Extensive Evaluation of Morphological Statistical Harmonization for Brain Age Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Morphological Features
- volume, intensity mean, standard deviation, minimum, maximum, and range of 40 sub-cortical brain structures and white matter parcellation of brain cortex;
- volume, surface area, Gaussian curvature, mean curvature, curvature index, folding index, thickness mean, and thickness standard deviation for the 34 cortical brain regions of each hemisphere; and,
- global brain metrics, including surface and volume statistics of each hemisphere; total cerebellar gray and white matter volume, brainstem volume, corpus callosum volume, and white matter hypointensities.
- global metrics (21 features);
- gortical metrics (544 features resulting from eight metrics for the 68 cortical regions of interest);
- sub-cortical metrics (240 features resulting from 6 metrics for the 40 sub-cortical regions of interest); and,
- WM metrics (408 features resulting from six metrics for the 68 regions of interest).
2.3. Overview of the Framework
- compare multiple harmonization strategies;
- identify the most effective age predictive model;
- select only the most significant features among the total set of features; and,
- compare the stability of the most age-related anatomical regions of interest across harmonization strategies.
2.4. Statistical Harmonization
2.5. Age Prediction
- Mean Absolute Error (MAE):
- Coefficient of determination ():
2.5.1. Support Vector Regression
2.5.2. Random Forest
2.5.3. Lasso
2.6. Feature Importance
- robust rank aggregation (RRA) algorithm [49] to combine the multiple base rankers into a final aggregated ranked list for RF and SVR; and,
- frequency-based criterion with a fixed threshold to retain the most frequent features selected in each round of Lasso regression.
2.7. Stability Index
2.8. Age Models
- for the ideal model;
- for an age overestimation prevalence of the model;
- for an age underestimation prevalence of the model.
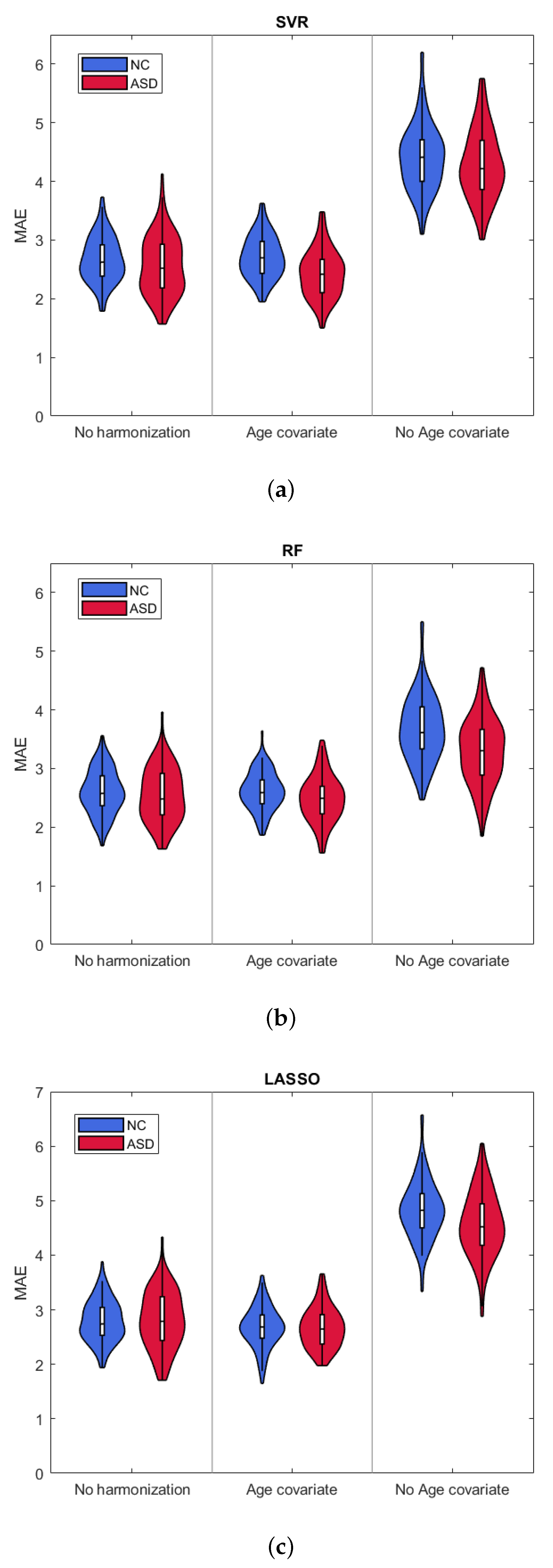
3. Results
3.1. Age Prediction
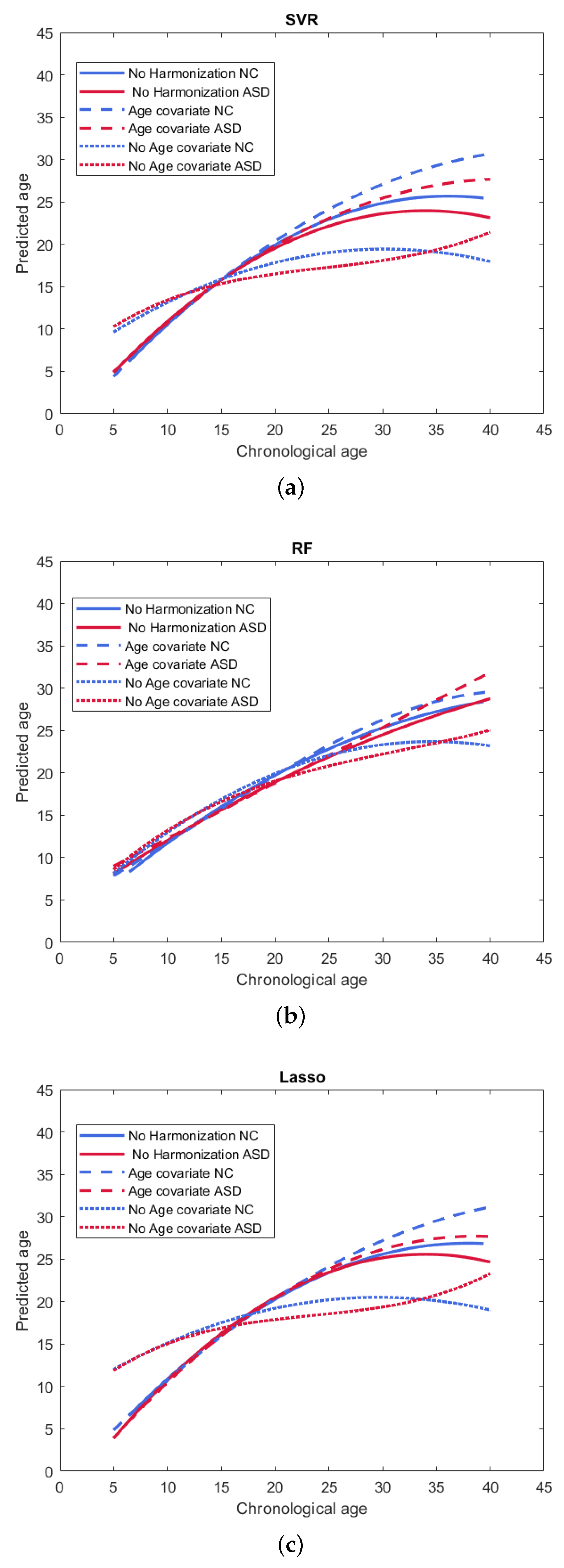
3.2. Age Models
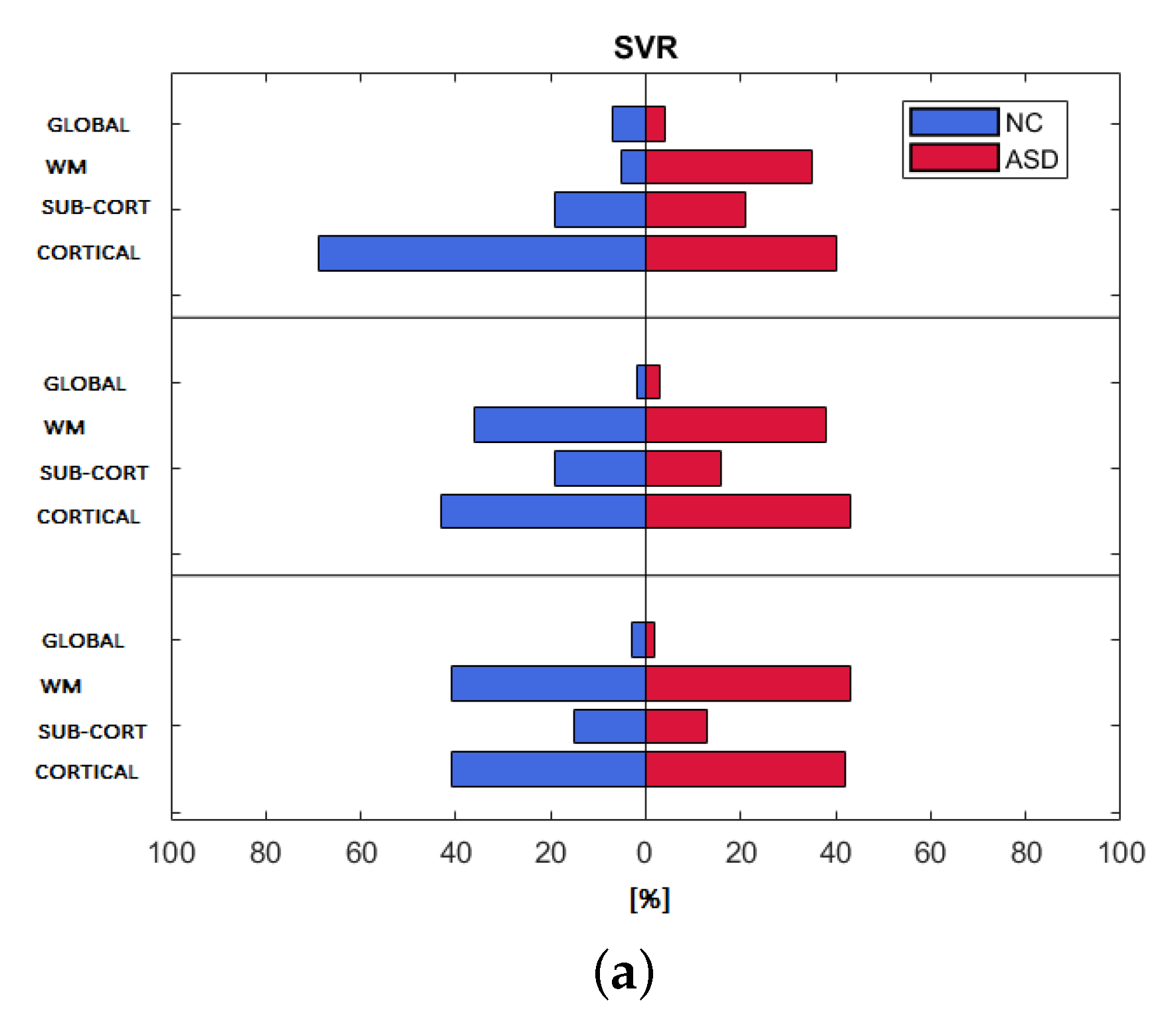
3.3. Feature Importance
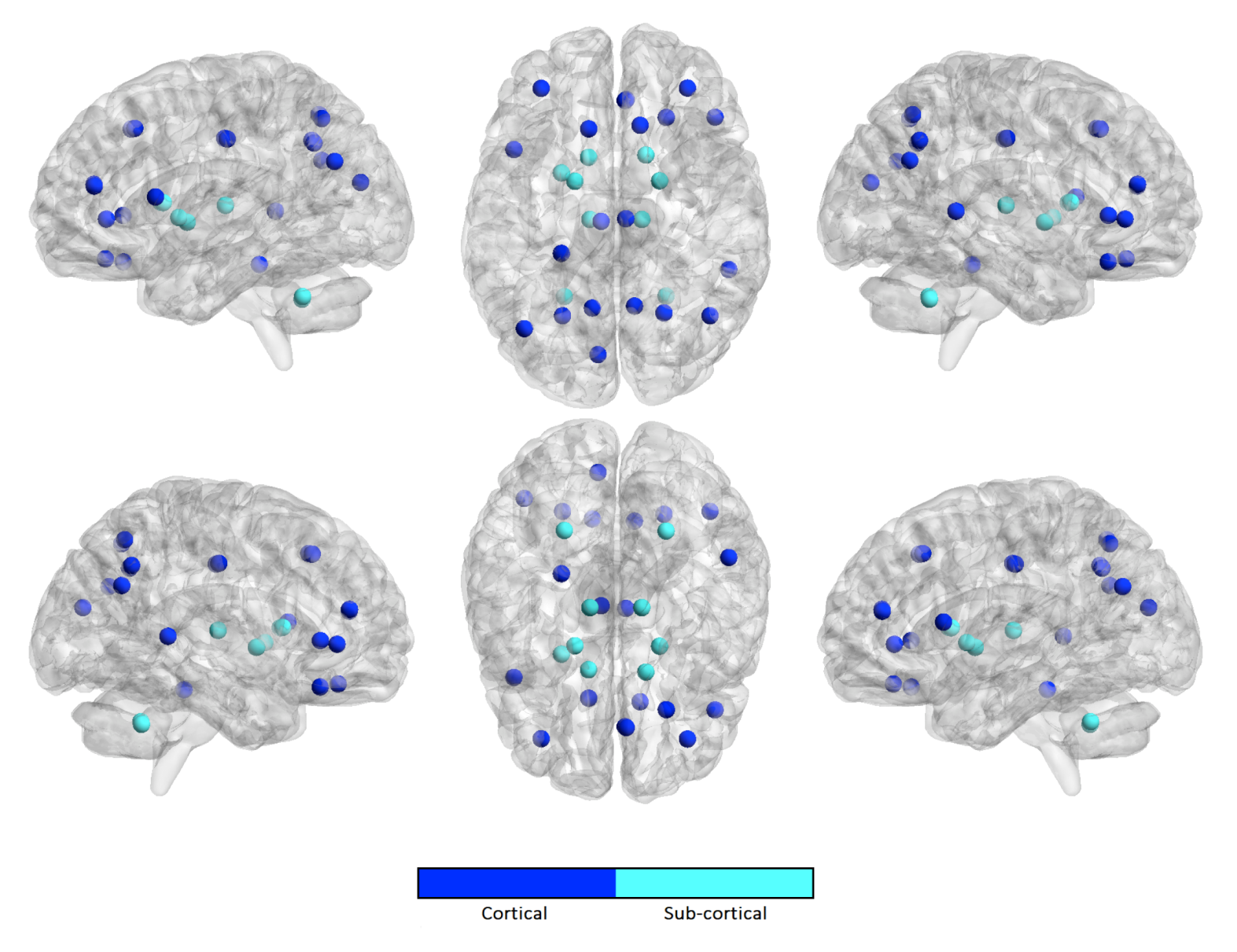
3.4. Stability Index
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ziegler, G.; Dahnke, R.; Jäncke, L.; Yotter, R.A.; May, A.; Gaser, C. Brain structural trajectories over the adult lifespan. Hum. Brain Mapp. 2012, 33, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Asato, M.; Terwilliger, R.; Woo, J.; Luna, B. White matter development in adolescence: A DTI study. Cereb. Cortex 2010, 20, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Inder, T.; Neil, J.; Dierker, D.; Harwell, J.; Van Essen, D. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 13135–13140. [Google Scholar] [CrossRef]
- Raznahan, A.; Shaw, P.; Lalonde, F.; Stockman, M.; Wallace, G.L.; Greenstein, D.; Clasen, L.; Gogtay, N.; Giedd, J.N. How does your cortex grow? J. Neurosci. 2011, 31, 7174–7177. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, L.M.; Langen, M.; Oranje, B.; Durston, S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage 2014, 87, 120–126. [Google Scholar] [CrossRef]
- Vértes, P.E.; Bullmore, E.T. Annual research review: Growth connectomics–the organization and reorganization of brain networks during normal and abnormal development. J. Child Psychol. Psychiatry 2015, 56, 299–320. [Google Scholar] [CrossRef]
- Tamnes, C.K.; Herting, M.M.; Goddings, A.L.; Meuwese, R.; Blakemore, S.J.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; Crone, E.A.; et al. Development of the cerebral cortex across adolescence: A multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017, 37, 3402–3412. [Google Scholar] [CrossRef]
- Tamnes, C.K.; stby, Y.; Walhovd, K.B.; Westlye, L.T.; Due-Tønnessen, P.; Fjell, A.M. Intellectual abilities and white matter microstructure in development: A diffusion tensor imaging study. Hum. Brain Mapp. 2010, 31, 1609–1625. [Google Scholar] [CrossRef]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Agartz, I.; Salat, D.H.; Greve, D.N.; Fischl, B.; et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex 2009, 19, 2001–2012. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev. Neurosci. 2010, 21, 187–222. [Google Scholar] [CrossRef]
- Gaser, C.; Franke, K.; Klöppel, S.; Koutsouleris, N.; Sauer, H.; Initiative, A.D.N. BrainAGE in mild cognitive impaired patients: Predicting the conversion to Alzheimer’s disease. PLoS ONE 2013, 8, e67346. [Google Scholar] [CrossRef] [PubMed]
- Kruggel, F.; Masaki, F.; Solodkin, A.; Initiative, A.D.N. Analysis of longitudinal diffusion-weighted images in healthy and pathological aging: An ADNI study. J. Neurosci. Methods 2017, 278, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mensen, V.T.; Wierenga, L.M.; van Dijk, S.; Rijks, Y.; Oranje, B.; Mandl, R.C.; Durston, S. Development of cortical thickness and surface area in autism spectrum disorder. Neuroimage Clin. 2017, 13, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, B.; Itahashi, T.; Fujino, J.; Ohta, H.; Takashio, O.; Nakamura, M.; Kato, N.; Mimura, M.; Hashimoto, R.; Aoki, Y.Y. Cortical surface architecture endophenotype and correlates of clinical diagnosis of autism spectrum disorder. Psychiatry Clin. Neurosci. 2019, 73, 409–415. [Google Scholar] [CrossRef]
- Cole, J.H.; Franke, K. Predicting age using neuroimaging: Innovative brain ageing biomarkers. Trends Neurosci. 2017, 40, 681–690. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C. Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer’s disease. GeroPsych J. Gerontopsychol. Geriatr. Psychiatry 2012, 25, 235. [Google Scholar] [CrossRef]
- Schnack, H.G.; Van Haren, N.E.; Nieuwenhuis, M.; Hulshoff Pol, H.E.; Cahn, W.; Kahn, R.S. Accelerated brain aging in schizophrenia: A longitudinal pattern recognition study. Am. J. Psychiatry 2016, 173, 607–616. [Google Scholar] [CrossRef]
- Cole, J.H.; Ritchie, S.J.; Bastin, M.E.; Hernández, M.V.; Maniega, S.M.; Royle, N.; Corley, J.; Pattie, A.; Harris, S.E.; Zhang, Q.; et al. Brain age predicts mortality. Mol. Psychiatry 2018, 23, 1385–1392. [Google Scholar] [CrossRef]
- Erus, G.; Battapady, H.; Satterthwaite, T.D.; Hakonarson, H.; Gur, R.E.; Davatzikos, C.; Gur, R.C. Imaging patterns of brain development and their relationship to cognition. Cereb. Cortex 2014, 25, 1676–1684. [Google Scholar] [CrossRef]
- Cole, J.H.; Leech, R.; Sharp, D.J.; Initiative, A.D.N. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015, 77, 571–581. [Google Scholar] [CrossRef]
- Cherubini, A.; Caligiuri, M.E.; Péran, P.; Sabatini, U.; Cosentino, C.; Amato, F. Importance of multimodal MRI in characterizing brain tissue and its potential application for individual age prediction. IEEE J. Biomed. Health Inform. 2016, 20, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Hänggi, J.; Mérillat, S.; Jäncke, L. Age prediction on the basis of brain anatomical measures. Hum. Brain Mapp. 2017, 38, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Poudel, R.P.; Tsagkrasoulis, D.; Caan, M.W.; Steves, C.; Spector, T.D.; Montana, G. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage 2017, 163, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Aycheh, H.M.; Seong, J.K.; Shin, J.H.; Na, D.L.; Kang, B.; Seo, S.W.; Sohn, K.A. Biological brain age prediction using cortical thickness data: A large scale cohort study. Front. Aging Neurosci. 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Miao, W.; Dai, D.; Hua, J.; He, H. Age estimation using cortical surface pattern combining thickness with curvatures. Med Biol. Eng. Comput. 2014, 52, 331–341. [Google Scholar] [CrossRef]
- Lewis, J.D.; Evans, A.C.; Tohka, J.; Brain Development Cooperative Group. T1 white/gray contrast as a predictor of chronological age, and an index of cognitive performance. Neuroimage 2018, 173, 341–350. [Google Scholar] [CrossRef]
- Madan, C.R.; Kensinger, E.A. Predicting age from cortical structure across the lifespan. Eur. J. Neurosci. 2018, 47, 399–416. [Google Scholar] [CrossRef]
- Madan, C.R. Advances in studying brain morphology: The benefits of open-access data. Front. Hum. Neurosci. 2017, 11, 405. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Fortin, J.P.; Cullen, N.; Sheline, Y.I.; Taylor, W.D.; Aselcioglu, I.; Cook, P.A.; Adams, P.; Cooper, C.; Fava, M.; McGrath, P.J.; et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018, 167, 104–120. [Google Scholar] [CrossRef]
- Fortin, J.P.; Parker, D.; Tunç, B.; Watanabe, T.; Elliott, M.A.; Ruparel, K.; Roalf, D.R.; Satterthwaite, T.D.; Gur, R.C.; Gur, R.E.; et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017, 161, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Yan, C.-G.; Li, Q.; Denio, E.; Castellanos, F.X.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Bookheimer, S.Y.; Dapretto, M.; et al. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 2014, 19, 659–667. [Google Scholar]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Liu, H.; Li, H.; Boimel, P.; Janopaul-Naylor, J.; Zhong, H.; Xiao, Y.; Ben-Josef, E.; Fan, Y. Collaborative clustering of subjects and radiomic features for predicting clinical outcomes of rectal cancer patients. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 1303–1306. [Google Scholar]
- Lombardi, A.; Guaragnella, C.; Amoroso, N.; Monaco, A.; Fazio, L.; Taurisano, P.; Pergola, G.; Blasi, G.; Bertolino, A.; Bellotti, R.; et al. Modelling cognitive loads in schizophrenia by means of new functional dynamic indexes. NeuroImage 2019, 195, 150–164. [Google Scholar] [CrossRef]
- Drucker, H.; Burges, C.J.; Kaufman, L.; Smola, A.J.; Vapnik, V. Support vector regression machines. Adv. Neural Inf. Process. Syst. 1997, 155–161. [Google Scholar]
- Smola, A.J.; Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef]
- Guyon, I.; Weston, J.; Barnhill, S.; Vapnik, V. Gene selection for cancer classification using support vector machines. Mach. Learn. 2002, 46, 389–422. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Grömping, U. Variable importance assessment in regression: Linear regression versus random forest. Am. Stat. 2009, 63, 308–319. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B (Methodological) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Kalousis, A.; Prados, J.; Hilario, M. Stability of feature selection algorithms: A study on high-dimensional spaces. Knowl. Inf. Syst. 2007, 12, 95–116. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xiao, G. A comparative study of rank aggregation methods for partial and top ranked lists in genomic applications. Briefings Bioinform. 2017, 20, 178–189. [Google Scholar] [CrossRef]
- Kolde, R.; Laur, S.; Adler, P.; Vilo, J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics 2012, 28, 573–580. [Google Scholar] [CrossRef]
- Kalousis, A.; Prados, J.; Hilario, M. Stability of feature selection algorithms. In Proceedings of the Fifth IEEE International Conference on Data Mining (ICDM’05), Houston, TX, USA, 27–30 November 2005; p. 8. [Google Scholar]
- Franke, K.; Luders, E.; May, A.; Wilke, M.; Gaser, C. Brain maturation: Predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 2012, 63, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; Kuperman, J.M.; Chung, Y.; Erhart, M.; McCabe, C.; Hagler, D.J., Jr.; Venkatraman, V.K.; Akshoomoff, N.; Amaral, D.G.; Bloss, C.S.; et al. Neuroanatomical assessment of biological maturity. Curr. Biol. 2012, 22, 1693–1698. [Google Scholar] [CrossRef]
- Corps, J.; Rekik, I. Morphological Brain Age prediction using Multi-View Brain Networks Derived from Cortical Morphology in Healthy and Disordered participants. Sci. Rep. 2019, 9, 9676. [Google Scholar] [CrossRef]
- Ball, G.; Beare, R.; Seal, M.L. Charting shared developmental trajectories of cortical thickness and structural connectivity in childhood and adolescence. Hum. Brain Mapp. 2019, 40, 4630–4644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Klein, A.; Castellanos, F.; Milham, M.P. Brain Age Prediction: Cortical and Subcortical Shape Covariation in the Developing Human Brain. BioRxiv 2019, 202, 570333. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, N.; La Rocca, M.; Bellantuono, L.; Diacono, D.; Fanizzi, A.; Lella, E.; Lombardi, A.; Maggipinto, T.; Monaco, A.; Tangaro, S.; et al. Deep learning and Multiplex networks for accurate modeling of brain age. Front. Aging Neurosci. 2019, 11, 115. [Google Scholar] [CrossRef]
- Tamnes, C.K.; stby, Y.; Fjell, A.M.; Westlye, L.T.; Due-Tønnessen, P.; Walhovd, K.B. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex 2009, 20, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Sotiras, A.; Toledo, J.B.; Gur, R.E.; Gur, R.C.; Satterthwaite, T.D.; Davatzikos, C. Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc. Natl. Acad. Sci. USA 2017, 114, 3527–3532. [Google Scholar] [CrossRef]
- Hoagey, D.A.; Rieck, J.R.; Rodrigue, K.M.; Kennedy, K.M. Joint contributions of cortical morphometry and white matter microstructure in healthy brain aging: A partial least squares correlation analysis. Hum. Brain Mapp. 2019, 40, 5315–5329. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Cohen, A.L.; Dosenbach, N.U.; Church, J.A.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. USA 2008, 105, 4028–4032. [Google Scholar] [CrossRef]
- Dennis, E.L.; Thompson, P.M. Mapping connectivity in the developing brain. Int. J. Dev. Neurosci. 2013, 31, 525–542. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Fjell, A.M.; Reinvang, I.; Lundervold, A.; Dale, A.M.; Eilertsen, D.E.; Quinn, B.T.; Salat, D.; Makris, N.; Fischl, B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol. Aging 2005, 26, 1261–1270. [Google Scholar] [CrossRef]
- Cherubini, A.; Péran, P.; Caltagirone, C.; Sabatini, U.; Spalletta, G. Aging of subcortical nuclei: Microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage 2009, 48, 29–36. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Westlye, L.T.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Agartz, I.; Salat, D.H.; Greve, D.N.; Fischl, B.; et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol. Aging 2011, 32, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, A.C.; McIntosh, A.M.; Spencer, M.D.; Philip, R.; Gaur, S.; Lawrie, S.M. Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur. Psychiatry 2008, 23, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.B.; Stoodley, C.J. Autism spectrum disorder and the cerebellum. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1–34. [Google Scholar]
- Le, T.T.; Kuplicki, R.T.; McKinney, B.A.; Yeh, H.w.; Thompson, W.K.; Paulus, M.P.; Aupperle, R.L.; Bodurka, J.; Cha, Y.H.; Feinstein, J.S.; et al. A nonlinear simulation framework supports adjusting for age when analyzing BrainAGE. Front. Aging Neurosci. 2018, 10, 317. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, F.; Niu, X. Investigating systematic bias in brain age estimation with application to post-traumatic stress disorders. Hum. Brain Mapp. 2019, 40, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vidaurre, D.; Alfaro-Almagro, F.; Nichols, T.E.; Miller, K.L. Estimation of brain age delta from brain imaging. Neuroimage 2019, 200, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Cawley, G.C.; Talbot, N.L. On over-fitting in model selection and subsequent selection bias in performance evaluation. J. Mach. Learn. Res. 2010, 11, 2079–2107. [Google Scholar]


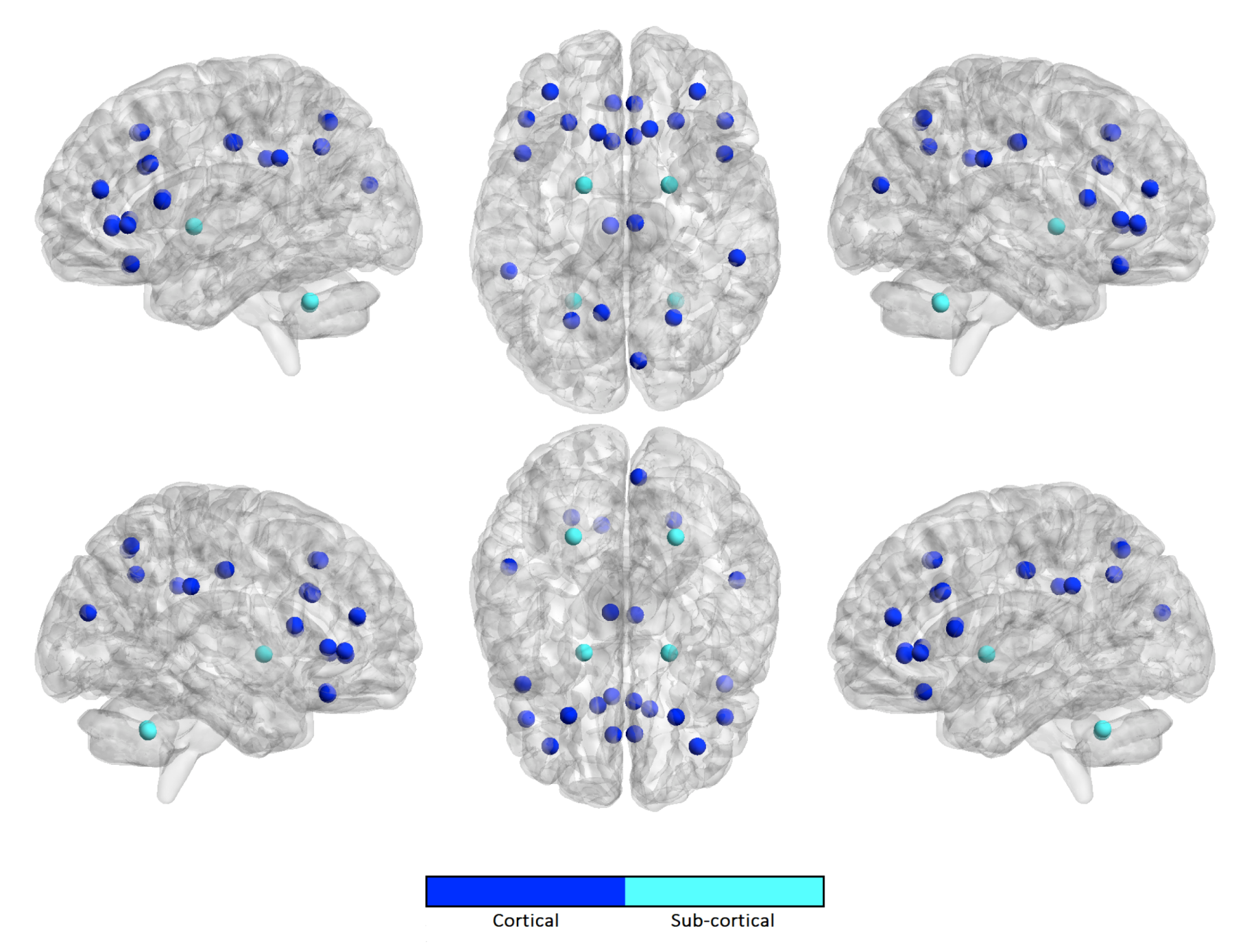

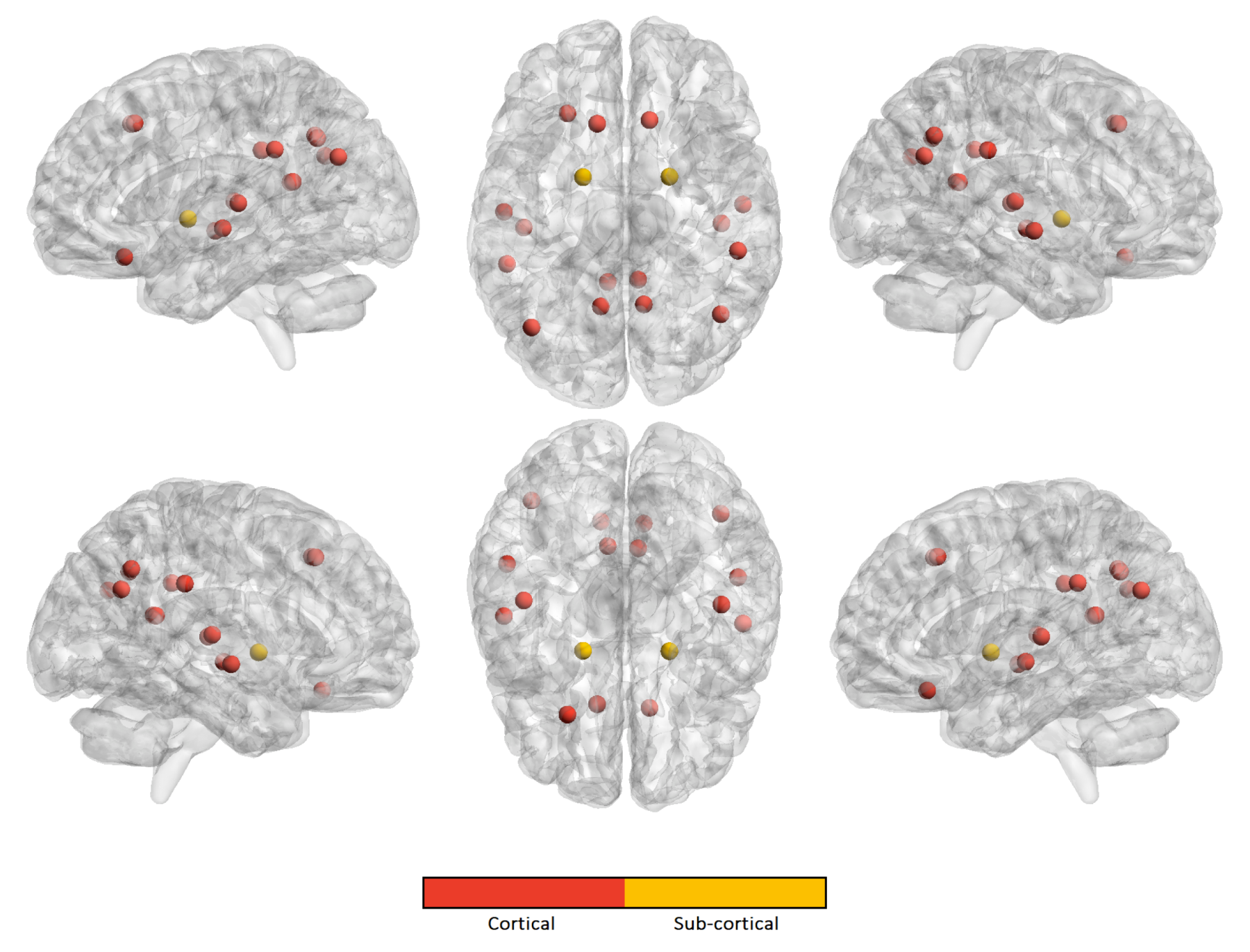
| Harmonization Technique | NC | ASD | ||||
|---|---|---|---|---|---|---|
| SVR | RF | Lasso | SVR | RF | Lasso | |
| No harmonization | 400 | 90 | 55 | 150 | 130 | 35 |
| Age covariate | 1000 | 100 | 100 | 400 | 50 | 65 |
| No age covariate | 40 | 30 | 15 | 50 | 20 | 15 |
| Harmonization Technique | NC | ASD | ||||
|---|---|---|---|---|---|---|
| SVR | RF | Lasso | SVR | RF | Lasso | |
| No harmonization | ||||||
| Age covariate | ||||||
| No age covariate | ||||||
| Harmonization Technique | NC | ASD | ||||
|---|---|---|---|---|---|---|
| SVR | RF | Lasso | SVR | RF | Lasso | |
| No harmonization | ||||||
| Age covariate | ||||||
| No age covariate | ||||||
| Harmonization Technique | Class | SVR | RF | Lasso |
|---|---|---|---|---|
| No harmonization | NC | |||
| ASD | ||||
| Age covariate | NC | |||
| ASD | ||||
| No age covariate | NC | |||
| ASD | ||||
| Harmonization Technique | Class | SVR | RF | Lasso |
|---|---|---|---|---|
| No harmonization | NC | |||
| ASD | ||||
| Age covariate | NC | |||
| ASD | ||||
| No age coviariate | NC | |||
| ASD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, A.; Amoroso, N.; Diacono, D.; Monaco, A.; Tangaro, S.; Bellotti, R. Extensive Evaluation of Morphological Statistical Harmonization for Brain Age Prediction. Brain Sci. 2020, 10, 364. https://doi.org/10.3390/brainsci10060364
Lombardi A, Amoroso N, Diacono D, Monaco A, Tangaro S, Bellotti R. Extensive Evaluation of Morphological Statistical Harmonization for Brain Age Prediction. Brain Sciences. 2020; 10(6):364. https://doi.org/10.3390/brainsci10060364
Chicago/Turabian StyleLombardi, Angela, Nicola Amoroso, Domenico Diacono, Alfonso Monaco, Sabina Tangaro, and Roberto Bellotti. 2020. "Extensive Evaluation of Morphological Statistical Harmonization for Brain Age Prediction" Brain Sciences 10, no. 6: 364. https://doi.org/10.3390/brainsci10060364
APA StyleLombardi, A., Amoroso, N., Diacono, D., Monaco, A., Tangaro, S., & Bellotti, R. (2020). Extensive Evaluation of Morphological Statistical Harmonization for Brain Age Prediction. Brain Sciences, 10(6), 364. https://doi.org/10.3390/brainsci10060364








