Characterizing Olfactory Function in Children with Autism Spectrum Disorder and Children with Sensory Processing Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
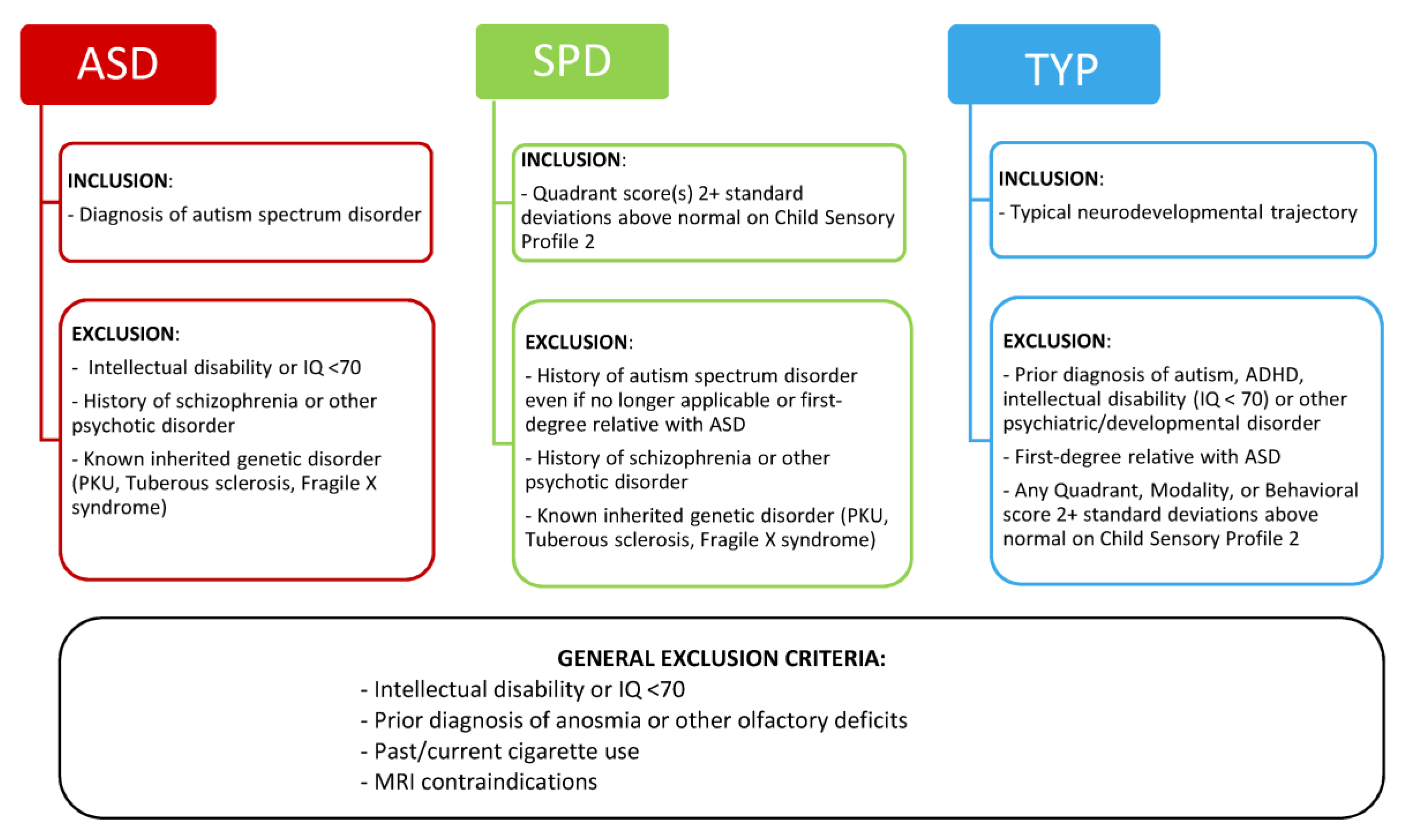
2.2.1. Clinical Evaluation and Group Assignment
2.2.2. Olfactory Testing
2.3. Statistical Analyses
3. Results
3.1. Olfactory Function by Group
3.2. Olfactory Function between Groups
3.3. Olfactory Function Correlations
3.4. Odor Identification and ASD Severity
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Tomchek, S.D.; Dunn, W. Sensory processing gin children with and without autism: A comparative study using the Short Sensory Profile. Am. J. Occup. Ther. 2007, 31, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.A. Sensory processing in children with autism spectrum disorders and impact on functioning. Pediatr. Clin. N. Am. 2012, 59, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.E.; Young, R.L.; Baker, E.Z.; Angley, M.T. Sensory processing subtypes in autism: Association with adaptive behavior. J. Autism Dev. Disord. 2010, 40, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Critchley, H.D.; Rowe, A.; Howlin, P.; Murphy, D.G. Impaired olfactory identification in Asperger’s syndrome. J. Neuropsychiatry Clin. Neurosci. 2003, 15, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Bennetto, L.; Kuschner, E.S. Olfaction and taste processing in autism. Biol. Psychiatry 2007, 62, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Galle, S.A.; Courchesne, V.; Mottron, L.; Frasnelli, J. Olfaction in the autism spectrum. Perception 2013, 42, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Wicker, B.; Monfardinia, E.; Royet, J.P. Olfactory processing in adults with autism spectrum disorders. Mol. Autism 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Muratori, F.; Tonacci, A.; Billeci, L.; Catalucci, T.; Igliozzi, R.; Calderoni, S.; Narzisi, A. Olfactory processing in male children with autism: Atypical odor threshold and identification. J. Autism Dev. Disord. 2017, 47, 3243–3251. [Google Scholar] [CrossRef]
- Koehler, L.; Fournel, A.; Albertowski, K.; Roessner, V.; Gerber, J.; Hummel, C.; Hummel, T.; Bensafi, M. Impaired odor perception in autism spectrum disorder is associated with decreased activity in olfactory cortex. Chem. Senses 2018, 43, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, T.; Baron-Cohen, S. Olfactory detection thresholds and adaptation in adults with autism spectrum condition. J. Autism Dev. Disord. 2012, 42, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Dudova, I.; Vodicka, J.; Havlovicova, M.; Sedlacek, Z.; Urbanek, T.; Hrdlicka, M. Odor detection threshold, but not odor identification, is impaired in children with autism. Eur. Child Adolesc. Psychiatry 2011, 20, 333–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ashwin, C.; Chapman, E.; Howells, J.; Rhydderch, D.; Walker, I.; Baron-Cohen, S. Enhanced olfactory sensitivity in autism spectrum conditions. Mol. Autism 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Andrade, G.; Kendrick, K.M. The main olfactory system and social learning in mammals. Behav. Brain Res. 2009, 200, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sakano, H. How is the olfactory map formed and interpreted in the mammalian brain? Annu. Rev. Neurosci. 2011, 34, 467–499. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.A.; Zald, D.H. On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates. Brain Res. Rev. 2005, 50, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Neville, K.R.; Haberly, L.B. Olfactory cortex. In The Synaptic Organization of the Brain; Shepherd, G., Ed.; Oxford University Press: New York, NY, USA, 2004; pp. 415–454. [Google Scholar]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef]
- Girgis, R.R.; Minshew, N.J.; Melhem, N.M.; Nutche, J.J.; Keshavan, M.S.; Hardan, A.Y. Volumetric alterations of the orbitofrontal cortex in autism. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2007, 31, 41–45. [Google Scholar] [CrossRef]
- Schumann, C.M.; Amaral, D.G. Stereological analysis of amygdala neuron number in autism. J. Neurosci. 2006, 26, 7674–7679. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, N.M.; Reiter, M.A.; Neuhaus, E.; Pauley, G.; Martin, N.; Dager, S.; Estes, A. Subregional differences in intrinsic amygdala hyperconnectivitiy and hypoconnectivity in autism spectrum disorder. Autism Res. 2015, 9, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Mandy, W.; Chilvers, R.; Chowdhury, U.; Salter, G.; Seigal, A.; Skuse, D. Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. J. Autism Dev. Disord. 2012, 42, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence; The Psychological Corporation: New York, NY, USA, 1999. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Dunn, W. Sensory Profile-2; Pearson Publishing: San Antonia, TX, USA, 2014. [Google Scholar]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef]
- Schandar, M.; Laugwitz, K.L.; Boekhoff, I.; Kroner, C.; Gudermann, T.; Schultz, G.; Breer, H. Odorants selectively activate distinct G protein subtypes in olfactory cilia. J. Biol. Chem. 1998, 273, 16669–16677. [Google Scholar] [CrossRef]
- Hummel, T.; Livermore, A. Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int. Arch. Occup. Environ. Health 2002, 75, 305–313. [Google Scholar] [CrossRef]
- Dalton, P.; Wysocki, C.J.; Brody, M.J.; Lawly, H.J. Perceived odor, irritation, and health symptoms following short-term exposure to acetone. Am. J. Ind. Med. 1997, 31, 558–569. [Google Scholar] [CrossRef]
- Doty, R.L.; Schaman, P.; Kimmelman, C.P.; Dann, M.S. University of Pennsylvania Smell Identification Test: A rapid quantitative olfactory function test for the clinic. Laryngoscope 1984, 94, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. The Smell Identification TestTM Administration Manual, 3rd ed.; Sensonics International: Haddon Heights, NJ, USA, 2013. [Google Scholar]
- SPSS Inc. PASW Statistics for Windows; Version 18.0; SPSS Inc.: Chicago, IL, USA, 2009. [Google Scholar]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Mesholam, R.I.; Moberg, P.J.; Mahr, R.N.; Doty, R.L. Olfaction in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. JAMA Neurol. 1998, 55, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Moberg, P.J.; Agrin, R.; Gur, R.E.; Gur, R.C.; Turetsky, B.I.; Doty, R.L. Olfactory dysfunction in schizophrenia: A qualitative and quantitative review. Neuropsychopharmacology 1999, 21, 325–340. [Google Scholar] [CrossRef]
- Atanasova, B.; Graux, J.; El Hage, W.; Hommet, C.; Camus, V.; Belzung, C. Olfaction: A potential cognitive marker of psychiatric disorders. Neurosci. Biobehav. Rev. 2008, 32, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, H.; Muramatsu, T.; Fujisawa, T.; Miyao, M.; Matsuura, E.; Okada, K.; Kosaka, H.; Tomoda, A.; Mimura, M. Assessment of olfactory detection thresholds in children with autism spectrum disorders using a pulse ejection system. Mol. Autism 2016, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Addo, R.; Wiens, S.; Nord, M.; Larsson, M. Olfactory functions in adults with autism spectrum disorders. Perception 2017, 46, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Schoen, S.A.; Miller, L.J.; Brett-Green, B.A.; Nielsen, D.M. Physiological and behavioral differences in sensory processing: A comparison of children with autism spectrum disorder and sensory modulation disorder. Front. Integr. Neurosci. 2009, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Romanos, M.; Renner, T.J.; Schecklmann, M.; Hummel, B.; Roos, M.; von Mering, C.; Pauli, P.; Reichmann, H.; Warnke, A.; Gerlack, M. Improved odor sensitivity in attention-deficit/hyperactivity disorder. Biol. Psychiatry 2008, 64, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, A.; Scholz-Hehn, D.; Wiesner, C.D.; Wolff, S.; Bergmann, T.O.; van Eimeren, T.; Lentfer, L.; Baving, L.; Prehn-Kristensen, A. Chemosensory processing in children with attention-deficit/hyperactivity disorder. J. Psychiatr. Res. 2016, 76, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Royet, J.P.; Plailly, J.; Delon-Marint, C.; Kareken, D.A.; Segebarth, C. fMRI of emotional responses to odors: Influence of hedonic valence and judgment, handedness, and gender. NeuroImage 2003, 20, 713–728. [Google Scholar] [CrossRef]
- Reske, M.; Kellermann, T.; Shah, N.J.; Schneider, F.; Habel, U. Impact of valence and age on olfactory induced brain activation in healthy women. Behav. Neurosci. 2010, 124, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, J.K.; Hurley, R.S.; Bowman, N.E.; Bao, X.; Mesulam, M.M.; Gottfried, J.A. A designated odor-language integration system in the human brain. J. Neurosci. 2014, 34, 14864–14873. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lopez, L.; Osher, J.; Howard, J.D.; Parrish, T.B.; Gottfried, J.A. Right orbitofrontal cortex mediates conscious olfactory perception. Psychol. Sci. 2010, 21, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Jones-Gotman, M.; Zatorre, R.J. Olfactory identification deficits in patients with focal cerebral excision. Neuropsychologia 1988, 26, 387–400. [Google Scholar] [CrossRef]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004, 55, 11–29. [Google Scholar] [CrossRef]
- Dalton, K.M.; Nacewicz, B.M.; Johnstone, T.; Schaefer, H.S.; Gernsbacher, M.A.; Goldsmith, H.H.; Alexander, A.L.; Davidson, R.J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005, 8, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Corbett, B.A.; Carmean, V.; Ravizza, S.; Wendelken, C.; Henry, M.L.; Carter, C.; Rivera, S.M. A functional and structural study of emotion and face processing in children with autism. Psychiatry Res. 2009, 173, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.K.; Chrsitoff, K.; Stappen, I.; Panitz, D.; Ghahremani, D.G.; Glover, G.; Gabrieli, J.D.; Sobel, N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003, 6, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kassir, M.A.; Wu, J.; Zhang, Q.; Zhou, S.; Xuan, S.Y.; Li, Q.; Ye, Y.; Hu, J. MR volumetric study of piriform-cortical amygdala and orbitofrontal cortices: The aging effect. PLoS ONE 2013, 8, e74526. [Google Scholar] [CrossRef] [PubMed]
- Rupp, C.I.; Fleischhacker, W.W.; Kemmler, G.; Kremser, C.; Bilder, R.M.; Mechtcheriakov, S.; Szeszko, P.R.; Walch, T.; Scholtz, A.W.; Klimbacher, M.; et al. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr. Res. 2005, 74, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ferdenzi, C.; Coureaud, G.; Camos, V.; Schaal, B. Human awareness and uses of odor cues in everyday life: Results from a questionnaire study in children. Int. J. Behav. Dev. 2008, 32, 422–431. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]

| ASD (n = 43) | SPD (n = 44) | TYP (n = 46) | F | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |||
| Gender (Male: Female) | 39:4 | 38:6 | 39:7 | |||||
| Age (yrs.) | 10.21 | (1.63) | 9.98 | (1.62) | 10.32 | (1.44) | 0.54 | 0.59 |
| Wechsler Abbreviated Scales of Intelligence | ||||||||
| Full-Scale IQ | 112.33 | (17.90) | 115.64 | (18.12) | 119.35 | (11.06) | 2.16 | 0.12 |
| Performance IQ | 114.91 | (14.33) | 113.02 | (16.80) | 115.28 | (13.12) | 0.297 | 0.74 |
| Verbal IQ | 107.33 | (21.21) | 114.75 | (18.07) | 119.11 | (11.19) | 5.29 | 0.006 a,c |
| Autism Diagnostic Observation Schedule d | ||||||||
| Social Affect | 7.37 | (1.94) | 3.73 | (2.56) | 1.98 | (1.22) | 85.54 | <0.001 a,b,c |
| Restricted/Repetitive Behav. | 6.67 | (2.33) | 3.50 | (2.26) | 1.80 | (1.80) | 59.31 | <0.001 a,b,c |
| Comparison Score | 7.12 | (1.95) | 3.16 | (2.22) | 1.43 | (0.81) | 121.08 | <0.001 a,b,c |
| Child Sensory Profile 2 | ||||||||
| Avoidant | 55.53 | (14.50) | 58.57 | (14.82) | 26.93 | (7.43) | 86.18 | <0.001 a,b |
| Seeking | 42.02 | (16.47) | 51.05 | (14.62) | 22.74 | (7.32) | 53.38 | <0.001 a,b,c |
| Sensitive | 51.47 | (13.10) | 52.18 | (13.56) | 22.28 | (6.77) | 97.51 | <0.001 a,b |
| Bystander | 52.14 | (15.56) | 54.27 | (16.80) | 25.02 | (8.54) | 61.03 | <0.001 a,b |
| Auditory | 24.16 | (6.64) | 25.57 | (6.69) | 11.85 | (4.01) | 74.39 | <0.001 a,b |
| Body Position | 17.28 | (7.76) | 19.11 | (9.21) | 8.26 | (3.26) | 29.80 | <0.001 a,b |
| Movement | 15.65 | (7.42) | 18.27 | (6.79) | 8.13 | (3.32) | 33.99 | <0.001 a,b |
| Oral | 25.58 | (10.13) | 26.70 | (10.38) | 11.39 | (4.68) | 43.08 | <0.001 a,b |
| Touch | 20.77 | (9.81) | 25.73 | (9.63) | 10.91 | (4.10) | 37.96 | <0.001 a,b,c |
| Visual | 14.09 | (5.22) | 14.80 | (4.80) | 10.00 | (3.29) | 15.03 | <0.001 a,b |
| Attention Behavior | 26.63 | (6.59) | 26.73 | (7.29) | 11.50 | (4.58) | 89.26 | <0.001 a,b |
| Conduct Behavior | 22.81 | (7.97) | 25.52 | (7.30) | 11.78 | (4.22) | 54.03 | <0.001 a,b |
| Social Emotional Behavior | 41.44 | (11.43) | 43.20 | (12.84) | 19.43 | (5.91) | 72.78 | <0.001 a,b |
| ASD | SPD | TYP | ||||
|---|---|---|---|---|---|---|
| s | (SD) | Mean | (SD) | Mean | (SD) | |
| Sniffin’ Sticks Threshold Test | ||||||
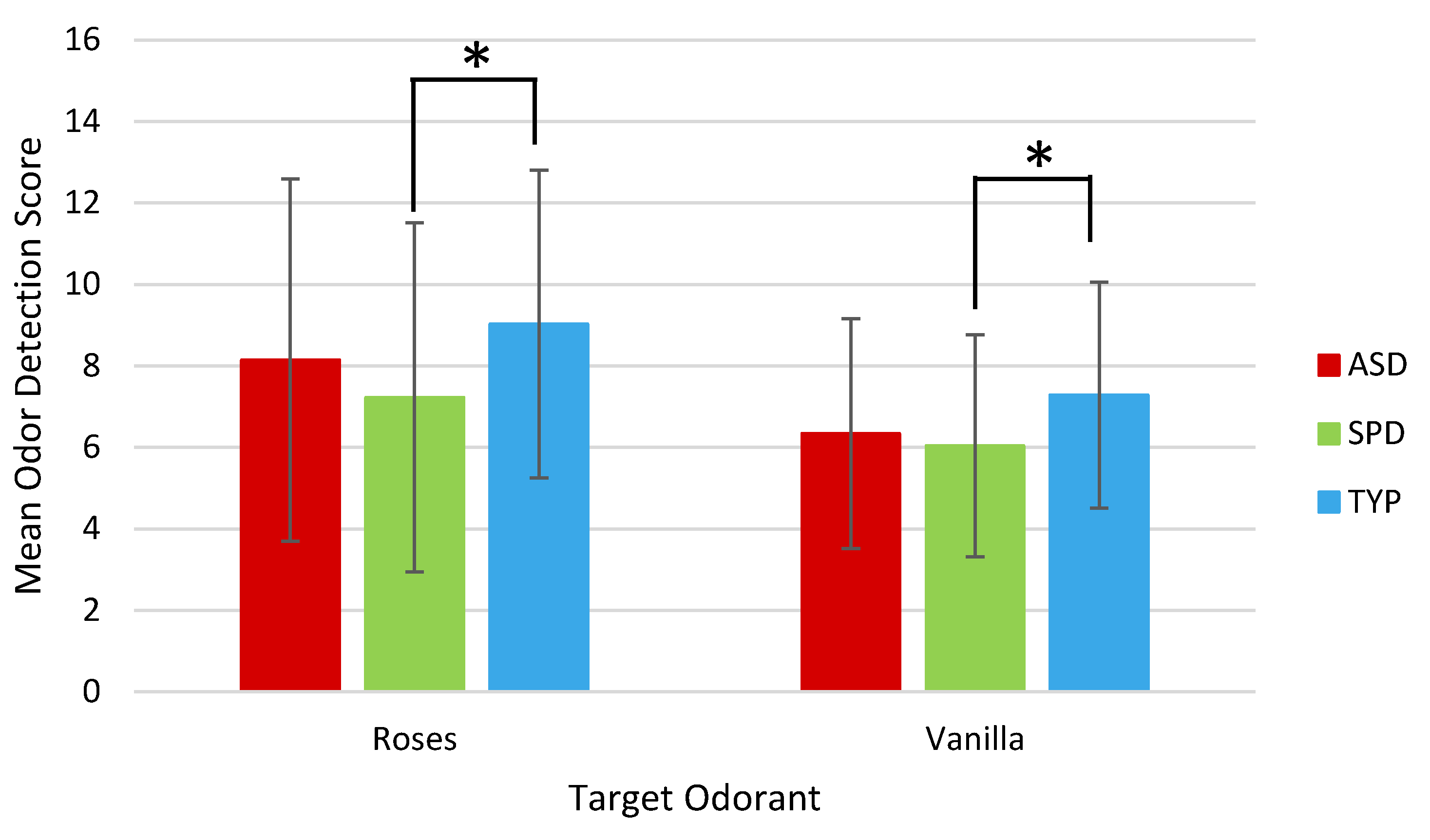
| Roses | 8.14 | 4.45 | 7.23 | 4.29 | 9.03 | 3.78 |
| Vanilla | 6.34 | 2.82 | 6.04 | 2.72 | 7.28 | 2.78 |
| Valence Rating | ||||||
| Roses | 1.31 | 1.52 | 0.58 | 1.96 | 1.39 | 1.30 |
| Vanilla | 2.08 | 1.55 | 1.81 | 1.58 | 2.00 | 1.16 |
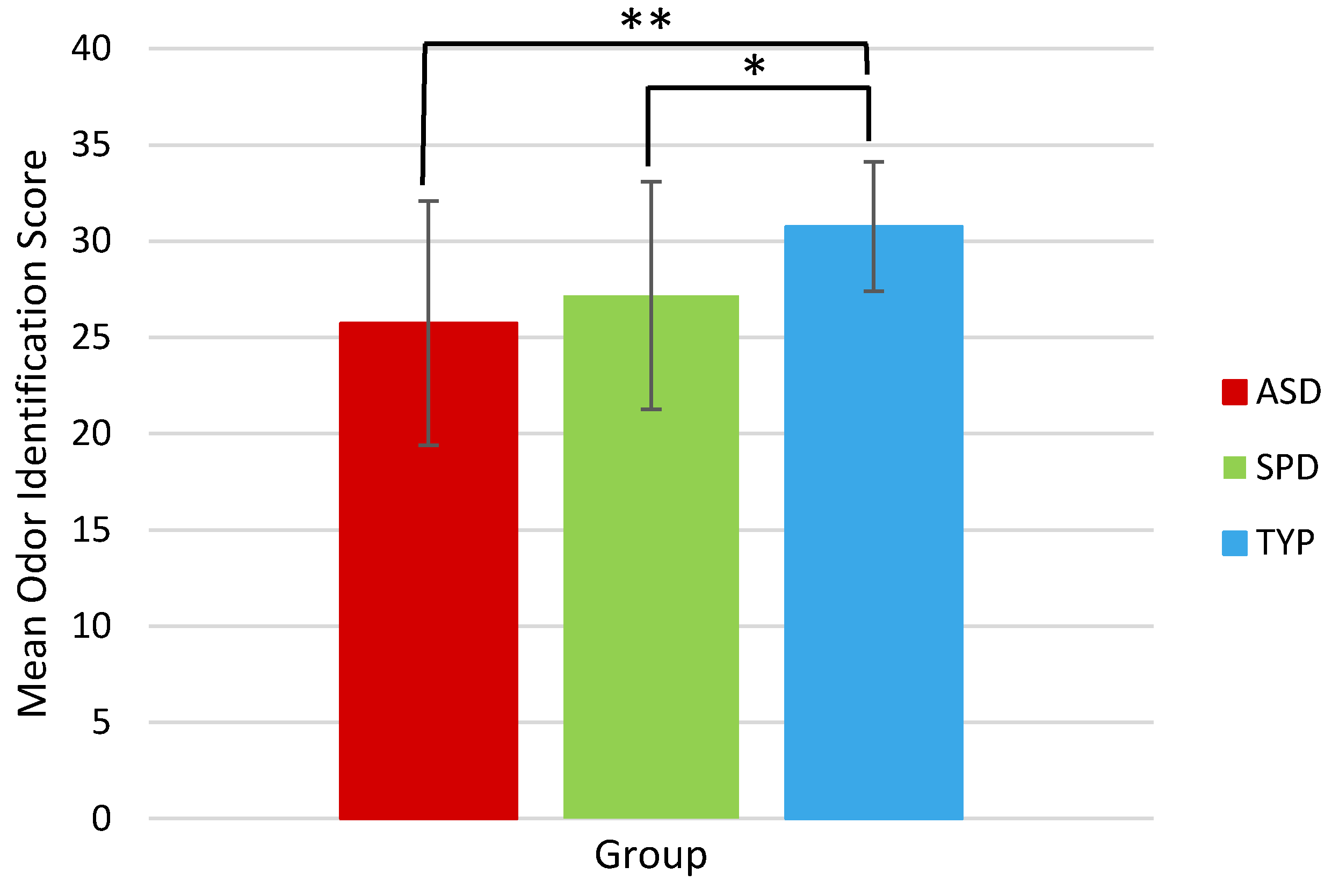
| UPSIT | 25.73 | 6.35 | 27.17 | 5.91 | 30.76 | 3.36 |
| ASD v. TYP | SPD v. TYP | ASD v. SPD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | t | p-Value | df | t | p-Value | Df | t | p-Value | |
| Sniffin’ Sticks Threshold Test | |||||||||
| Roses | 82 | 0.991 | 0.33 | 86 | 2.095 | 0.039 * | 78 | 0.933 | 0.35 |
| Vanilla | 83 | 1.547 | 0.126 | 88 | 2.146 | 0.035 * | 81 | 0.495 | 0.62 |
| Valence Rating | |||||||||
| Roses | 57 | 0.235 | 0.82 | 62 | 1.967 | 0.054 | 55 | 1.54 | 0.13 |
| Vanilla | 55 | −0.222 | 0.83 | 61 | .555 | 0.581 | 54 | 0.649 | 0.52 |
| UPSIT | 84 | 4.683 | <0.001 *** | 85 | 3.532 | 0.001 ** | 79 | −1.062 | 0.29 |
| ASD Group | SPD Group | TYP Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | r | p-Value | DF | R | p-Value | DF | r | p-Value | |
| Sniffin’ Sticks—Roses | |||||||||
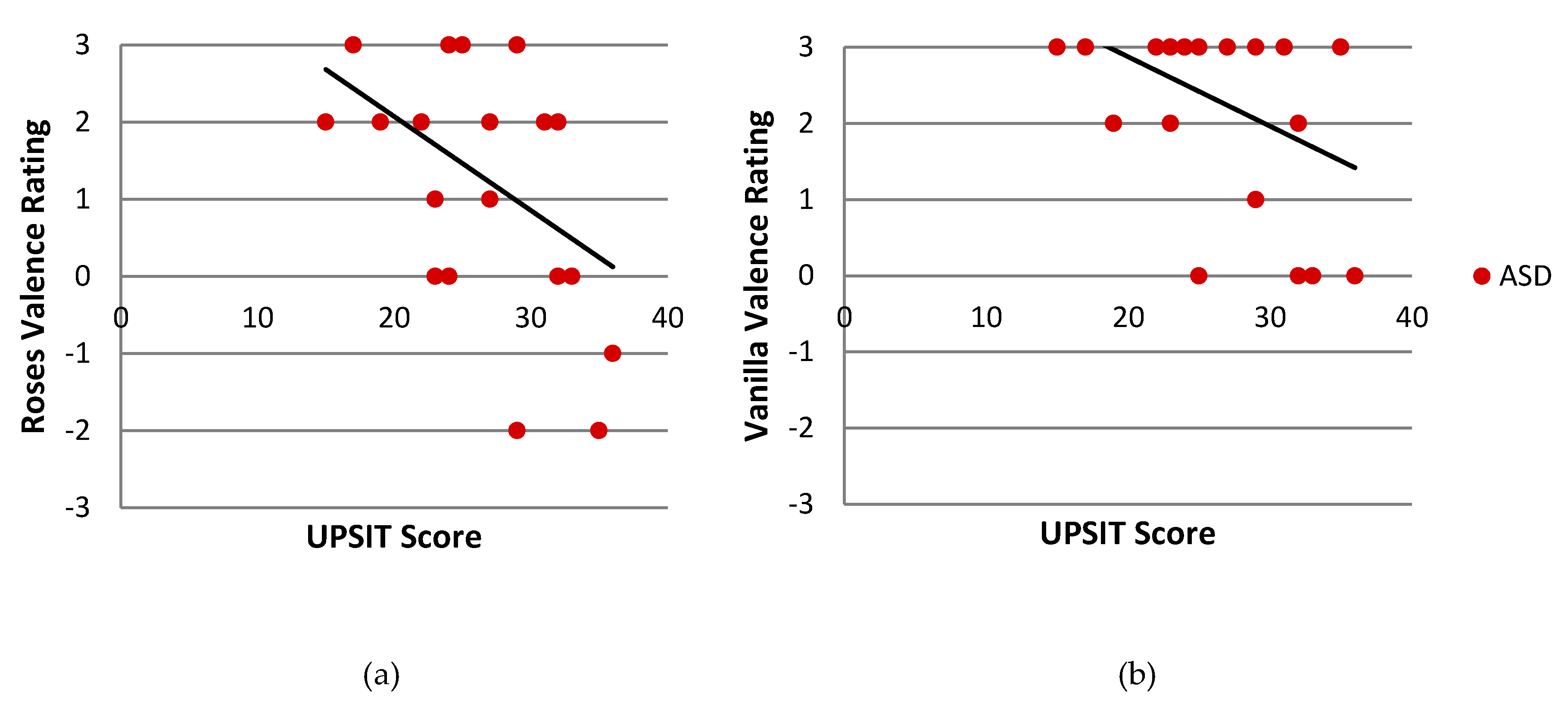
| Valence Rating—Roses | 21 | −0.043 | 0.85 | 27 | −0.116 | 0.55 | 31 | 0.115 | 0.53 |
| Sniffin’ Sticks—Vanilla | |||||||||
| Valence Rating—Vanilla | 21 | −0.539 | 0.008 ** | 29 | .053 | 0.78 | 30 | −0.048 | 0.793 |
| UPSIT | |||||||||
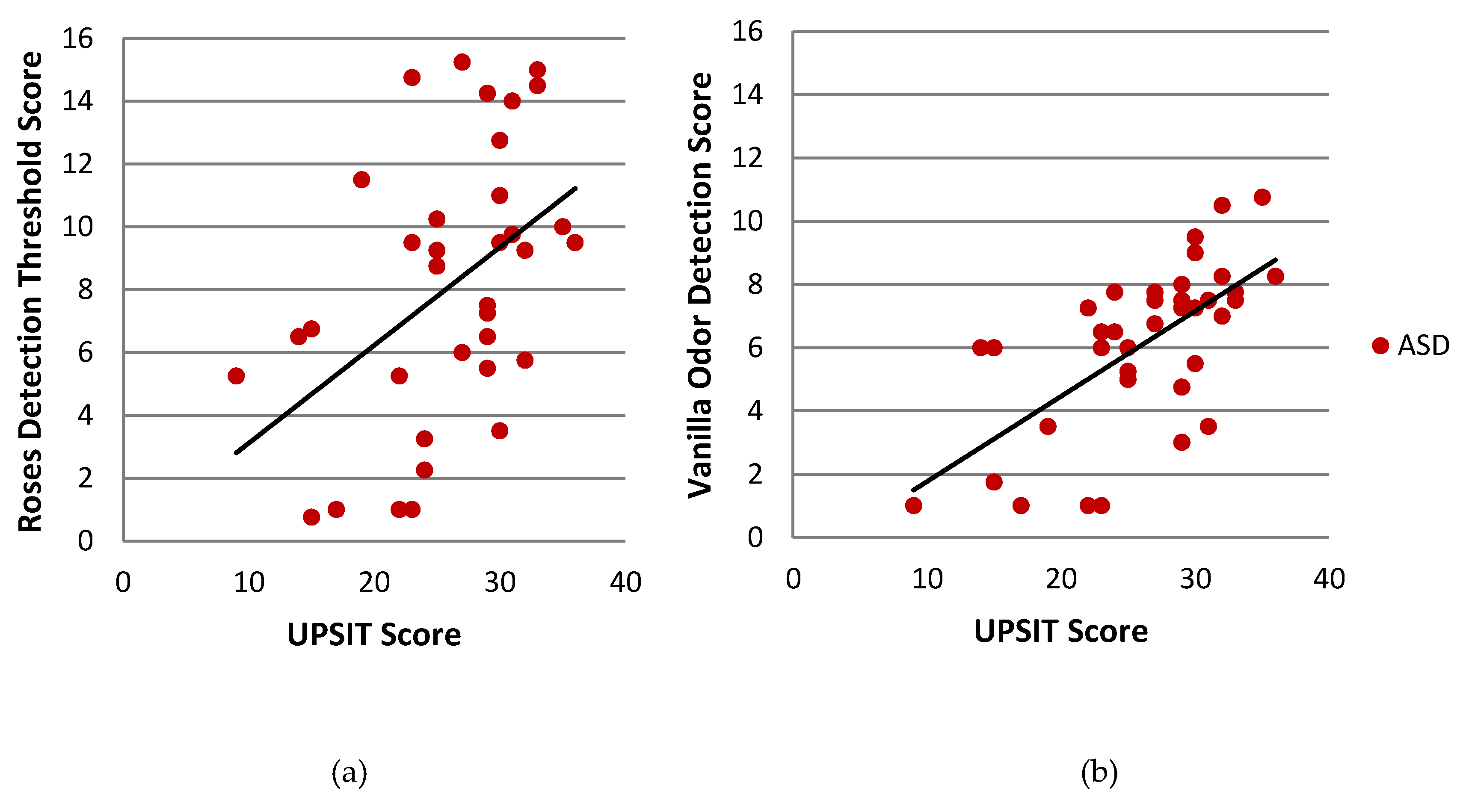
| Sniffin’ Sticks—Roses | 34 | 0.463 | 0.004 ** | 37 | 0.344 | 0.032 * | 44 | 0.089 | 0.56 |
| Sniffin’ Sticks—Vanilla | 35 | 0.649 | 0.000 *** | 39 | 0.217 | 0.17 | 44 | −0.196 | 0.19 |
| UPSIT | |||||||||
| Valence Rating—Roses | 23 | −0.422 | 0.036 * | 27 | 0.271 | 0.16 | 31 | −0.361 | 0.039 * |
| Valence Rating—Vanilla | 22 | −0.425 | 0.038 * | 27 | 0.107 | 0.58 | 30 | 0.050 | 0.79 |
| ASD Group | SPD Group | |||||
|---|---|---|---|---|---|---|
| DF | r-Value | p-Value | DF | r-Value | p-Value | |
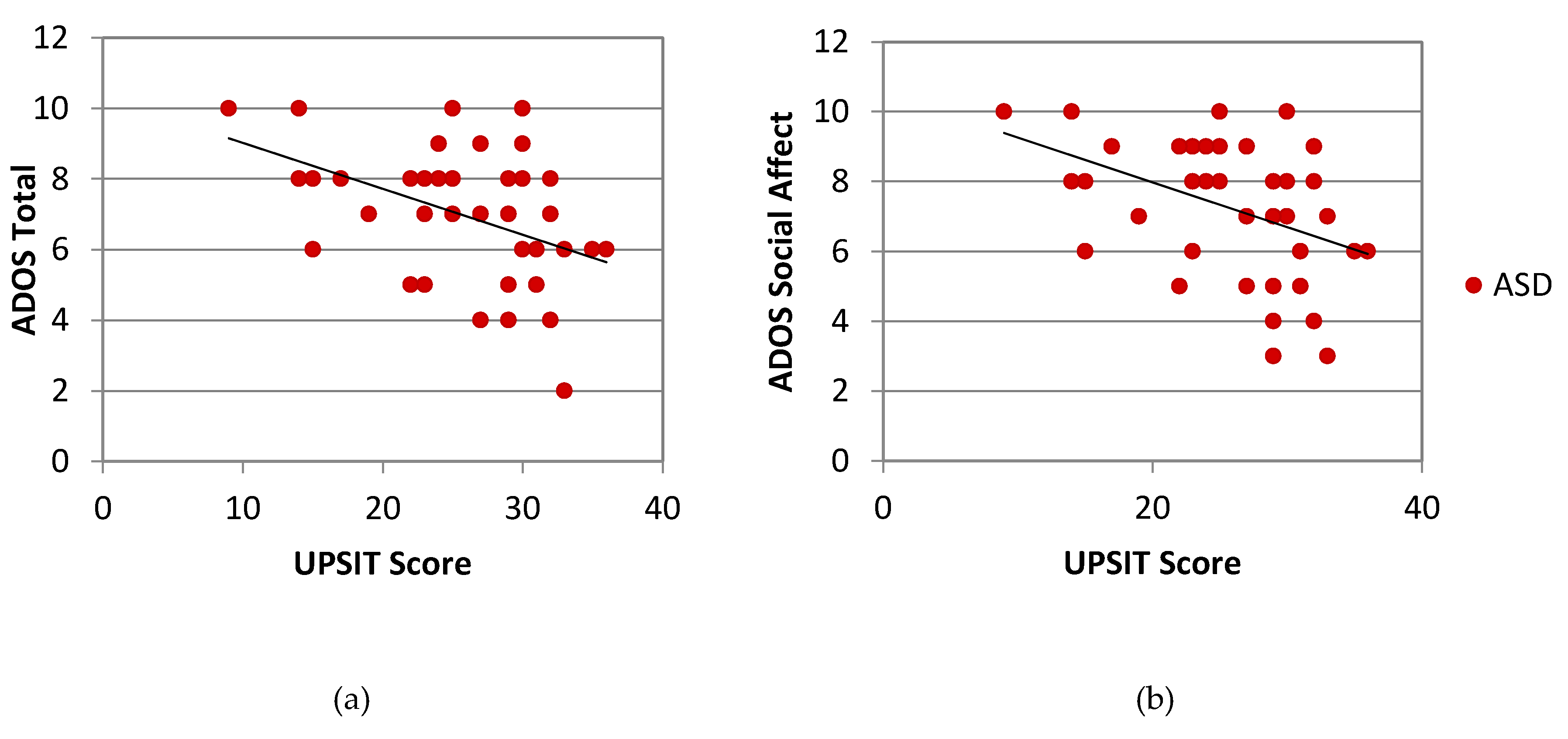
| ADOS Total | 38 | −0.431 | 0.005 ** | 42 | −0.230 | 0.13 |
| ADOS Social Affect | 38 | −0.424 | 0.006 ** | 42 | −0.222 | 0.15 |
| ADOS Restricted/Repetitive Behavior | 38 | −0.171 | 0.29 | 42 | −0.044 | 0.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweigert, J.R.; St. John, T.; Begay, K.K.; Davis, G.E.; Munson, J.; Shankland, E.; Estes, A.; Dager, S.R.; Kleinhans, N.M. Characterizing Olfactory Function in Children with Autism Spectrum Disorder and Children with Sensory Processing Dysfunction. Brain Sci. 2020, 10, 362. https://doi.org/10.3390/brainsci10060362
Sweigert JR, St. John T, Begay KK, Davis GE, Munson J, Shankland E, Estes A, Dager SR, Kleinhans NM. Characterizing Olfactory Function in Children with Autism Spectrum Disorder and Children with Sensory Processing Dysfunction. Brain Sciences. 2020; 10(6):362. https://doi.org/10.3390/brainsci10060362
Chicago/Turabian StyleSweigert, Julia R., Tanya St. John, Kristin Kawena Begay, Greg E. Davis, Jeffrey Munson, Eric Shankland, Annette Estes, Stephen R. Dager, and Natalia M. Kleinhans. 2020. "Characterizing Olfactory Function in Children with Autism Spectrum Disorder and Children with Sensory Processing Dysfunction" Brain Sciences 10, no. 6: 362. https://doi.org/10.3390/brainsci10060362
APA StyleSweigert, J. R., St. John, T., Begay, K. K., Davis, G. E., Munson, J., Shankland, E., Estes, A., Dager, S. R., & Kleinhans, N. M. (2020). Characterizing Olfactory Function in Children with Autism Spectrum Disorder and Children with Sensory Processing Dysfunction. Brain Sciences, 10(6), 362. https://doi.org/10.3390/brainsci10060362





