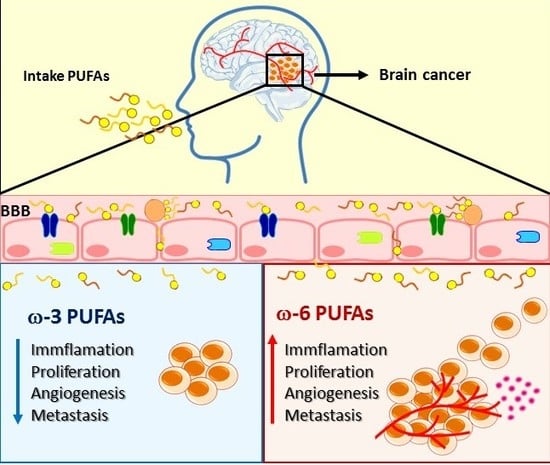
Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer
Abstract
1. Overview Brain Cancer
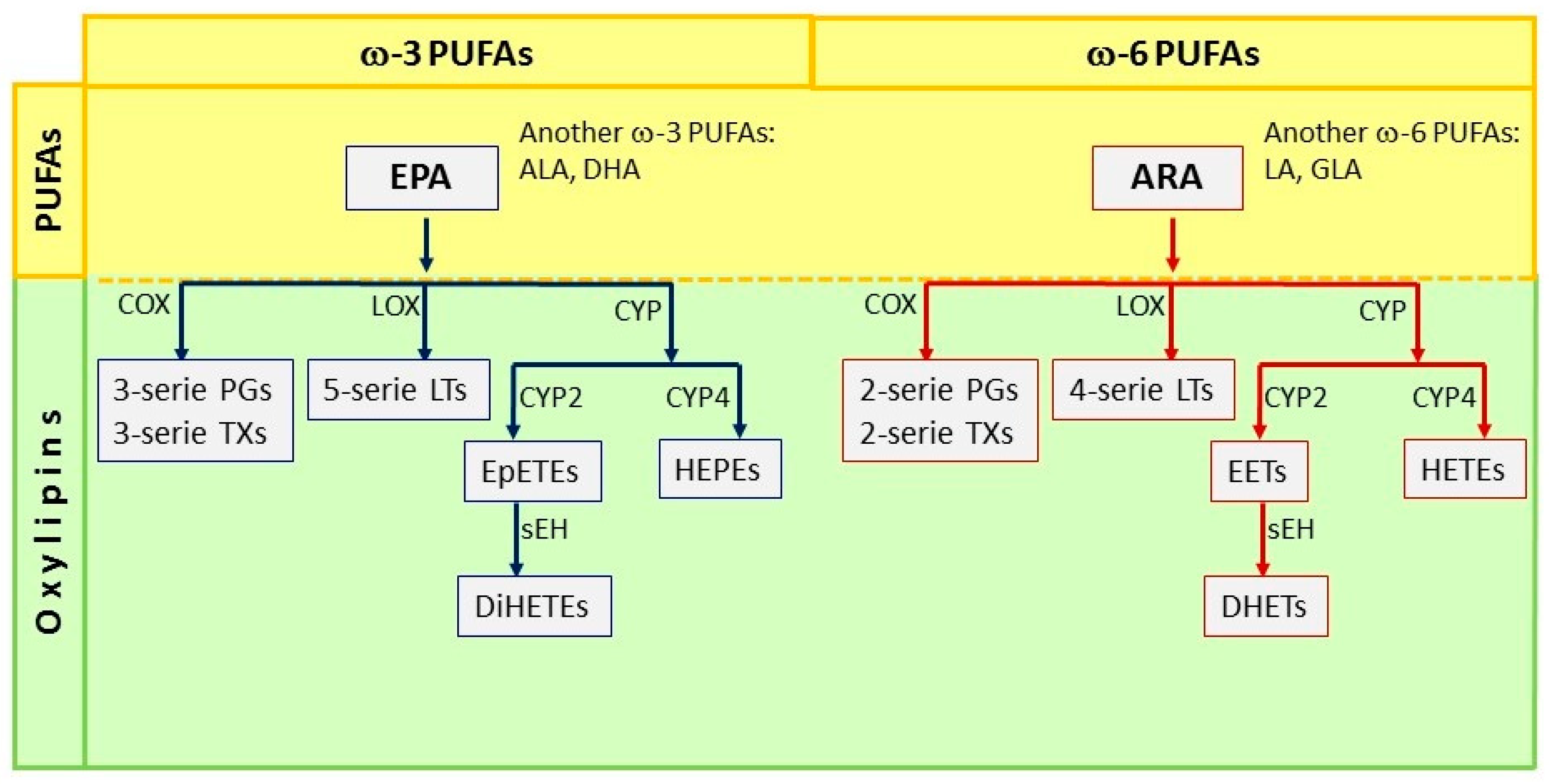
2. Polyunsaturated Fatty Acids (PUFAs)
3. Importance of PUFAs in Brain Functioning and Brain Cancer
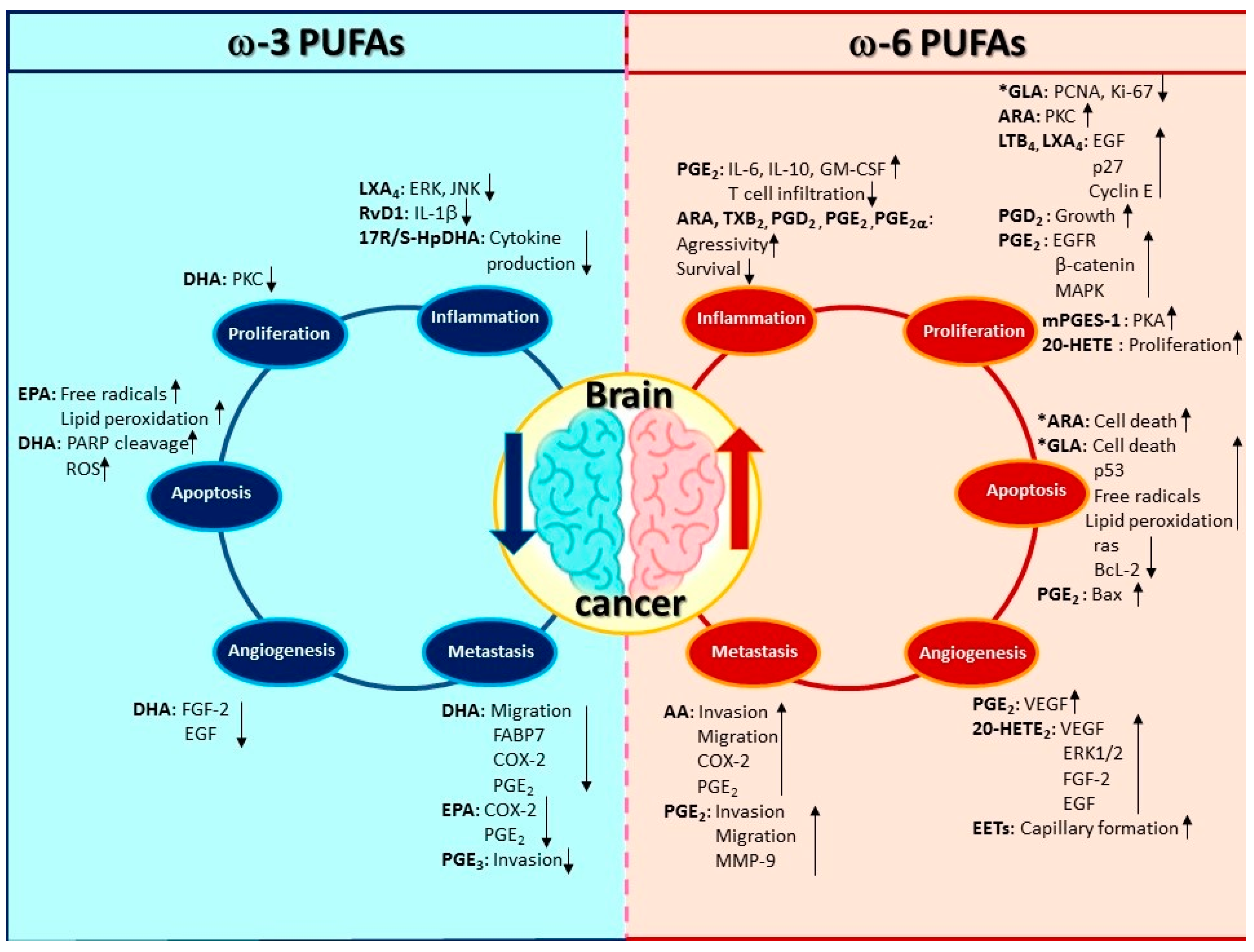
3.1. The Implication of PUFAs on Inflammation in Brain Cancer
3.2. Regulation of Proliferation in Brain Cancer Cells by PUFAs
3.3. The Effect of PUFAs on Apoptosis in Brain Cancer
3.4. Induction of Angiogenesis and Metastasis by PUFAs and Its Implication in Brain Cancer Progression
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALA | α-linolenic acid |
| ARA | Arachidonic acid |
| Bax | Bcl-2-associated X protein |
| BBB | Blood-Brain-Barrier |
| Bcl-2 | B-cell lymphoma 2 |
| B-FABP | Fatty acid-binding protein |
| BMEC | Cerebral microvascular endothelial cells |
| CBTRUS | Central Brain Tumor Registry of the United States |
| CD36 | Fatty acid translocase |
| CHD | Coronary heart disease |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| COX-2 | Cyclooxygenase 2 |
| CYP450 | Cytochrome P450 |
| CYP2s | Cytochrome P450 Family 2 |
| CYP2C11 | Cytochrome P450 Family 2 subfamily C member 11 |
| CYP4s | Cytochrome P450 Family 4 |
| CYP4A1 | Cytochrome P450 Family 4 subfamily A member 1 |
| CYP4X1 | Cytochrome P450 Family 4 subfamily X member 1 |
| DHA | Docosahexaenoic acid |
| DiHETEs | Dihydroxy eicosatetraenoic acids |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| EPA | Eicosapentaenoic acid |
| 16,17-EpDPE | 16,17-Epoxy docosapentaenoic acid |
| EpETEs | Epoxyeicosatetraenoic acids |
| EETs | Epoxy-eicosatrienoic cis-acids |
| 5,6-EET | 5,6-Epoxy-eicosatrienoic acid |
| 8,9-EET | 8,9-Epoxy-eicosatrienoic acid |
| 11,12-EET | 11,12-Epoxy-eicosatrienoic acid |
| 14,15-EET | 14,15-Epoxy-eicosatrienoic acid |
| EP1 | Prostaglandin E2 receptor 1 |
| EP2 | Prostaglandin E2 receptor 2 |
| EP4 | Prostaglandin E4 receptor 4 |
| EPHX2 | Epoxy-hydroxylase |
| ERK | Extracellular regulated kinase |
| FABP3 | Fatty acid-binding protein 3 |
| FABP4 | Fatty acid-binding protein 4 |
| FABP5 | Fatty acid-binding protein 5 |
| FABP7 | Fatty acid-binding protein 7 |
| FAT | Fatty acid translocase |
| FATP1 | Fatty acid transport protein 1 |
| FATP4 | Fatty acid transport protein 4 |
| FGF-2 | Fibroblast Growth Factor 2 |
| GBM | Glioblastoma |
| GLA | γ-linolenic acid |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HET0016 | N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine |
| HETEs | Hydroxy-eicosatetraenoic acids |
| 5-HETE | 5-Hydroxy-eicosatetraenoic acid |
| 12-HETE | 12-Hydroxy-eicosatetraenoic acid |
| 15-HETE | 15-Hydroxy-eicosatetraenoic acid |
| 19-HETE | 19-Hydroxy-eicosatetraenoic acid |
| 20-HETE | 20-Hydroxy-eicosatetraenoic acid |
| HEPEs | Hydroxy-eicosapentaenoic acids |
| HpETE | Hydroperoxyl-eicosatetraenoic acids |
| 5-HpETE | 5-Hydroperoxyl-eicosatetraenoic acid |
| IL-1 | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| JNK | c-Jun N-terminal kinase |
| LA | Linoleic acid |
| LDL | Low density lipoprotein |
| LDL-R | Low density lipoprotein receptor |
| Leuk 4 | 4-series leukotrienes |
| LOX | Lipoxygenase |
| LPC | Lysophosphatidylcholine |
| LPC-DHA | Lysophosphatidylcholine-Docosahexaenoic acid |
| LPC-PUFAs | Lysophosphatidylcholine-Polyunsaturated fatty acids |
| LPL | Lipoprotein lipase |
| LTs | Leukotrienes |
| LTB4 | Leukotriene B4 |
| LTB5 | Leukotriene B5 |
| LTC4 | Leukotriene C4 |
| LTC5 | Leukotriene C5 |
| LTD4 | Leukotriene D4 |
| LTD5 | Leukotriene D5 |
| LXA4 | Lipoxin A4 |
| MAPK | Mitogen-Activated Protein Kinases |
| Mfsd2a | Major facilitator superfamily domain-containing protein 2 |
| MMP-9 | Matrix metalloproteinase-9 |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| mRNA | Messenger Ribonucleic acid |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NE-DHA | Non esterified-Docosahexaenoic acid |
| NE-PUFAs | Non esterified-Polyunsaturated fatty acids |
| NFATC1 | Nuclear factor of activated T cells 1 |
| NSCs | Neuronal stem cells |
| 17-ODYA | 17-octadecynoic acid |
| p27 | Cyclin-dependent kinase inhibitor 1B |
| p38 | Mitogen-activated protein kinase |
| p53 | Tumor protein p53 |
| PARP | Poly (ADP-ribose) polymerase |
| PCNA | Proliferating cell nuclear antigen |
| pERK1/2 | Extracellular signal-regulated kinase 1/2 |
| PGEs | Prostaglandins |
| PGD2 | Prostaglandin D2 |
| PGE1 | Prostaglandin E1 |
| PGE2 | Prostaglandin E2 |
| PGE3 | Prostaglandin E3 |
| PGF2α | Prostaglandin F2α |
| PGF3 | Prostaglandin F3 |
| PGES | Prostaglandin E synthase |
| PI3K | Phosphatidylinositol 3-kinase |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PUFAs | Polyunsaturated fatty acids |
| -3 PUFAs | Omega-3-polyunsaturated fatty acids |
| -6 PUFAs | Omega-6-polyunsaturated fatty acids |
| Sp1 | Specificity protein 1 |
| Sp3 | Specificity protein 3 |
| ROS | Reactive oxygen species |
| 17RS-HpDHA | 17S-hydroxy-containing docosanoids |
| sEH | Soluble Epoxy-Hydrolase |
| TNF-α | Tumor necrosis factor-alpha |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling |
| TXs | Thromboxanes |
| TXA2 | Thromboxane A2 |
| TXA3 | Thromboxane A3 |
| TXB2 | Thromboxane B2 |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | World Health Organization |
References
- De Robles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; Germaine-Smith, C.S.; Day, L.; Lam, D.; Jette, N. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro-Oncology 2014, 17, 776–783. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. All Cancers—WHO 2018. In All Cancers 2018; International Agency for Research on Cancer: Lyon, France, 2018; Volume 876, p. 2. [Google Scholar]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Kochar, P. Brain Cancer: Implication to Disease, Therapeutic Strategies and Tumor Targeted Drug Delivery Approaches. Recent Pat. Anti. Cancer Drug Discov. 2018, 13, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Tandel, G.S.; Biswas, M.; Kakde, O.G.; Tiwari, A.; Suri, H.S.; Turk, M.; Laird, J.R.; Asare, C.K.; Ankrah, A.A.; Khanna, N.N.; et al. A Review on a Deep Learning Perspective in Brain Cancer Classification. Cancers 2019, 11, 111. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Newton, H.B. Primary brain tumors: Review of etiology, diagnosis and treatment. Am. Fam. Physician 1994, 49, 787–797. [Google Scholar]
- Chan, M.-Y.; Teo, W.-Y.; Seow, W.-T.; Tan, A.-M. Epidemiology, management and treatment outcome of medulloblastoma in singapore. Ann. Acad. Med. Singap. 2007, 36, 314–318. [Google Scholar]
- Brain Cancer & Brain Tumor: Symptoms & More, Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/articles/6149-brain-cancer-brain-tumor-adult (accessed on 23 November 2019).
- Fisher, J.L.; Schwartzbaum, J.; Wrensch, M.; Berger, M.S. Evaluation of Epidemiologic Evidence for Primary Adult Brain Tumor Risk Factors Using Evidence-Based Medicine. Prog. Neurol. Surg. 2006, 19, 54–79. [Google Scholar] [CrossRef]
- Karipidis, K.; Benke, G.; Sim, M.R.; Kauppinen, T.; Giles, G.G. Occupational exposure to ionizing and non-ionizing radiation and risk of glioma. Occup. Med. 2007, 57, 518–524. [Google Scholar] [CrossRef]
- Farrell, C.J.; Plotkin, S.R. Genetic Causes of Brain Tumors: Neurofibromatosis, Tuberous Sclerosis, von Hippel-Lindau, and Other Syndromes. Neurol. Clin. 2007, 25, 925–946. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Ahluwalia, M.S. Targeted therapy of brain metastases: Latest evidence and clinical implications. Ther. Adv. Med. Oncol. 2017, 9, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, K.J. Epidemiology and prognosis of brain metastases. Surg. Neurol. Int. 2013, 4, S192–S202. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, E.S.; Djalilian, H.R.; Cho, K.H.; Hall, W.A. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996, 78, 1781–1788. [Google Scholar] [CrossRef]
- DeAngelis, L.M. Anaplastic Glioma: How to Prognosticate Outcome and Choose a Treatment Strategy. J. Clin. Oncol. 2009, 27, 5861–5862. [Google Scholar] [CrossRef] [PubMed]
- Kebudi, R.; Ayan, I.; Görgün, Ö.; Agaoglu, F.Y.; Vural, S.; Darendeliler, E. Brain metastasis in pediatric extracranial solid tumors: Survey and literature review. J. Neuro-Oncol. 2005, 71, 43–48. [Google Scholar] [CrossRef]
- Qiu, J.; Shi, Z.; Jiang, J. Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov. Today 2017, 22, 148–156. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Bent, M.J.V.D. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 27, 15–16. [Google Scholar] [CrossRef]
- Imperato, J.P.; Paleologos, N.A.; Vick, N.A. Effects of treatment on long-term survivors with malignant astrocytomas. Ann. Neurol. 1990, 28, 818–822. [Google Scholar] [CrossRef]
- Laramore, G.; Martz, K.; Nelson, J.; Griffin, T.; Chang, C.; Horton, J. Radiation therapy oncology group (rtog) survival data on anaplastic astrocytomas of the brain: Does a more aggressive form of treatment adversely impact survival? Int. J. Radiat. Oncol. 1989, 17, 1351–1356. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Lee, G.S.; Pan, Y.; Scanlon, M.J.; Porter, C.J.H.; Nicolazzo, J.A. Fatty Acid–Binding Protein 5 Mediates the Uptake of Fatty Acids, but not Drugs, Into Human Brain Endothelial Cells. J. Pharm. Sci. 2018, 107, 1185–1193. [Google Scholar] [CrossRef]
- Bernardi, A.; Braganhol, E.; Jäger, E.; Figueiró, F.; Edelweiss, M.I.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Indomethacin-loaded nanocapsules treatment reduces in vivo glioblastoma growth in a rat glioma model. Cancer Lett. 2009, 281, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Burr, G.O.; Burr, M.M. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr. Rev. 2009, 31, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A.; Kim, H.Y. Discovery of essential fatty acids. J. Lipid Res. 2014, 56, 11–21. [Google Scholar] [CrossRef]
- Moghadasian, M.H. Advances in Dietary Enrichment with N-3 Fatty Acids. Crit. Rev. Food Sci. Nutr. 2008, 48, 402–410. [Google Scholar] [CrossRef]
- Kim, D.E.; Shang, X.; Assefa, A.; Keum, Y.S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 fatty acids for nutrition and medicine: Considering microalgae oil as a vegetarian source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef]
- Sontrop, J.M.; Campbell, M.K. ω-3 polyunsaturated fatty acids and depression: A review of the evidence and a methodological critique. Prev. Med. 2006, 42, 4–13. [Google Scholar] [CrossRef]
- Parker, G. Omega-3 Fatty Acids and Mood Disorders. Am. J. Psychiatry 2006, 163, 969–978. [Google Scholar] [CrossRef]
- Buddhachat, K.; Siengdee, P.; Chomdej, S.; Soontornvipart, K.; Nganvongpanit, K. Effects of different omega-3 sources, fish oil, krill oil, and green-lipped mussel against cytokine-mediated canine cartilage degradation. Vitr. Cell. Dev. Boil. Anim. 2017, 53, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the Cyclooxygenase Pathways of Prostanoid Biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.; Shankaranarayanan, P.; Schewe, T.; Nigam, S. Evidence for the presence of phospholipid hydroperoxide glutathione peroxidase in human platelets: Implications for its involvement in the regulatory network of the 12-lipoxygenase pathway of arachidonic acid metabolism. Biochem. J. 2000, 353, 91–100. [Google Scholar] [CrossRef]
- Feltenmark, S.; Gautam, N.; Brunnström, Å.; Griffiths, W.J.; Backman, L.; Edenius, C.; Lindbom, L.; Björkholm, M.; Claesson, H.-E. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc. Natl. Acad. Sci. USA 2008, 105, 680–685. [Google Scholar] [CrossRef]
- Yuan, D.; Zou, Q.; Yu, T.; Song, C.; Huang, S.; Chen, S.; Ren, Z.; Xu, A. Ancestral genetic complexity of arachidonic acid metabolism in Metazoa. Biochim. Biophys. Acta (BBA) Mol. Cell Boil. Lipids 2014, 1841, 1272–1284. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- DeFilippis, A.P.; Sperling, L.S. Understanding omega-3′s. Am. Hear. J. 2006, 151, 564–570. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, D.; Cao, D.; Xie, P. Association between processed meat and red meat consumption and risk for glioma: A meta-analysis from 14 articles. Nutrition 2015, 31, 45–50. [Google Scholar] [CrossRef]
- Holick, C.N.; Giovannucci, E.L.; Rosner, B.; Stampfer, M.J.; Michaud, M.S. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am. J. Clin. Nutr. 2007, 85, 877–886. [Google Scholar] [CrossRef]
- Lian, W.; Wang, R.; Xing, B.; Yao, Y. Fish intake and the risk of brain tumor: A meta-analysis with systematic review. Nutr. J. 2017, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Yépez, S.; Tirado-Rodriguez, A.B.; Hankinson, O. Papel de las dietas ricas en omega-3 y omega-6 en el desarrollo del cancer. Med. Bull. Child. Hosp. Mex. 2016, 73, 446–456. [Google Scholar]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Steingrimsdottir, L.; Ueshima, H.; Shin, C.; Curb, J.D.; Evans, R.W.; Hauksdottir, A.M.; Kadota, A.; Choo, J.; Masaki, K.; et al. Serum levels of marine-derived n-3 fatty acids in Icelanders, Japanese, Koreans, and Americans--a descriptive epidemiologic study. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 11–16. [Google Scholar] [CrossRef]
- Stark, K.D.; Van Elswyk, M.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- De Turco, E.B.; Gordon, W.C.; Peyman, G.A.; Bazan, N.G. Preferential uptake and metabolism of docosahexaenoic acid in membrane phospholipids from rod and cone photoreceptor cells of human and monkey retinas. J. Neurosci. Res. 1990, 27, 522–532. [Google Scholar] [CrossRef]
- Ferdouse, A.; Leng, S.; Winter, T.; Aukema, H.M. The Brain Oxylipin Profile Is Resistant to Modulation by Dietary n-6 and n-3 Polyunsaturated Fatty Acids in Male and Female Rats. Lipids 2019, 54, 67–80. [Google Scholar] [CrossRef]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Lacombe, R.S.; Bazinet, R.P. Mechanisms regulating brain docosahexaenoic acid uptake. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 71–77. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Hennebelle, M.; Schuster, S.; Keyes, G.S.; Johnson, C.D.; Kirpich, I.A.; Dahlen, J.E.; Horowitz, M.S.; Zamora, D.; Feldstein, A.E.; et al. Effects of diets enriched in linoleic acid and its peroxidation products on brain fatty acids, oxylipins, and aldehydes in mice. Biochim. Biophys. Acta (BBA) Mol. Cell Boil. Lipids 2018, 1863, 1206–1213. [Google Scholar] [CrossRef]
- Su, K.P. Biological Mechanism of Antidepressant Effect of Omega–3 Fatty Acids: How Does Fish Oil Act as a ‘Mind-Body Interface’? Neurosignals 2009, 17, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Weisinger, H.S.; Vingrys, A.J.; Sinclair, A.J. Dietary manipulation of long-chain polyunsaturated fatty acids in the retina and brain of guinea pigs. Lipids 1995, 30, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Nariai, T.; DeGeorge, J.J.; Greig, N.H.; Genka, S.; Rapoport, S.I.; Purdon, A.D. Differences in rates of incorporation of intravenously injected radiolabeled fatty acids into phospholipids of intracerebrally implanted tumor and brain in awake rats. Clin. Exp. Metastasis 1994, 12, 213–225. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Pawełczyk, T.; Grancow-Grabka, M.; Kotlicka-Antczak, M.; Trafalska, E.; Gębski, P.; Szemraj, J.; Żurner, N.; Pawełczyk, A. Omega-3 fatty acids in first-episode schizophrenia—A randomized controlled study of efficacy and relapse prevention (OFFER): Rationale, design, and methods. BMC Psychiatry 2015, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Ochiai, Y.; Uchida, Y.; Ohtsuki, S.; Tachikawa, M.; Aizawa, S.; Terasaki, T. The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport. J. Neurochem. 2017, 141, 400–412. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Lee, G.S.; Kappler, K.; Porter, C.J.H.; Scanlon, M.J.; Nicolazzo, J.A. Fatty Acid Binding Proteins Expressed at the Human Blood–Brain Barrier Bind Drugs in an Isoform-Specific Manner. Pharm. Res. 2015, 32, 3432–3446. [Google Scholar] [CrossRef]
- Pan, Y.; Scanlon, M.J.; Owada, Y.; Yamamoto, Y.; Porter, C.J.H.; Nicolazzo, J.A. Fatty Acid-Binding Protein 5 Facilitates the Blood–Brain Barrier Transport of Docosahexaenoic Acid. Mol. Pharm. 2015, 12, 4375–4385. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E. Blood-brain barrier and brain fatty acid uptake: Role of arachidonic acid and PGE2. J. Neurochem. 2015, 135, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, J.H.; Zimmerman, A.W. Fatty Acid-Binding Proteins of Nervous Tissue. J. Mol. Neurosci. 2001, 16, 133–142. [Google Scholar] [CrossRef]
- Murphy, E.J.; Owada, Y.; Kitanaka, N.; Kondo, H.; Glatz, J.F.C. Brain Arachidonic Acid Incorporation Is Decreased in Heart Fatty Acid Binding Protein Gene-Ablated Mice. Biochemistry 2005, 44, 6350–6360. [Google Scholar] [CrossRef]
- Liu, R.Z.; Mita, R.; Beaulieu, M.; Gao, Z.; Godbout, R. Fatty acid binding proteins in brain development and disease. Int. J. Dev. Boil. 2010, 54, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Almaguel, F.G.; Bu, L.; De Leon, D.D.; De Leon, M. Expression of E-FABP in PC12 cells increases neurite extension during differentiation: Involvement of n-3 and n-6 fatty acids. J. Neurochem. 2008, 106, 2015–2029. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Sánchez, R.; Sali, A.; Heintz, N. Ligand Specificity of Brain Lipid-binding Protein. J. Boil. Chem. 1996, 271, 24711–24719. [Google Scholar] [CrossRef]
- Fukumoto, K.-I.; Takagi, N.; Yamamoto, R.; Moriyama, Y.; Takeo, S.; Tanonaka, K. Prostanoid EP1 receptor antagonist reduces blood–brain barrier leakage after cerebral ischemia. Eur. J. Pharmacol. 2010, 640, 82–86. [Google Scholar] [CrossRef]
- Jiang, J.; Dingledine, R.; And, J.J. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol. Sci. 2013, 34, 413–423. [Google Scholar] [CrossRef]
- Liu, J.J.; Green, P.; Mann, J.J.; Rapoport, S.I.; Sublette, M.E. Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. 2014, 1597, 220–246. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Vattulainen, I. Molecular Mechanism for Lipid Flip-Flops. J. Phys. Chem. B 2007, 111, 13554–13559. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Shie, F.-S.; Breyer, R.M.; Montine, T.J. Microglia Lacking E Prostanoid Receptor Subtype 2 Have Enhanced Aβ Phagocytosis yet Lack Aβ-Activated Neurotoxicity. Am. J. Pathol. 2005, 166, 1163–1172. [Google Scholar] [CrossRef]
- Payner, T.; Leaver, H.A.; Knapp, B.; Whittle, I.R.; Trifan, O.C.; Miller, S.; Rizzo, M.T. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E2-dependent activation of type II protein kinase A. Mol. Cancer Ther. 2006, 5, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Bylund, J.; Zhang, C.; Harder, D.R. Identification of a novel cytochrome P450, CYP4X1, with unique localization specific to the brain. Biochem. Biophys. Res. Commun. 2002, 296, 677–684. [Google Scholar] [CrossRef]
- Guo, A.M.; Sheng, J.; Scicli, G.M.; Arbab, A.S.; Lehman, N.L.; Edwards, P.A.; Falck, J.R.; Roman, R.J.; Scicli, A.G. Expression of CYP4A1 in U251 human glioma cell induces hyperproliferative phenotype in vitro and rapidly growing tumors in vivo. J. Pharmacol. Exp. Ther. 2008, 327, 10–19. [Google Scholar] [CrossRef]
- Shono, T.; Tofilon, P.J.; Bruner, J.M.; Owolabi, O.; Lang, F.F. Cyclooxygenase-2 expression in human gliomas: Prognostic significance and molecular correlations. Cancer Res. 2001, 61, 4375–4381. [Google Scholar]
- Svensson, C.I.; Zattoni, M.; Serhan, C.N. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 2007, 204, 245–252. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Gomes, R.N.; Panagopoulos, A.T.; De Almeida, F.G.; Veiga, J.C.E.; Colquhoun, A. Opposing roles of PGD 2 in GBM. Prostaglandins Other Lipid Mediat. 2018, 134, 66–76. [Google Scholar] [CrossRef]
- Authier, A.; Farrand, K.J.; Broadley, K.W.; Ancelet, L.R.; Hunn, M.K.; Stone, S.; McConnell, M.J.; Hermans, I.F. Enhanced immunosuppression by therapy-exposed glioblastoma multiforme tumor cells. Int. J. Cancer 2014, 136, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, M.; Chen, H.; Emara, M.; Godbout, R. ω-3 and ω-6 Fatty Acids Modulate Conventional and Atypical Protein Kinase C Activities in a Brain Fatty Acid Binding Protein Dependent Manner in Glioblastoma Multiforme. Nutrients 2018, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Leaver, H.; Bell, H.; Rizzo, M.; Ironside, J.W.; Gregor, A.; Wharton, S.; Whittle, I. Antitumour and pro-apoptotic actions of highly unsaturated fatty acids in glioma. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 19–29. [Google Scholar] [CrossRef][Green Version]
- Matsuo, M.; Yoshida, N.; Zaitsu, M.; Ishii, K.; Hamasaki, Y. Inhibition of human glioma cell growth by a PHS-2 inhibitor, NS398, and a prostaglandin E receptor subtype EP1-selective antagonist, SC51089. J. Neuro-Oncol. 2004, 66, 285–292. [Google Scholar] [CrossRef]
- Brocard, E.; Oizel, K.; Lalier, L.; Pecqueur, C.; Paris, F.; Vallette, F.M.; Oliver, L. Radiation-induced PGE2 sustains human glioma cell growth and survival through EGF signaling. Oncotarget 2015, 6, 6840–6849. [Google Scholar] [CrossRef]
- Cook, P.J.; Thomas, R.; Kingsley, P.J.; Shimizu, F.; Montrose, D.C.; Marnett, L.J.; Tabar, V.S.; Dannenberg, A.J.; Benezra, R. Cox-2-derived PGE2induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro-Oncol. 2016, 18, 1379–1389. [Google Scholar] [CrossRef]
- Gomes, R.N.; Colquhoun, A. E series prostaglandins alter the proliferative, apoptotic and migratory properties of T98G human glioma cells in vitro. Lipids Heal. Dis. 2012, 11, 171. [Google Scholar] [CrossRef]
- Wada, K.; Arita, M.; Nakajima, A.; Katayama, K.; Kudo, C.; Kamisaki, Y.; Serhan, C.N. Leukotriene B 4 and lipoxin A 4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006, 20, 1785–1792. [Google Scholar] [CrossRef]
- Kim, S.; Jing, K.; Shin, S.; Jeong, S.; Han, S.H.; Oh, H.; Yoo, Y.S.; Han, J.; Jeon, Y.J.; Heo, J.Y.; et al. ω3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: In vitro and in vivo. Oncol. Rep. 2018, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Vartak, S.; Robbins, M.E.; Spector, A.A. Polyunsaturated fatty acids increase the sensitivity of 36B10 rat astrocytoma cells to radiation-induced cell kill. Lipids 1997, 32, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N.; Huang, Y.S.; Bégin, M.E.; Ells, G.; Horrobin, D.F. Uptake and distribution of cis-unsaturated fatty acids and their effect on free radical generation in normal and tumor cells in vitro. Free Radic. Boil. Med. 1987, 3, 9–14. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, W.; Che, J.; Yuan, D.; Zhang, L.; Sun, Y.; Yue, X.; Xiao, L.; Jin, Y. 12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-κB pathway in ovarian cancer. Cancer Manag. Res. 2018, 10, 5825–5838. [Google Scholar] [CrossRef]
- Bell, H.S.; Wharton, S.B.; Leaver, H.A.; Whittle, I.R. Effects of N-6 essential fatty acids on glioma invasion and growth: Experimental studies with glioma spheroids in collagen gels. J. Neurosurg. 1999, 91, 989–996. [Google Scholar] [CrossRef]
- Lalier, L.; Cartron, P.F.; Pedelaborde, F.; Olivier, C.; Loussouarn, D.; Martin, S.A.; Meflah, K.; Menanteau, J.; Vallette, F.M. Increase in PGE2 biosynthesis induces a Bax dependent apoptosis correlated to patients’ survival in glioblastoma multiforme. Oncogene 2007, 26, 4999–5009. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, J.; Lyu, F.; Panigrahy, D.; Ferrara, K.W.; Hammock, B.; Zhang, G. ω-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014, 113, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chang, C.M.; Gao, H.; Shu, H.K.G. Epidermal growth factor-dependent cyclooxygenase-2 induction in gliomas requires protein kinase C-δ. Oncogene 2009, 28, 1410–1420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, F.; Bhat, K.; Doucette, M.; Zhou, S.; Gu, Y.; Law, B.; Liu, X.; Wong, E.T.; Kang, J.X.; Hsieh, T.C.; et al. Docosahexaenoic acid (DHA) sensitizes brain tumor cells to etoposide-induced apoptosis. Curr. Mol. Med. 2011, 11, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Shu, H.K.G. EGFR activation results in enhanced cyclooxygenase-2 expression through p38 mitogen-activated protein kinase–dependent activation of the SP1/SP3 transcription factors in human gliomas. Cancer Res. 2007, 67, 6121–6129. [Google Scholar] [CrossRef]
- Chen, P.; Guo, M.; Wygle, D.; Edwards, P.A.; Falck, J.R.; Roman, R.J.; Scicli, A.G. Inhibitors of Cytochrome P450 4A Suppress Angiogenic Responses. Am. J. Pathol. 2005, 166, 615–624. [Google Scholar] [CrossRef]
- Alkayed, N.J.; Narayanan, J.; Gebremedhin, D.; Medhora, M.; Roman, R.J.; Harder, D.R. Molecular Characterization of an Arachidonic Acid Epoxygenase in Rat Brain Astrocytes. Stroke 1996, 27, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Harder, D.R. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke 2002, 33, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Zagorac, A.; Jakovcevic, D.; Gebremedhin, D.; Harder, D.R. Antiangiogenic Effect of Inhibitors of Cytochrome P450 on Rats with Glioblastoma Multiforme. Br. J. Pharmacol. 2008, 28, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Mita, R.; Beaulieu, M.J.; Field, C.; Godbout, R. Brain Fatty Acid-binding Protein and ω-3/ω-6 Fatty Acids: Mechanistic insight into malignant glioma cell migration. J. Biol. Chem. 2010, 285, 37005–37015. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Watters, A.; Cheng, N.; Perry, C.E.; Xu, K.; Alicea, G.M.; Parris, J.L.; Baraban, E.; Ray, P.; Nayak, A.; et al. Polyunsaturated Fatty Acids from Astrocytes Activate PPARγ Signaling in Cancer Cells to Promote Brain Metastasis. Cancer Discov. 2019, 9, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- Denkins, Y.; Kempf, D.; Ferniz, M.; Nileshwar, S.; Marchetti, D. Role of ω-3 polyunsaturated fatty acids on cyclooxygenase-2 metabolism in brain-metastatic melanoma. J. Lipid Res. 2005, 4, 1278–1284. [Google Scholar] [CrossRef]
- Chiu, W.T.; Shen, S.C.; Chow, J.M.; Lin, C.W.; Shia, L.T.; Chen, Y.C. Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via ERK-dependent COX-2/PGE2 activation. Neurobiol. Dis. 2010, 37, 118–129. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Li, J.; Zhang, W.; Ren, F.; Yue, W. NFATc1 activation promotes the invasion of U251 human glioblastoma multiforme cells through COX-2. Int. J. Mol. Med. 2015, 35, 1333–1340. [Google Scholar] [CrossRef]
- Gomes, R.N.; Colquhoun, A. CBIO-11EFFECT OF PROSTAGLANDIN E2ON CELL MIGRATION IN U251 MG AND U87 MG HUMAN GLIOMA CELLS. Neuro-Oncology 2015, 17, v57. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Tong, Z.; Vega, O.M.; Morales-Martínez, M.; Abkenari, S.; Pedraza-Chaverri, J.; Huerta-Yepez, S. Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer. Brain Sci. 2020, 10, 381. https://doi.org/10.3390/brainsci10060381
Montecillo-Aguado M, Tirado-Rodriguez B, Tong Z, Vega OM, Morales-Martínez M, Abkenari S, Pedraza-Chaverri J, Huerta-Yepez S. Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer. Brain Sciences. 2020; 10(6):381. https://doi.org/10.3390/brainsci10060381
Chicago/Turabian StyleMontecillo-Aguado, Mayra, Belen Tirado-Rodriguez, Zhen Tong, Owen M. Vega, Mario Morales-Martínez, Shaheen Abkenari, José Pedraza-Chaverri, and Sara Huerta-Yepez. 2020. "Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer" Brain Sciences 10, no. 6: 381. https://doi.org/10.3390/brainsci10060381
APA StyleMontecillo-Aguado, M., Tirado-Rodriguez, B., Tong, Z., Vega, O. M., Morales-Martínez, M., Abkenari, S., Pedraza-Chaverri, J., & Huerta-Yepez, S. (2020). Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer. Brain Sciences, 10(6), 381. https://doi.org/10.3390/brainsci10060381