pediatric obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef]
- Hvolby, A. Associations of sleep disturbance with ADHD: Implications for treatment. Atten. Deficit Hyperact. Disord. 2015, 7, 1–18. [Google Scholar] [CrossRef]
- Philby, M.F.; Macey, P.M.; Ma, R.A.; Kumar, R.; Gozal, D.; Kheirandish-Gozal, L. Reduced Regional Grey Matter Volumes in Pediatric Obstructive Sleep Apnea. Sci. Rep. 2017, 7, 44566. [Google Scholar] [CrossRef]
- Russo, S.; Fardell, J.E.; Signorelli, C.; Wakefield, C.E.; McLoone, J.K.; Cohn, R.J. Sleep Disturbances in Childhood Cancer Survivors. Pediatric Blood Cancer 2016, 63, 759–760. [Google Scholar] [CrossRef]
- Konstantinopoulou, S.; Tapia, I.E. Neurocognitive and Behavioural Outcomes Following Intervention for Obstructive Sleep Apnoea Syndrome in Children. Paediatr. Respir. Rev. 2016, 20, 51–54. [Google Scholar] [CrossRef]
- Xanthopoulos, M.S.; Kim, J.Y.; Blechner, M.; Chang, M.Y.; Menello, M.K.; Brown, C.; Matthews, E.; Weaver, T.E.; Shults, J.; Marcus, C.L. Self-Efficacy and Short-Term Adherence to Continuous Positive Airway Pressure Treatment in Children. Sleep 2017, 40. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogavero, M.P.; Bruni, O.; DelRosso, L.M.; Ferri, R. Neurodevelopmental Consequences of Pediatric Cancer and Its Treatment: The Role of Sleep. Brain Sci. 2020, 10, 411. https://doi.org/10.3390/brainsci10070411
Mogavero MP, Bruni O, DelRosso LM, Ferri R. Neurodevelopmental Consequences of Pediatric Cancer and Its Treatment: The Role of Sleep. Brain Sciences. 2020; 10(7):411. https://doi.org/10.3390/brainsci10070411
Chicago/Turabian StyleMogavero, Maria Paola, Oliviero Bruni, Lourdes M. DelRosso, and Raffaele Ferri. 2020. "Neurodevelopmental Consequences of Pediatric Cancer and Its Treatment: The Role of Sleep" Brain Sciences 10, no. 7: 411. https://doi.org/10.3390/brainsci10070411
APA StyleMogavero, M. P., Bruni, O., DelRosso, L. M., & Ferri, R. (2020). Neurodevelopmental Consequences of Pediatric Cancer and Its Treatment: The Role of Sleep. Brain Sciences, 10(7), 411. https://doi.org/10.3390/brainsci10070411





