Effect of Olfactory and Gustatory Dysfunction and Motor Symptoms on Body Weight in Patients with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Procedures
2.3. Olfactory Function
2.4. Gustatory Function
2.5. Statistical Analysis
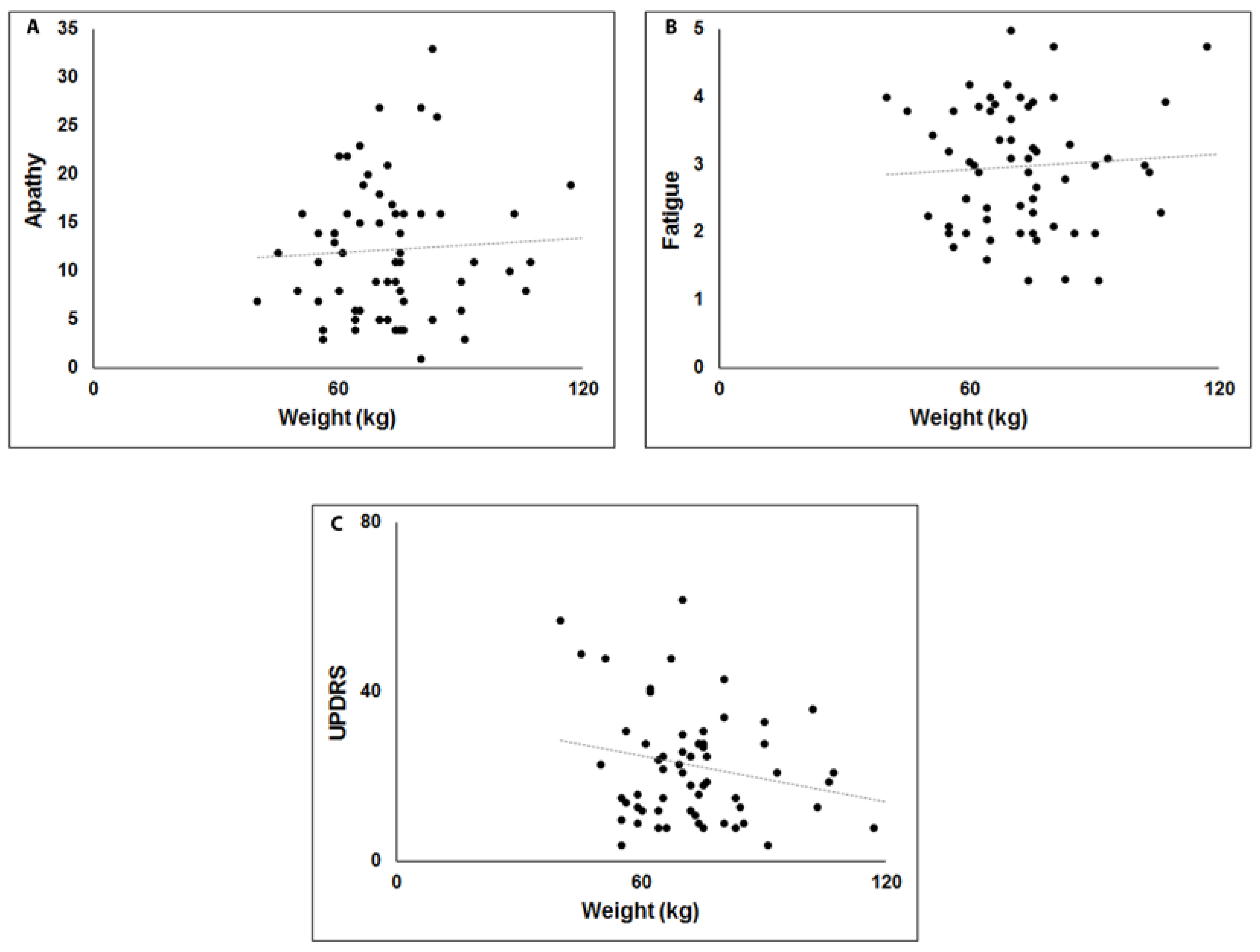
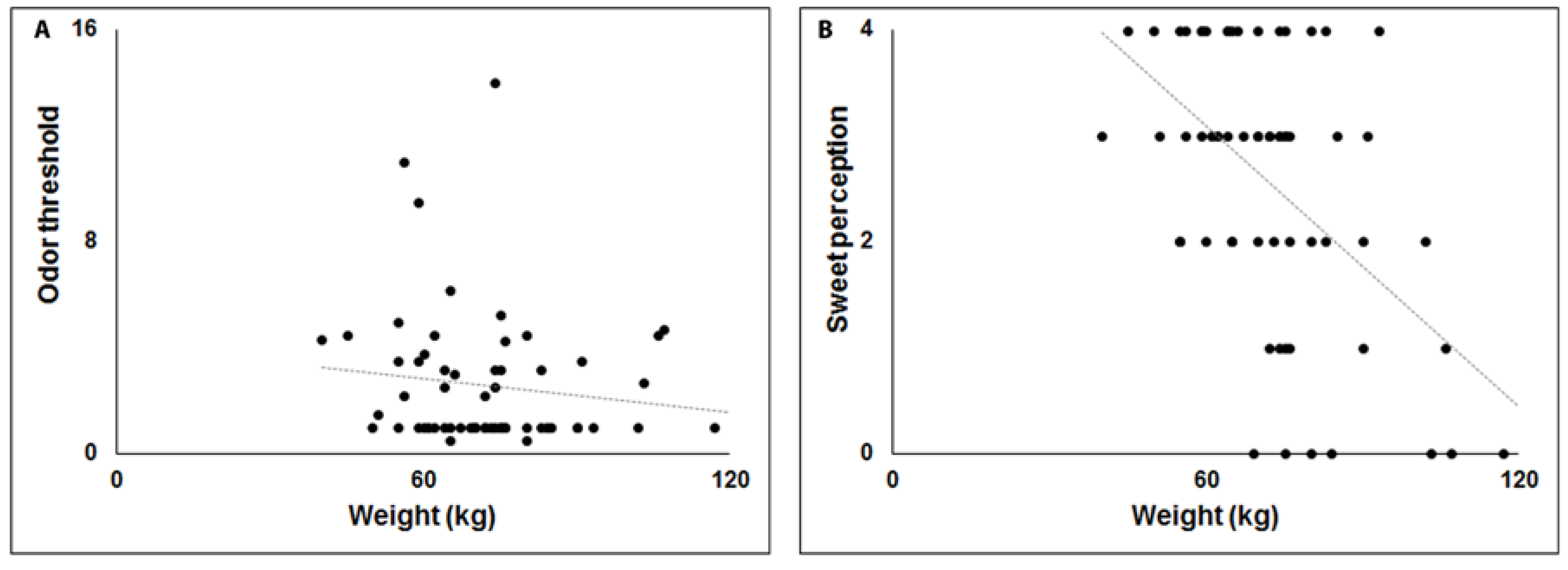
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Standard
Informed Consent
References
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Cecchini, M.P.; Osculati, F.; Ottaviani, S.; Boschi, F.; Fasano, A.; Tinazzi, M. Taste performance in Parkinson’s disease. J. Neural Transm. 2014, 121, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.P.; Fasano, A.; Boschi, F.; Osculati, F.; Tinazzi, M. Taste in Parkinson’s disease. J. Neurol. 2015, 262, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.P.; Federico, A.; Zanini, A.; Mantovani, E.; Masala, C.; Tinazzi, M.; Tamburin, S. Olfaction and taste in Parkinson’s disease: The association with mild cognitive impairment and the single cognitive domain dysfunction. J. Neural. Transm. 2019, 126, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.M.; Hernàn, M.A.; Willet, W.C.; Aschenio, A. Weight loss in Parkinson’s disease. Ann. Neurol. 2003, 53, 676–679. [Google Scholar] [CrossRef]
- Barichella, M.; Cereda, E.; Pezzoli, G. Major Nutritional issues in the management of Parkinson’s disease. Mov. Disord. 2009, 24, 1881–1892. [Google Scholar] [CrossRef]
- Aiello, M.; Eleopra, R.; Rumiati, R.I. Body weight and food intake in Parkinson’s disease. A review of the association to non-motor symptoms. Appetite 2015, 84, 204–211. [Google Scholar] [CrossRef]
- Ma, K.; Xiong, N.; Shen, Y.; Han, C.; Liu, L.; Zhang, G.; Wang, L.; Guo, S.; Guo, X.; Xia, Y.; et al. Weight loss and malnutrition in patients with Parkinson’s disease: Current knowledge and future prospects. Front. Aging Neuro. Sci. 2018, 10, 1. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, E.S.; Lee, J.H.; Moon, J.S.; Oh, J.E.; Shin, J.W.; Lee, K.J.; Baek, I.C.; Jeong, S.H.; Song, H.J.; et al. Relationship between changes of body mass index (BMI) and cognitive decline in Parkinson’s disease (PD). Arch. Gerontol. Geriatr. 2012, 55, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Fluitman, K.S.; Nadar, H.J.; Roos, D.S.; Berendse, H.W.; Keijser, B.J.F.; Nieuwdorp, M.; Ijzerman, R.G.; Visser, M. The association of olfactory function with BMI, appetite, and prospective weight change in Dutch community-dwelling older adults. J. Nutr. Health Aging 2019, 23, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Skrandies, W.; Zschieschang, R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.M.; Del Gaudio, J.M.; Wise, S.K. Higher body mass index in associated with subjective olfactory dysfunction. Behav. Neurol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life-an updated review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Masala, C.; Saba, L.; Cecchini, M.P.; Solla, P.; Loy, F. Olfactory function and age: A Sniffin’ Sticks extended test study performed in Sardinia. Chemosens. Percept. 2018, 11, 19–26. [Google Scholar] [CrossRef]
- Solla, P.; Masala, C.; Liscia, A.; Piras, R.; Ercoli, T.; Fadda, L.; Hummel, T.; Hähner, A.; Defazio, G. Sex-related differences in olfactory function and evaluation of possible confounding factor among patients with Parkinson’s disease. J. Neurol. 2020, 267, 57–63. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Litvan, I.; Bhatia, K.P.; Burn, D.J.; Goetz, C.G.; Lang, A.E.; McKeith, I.; Quinn, N.; Sethi, K.D.; Shults, C.; Wenning, G.K. Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov. Disord. 2003, 18, 467–486. [Google Scholar] [CrossRef]
- Siciliano, M.; Trojano, L.; De Micco, R.; De Mase, A.; Garramone, F.; Russo, A.; Tedeschi, G.; Tessitore, A. Motor, behavioural, and cognitive correlates of fatigue in early, de novo Parkinson disease patients. Parkinsonism Relat. Disord. 2017, 45, 63–68. [Google Scholar] [CrossRef]
- Conti, S.; Bonazzi, S.; Laiacona, M.; Masina, M.; Coralli, M.V. Montreal cognitive assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol. Sci. 2015, 36, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Dittner, A.; Findley, L.; Wessely, S.C. The Parkinson fatigue scale. Parkinsonism Relat. Disord. 2005, 11, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.F.; Alves, G.; Larsen, J.P.; Tysnes, O.B.; Møller, S.G.; Brønnick, K. Psychometric properties of the Starkstein apathy scale in patients with early untreated Parkinson disease. Am. J. Geriatr. Psychiatry 2012, 20, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 50, 318–334. [Google Scholar] [CrossRef]
- Fahn, S.; Elton, R.; Members of the UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Calne, D.B., Goldstein, M., Eds.; McMellam Health Care Information: Florham Park, NJ, USA, 1987; Volume 2, pp. 153–163. [Google Scholar]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. Sniffin’ Sticks’ Olfactory performances assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Masala, C.; Solla, P.; Liscia, A.; Defazio, G.; Saba, L.; Cannas, A.; Cavazzana, A.; Hummel, T.; Haehner, A. Correlation among olfactory function, motors’ symptoms, cognitive impairment, apathy, and fatigue in patients with Parkinson’s disease. J. Neurol. 2018, 265, 1764–1771. [Google Scholar] [CrossRef]
- Masala, C.; Käehling, C.; Fall, F.; Hummel, T. Correlation between olfactory function, trigeminal sensitivity, and nasal anatomy in healthy subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 1649–1654. [Google Scholar] [CrossRef]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Schriver, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Stick normative data based on an extended sample of 9139 subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Masala, C.; Walter, S.; Reichmann, H.; Hummel, T. Incidence of Parkinson’s disease in a large patient cohort with idiopathic smell and taste loss. J. Neurol. 2019, 266, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lorefalt, B.; Ganowiak, W.; Palhagen, S.; Toss, G.; Unosson, M.; Granerus, A.K. Factors of importance for weight loss in elderly patients with Parkinson’s disease. Acta Neurol. Scand. 2004, 110, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Raina, G.B.; Pellene, L.A.; Micheli, F.E.; Calandra, C.R.; Maiola, R. Weight loss in Parkinson’s Disease: The relationship with motor symptoms and disease progression. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef]
- Markus, H.S.; Cox, M.; Tomkins, A.M. Raised resting energy expenditure in Parkinson’s disease and its relationship to muscle rigidity. Clin. Sci. 1992, 83, 199–204. [Google Scholar] [CrossRef]
- Stafford, L.D.; Welbeck, K. High hunger state increases olfactory sensitivity to neutral but not food odors. Chem. Senses 2011, 36, 189–198. [Google Scholar] [CrossRef]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Nirenberg, M.J.; Waters, C. Compulsive eating, and weight gain related to dopamine agonist use. Mov. Disord. 2006, 21, 524–529. [Google Scholar] [CrossRef]
- Miwa, H.; Kondo, T. Alteration of eating behaviors in patients with Parkinson’s disease: Possibly overlooked? Neurocase 2008, 14, 480–484. [Google Scholar] [CrossRef]
- Dujardin, K.; Sockeel, P.; Devos, D.; Delliaux, M.; Krystkowiak, P.; Destée, A.; Defebvre, L. Characteristics of apathy in Parkinson’disease. Mov. Disord. 2007, 22, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.K.; Friedman, J.H.; Amick, M.M. Olfaction and apathy in Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Sunwoo, M.K.; Ham, J.H.; Lee, J.J.; Lee, P.H.; Son, Y.H. Apathy and olfactory dysfunction in early Parkinson’s disease. J. Mov. Disord. 2015, 8, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Spirgi, S.; Meyer, A.; Calabrese, P.; Gschwandtner, U.; Fuhr, P. Effects of Cognitive Performance and Affective Status on Fatigue in Parkinson’s Disease. Dement. Geriatr. Cogn. Dis. Extra 2019, 9, 344–351. [Google Scholar] [CrossRef]
- Uc, E.Y.; Struck, L.K.; Rodnitzky, R.L.; Zimmerman, B.; Dobson, J.; Evans, W.J. Predictors of weight loss in Parkinson’s disease. Mov. Disord. 2006, 21, 930–936. [Google Scholar] [CrossRef]
- Reas, D.L.; Nygård, J.F.; Svensson, E.; Sørensen, T.; Sandanger, I. Changes in body mass index by age, gender, and socio-economic status among a cohort of Norwegian men and women (1990–2001). BMC Public Health 2007, 7, 269. [Google Scholar] [CrossRef]
- Yi, S.W.; Ohrr, H.; Shin, S.A.; Yi, J.J. Sex-age-specific association of body mass index with all-cause mortality among 12.8 million Korean adults: A prospective cohort study. Int. J. Epidemiol. 2015, 44, 1696–1705. [Google Scholar] [CrossRef]
- Cossu, G.; Melis, M.; Sarchioto, M.; Melis, M.; Melis, M.; Morelli, M.; Tomassini Barbarossa, I. 6-n-propylthiouracil taste disruption and TAS2R38 nontasting form in Parkinson’s disease. Mov. Disord. 2018, 33, 1331–1339. [Google Scholar] [CrossRef]
- Melis, M.; Sollai, G.; Masala, C.; Pisanu, C.; Cossu, G.; Melis, M.; Sarchioto, M.; Oppo, V.; Morelli, M.; Crnjar, R.; et al. Odor identification performance in idiopathic Parkinson’s disease is associated with gender and the genetic variability of the olfactory binding protein. Chem. Senses 2019, 44, 311–318. [Google Scholar] [CrossRef]
| Controls (Mean ± SD) | PD (Mean ± SD) | Significance | |
|---|---|---|---|
| Age (years) | 67.9 ± 9.6 | 69.2 ± 10.1 | p = 0.503 |
| Height (m) | 1.63 ± 0.104 | 1.64 ± 0.103 | p = 0.442 |
| Weight (kg) 45–65 years | 68.2 ± 12.2 | 78.9 ± 22.03 | p = 0.047 |
| Weight (kg) ≥ 66 years | 67.72 ± 14.01 | 70.4 ± 13.133 | p = 0.433 |
| Controls (Mean ± SD) | PD (Mean ± SD) | Significance | |
|---|---|---|---|
| Threshold | 5.6 ± 4.4 | 2.6 ± 2.5 | p ≤ 0.005 |
| Discrimination | 10.8 ± 2.7 | 7.3 ± 3.1 | p ≤ 0.005 |
| Identification | 12.1 ± 2.6 | 7.6 ± 3.5 | p ≤ 0.005 |
| TDI score | 28.6 ± 7.3 | 17.6 ± 7.3 | p ≤ 0.005 |
| Cognitive ability | 25.9 ± 3.7 | 21.4 ± 5.8 | p ≤ 0.005 |
| Apathy | 7.3 ± 3.7 | 12.5 ± 6.9 | p ≤ 0.005 |
| Fatigue | 2.1 ± 0.70 | 2.9 ± 0.9 | p ≤ 0.005 |
| Depression | 5.4 ± 5.1 | 14.3 ± 8.7 | p ≤ 0.005 |
| Controls (Mean ±SD) | PD (Mean ± SD) | Significance | |
|---|---|---|---|
| Sweet | 3.3 ± 0.8 | 2.5 ± 1.3 | p ≤ 0.005 |
| Salty | 3.1 ± 1.1 | 1.9 ± 1.5 | p ≤ 0.005 |
| Sour | 2.3 ± 1.5 | 1.7 ± 1.2 | p = 0.012 |
| Bitter | 2.6 ± 1.5 | 1.9 ± 1.5 | p = 0.008 |
| Total taste score | 11.3 ± 3.1 | 8 ± 3.9 | P ≤ 0.005 |
| Unstandardized Coefficients | Standard Coefficients | ||||
|---|---|---|---|---|---|
| B | Std Error | β | t | Significance | |
| Model 1 | |||||
| Fatigue | −12.562 | 5.314 | −0.726 | −2.364 | 0.032 |
| Apathy | 0.815 | 0.358 | 0.501 | 2.275 | 0.038 |
| UPDRS | −0.386 | 0.171 | −0.492 | −2.260 | 0.039 |
| Depression | 0.454 | 0.397 | 0.290 | 1.143 | 0.271 |
| LEDD | 0.010 | 0.013 | 0.168 | 0.752 | 0.463 |
| Model 2 | |||||
| Threshold | −0.781 | 0.346 | −0.287 | −2.257 | 0.028 |
| Discrimination | 0.302 | 0.863 | 0.055 | 0.350 | 0.728 |
| Identification | 0.384 | 0.758 | 0.080 | 0.506 | 0.615 |
| Model 3 | |||||
| Sweet | −5.375 | 1.625 | −0.426 | −3.308 | 0.002 |
| Salty | −0.249 | 1.356 | −0.022 | −0.184 | 0.855 |
| Sour | −0.851 | 1.558 | −0.060 | −0.546 | 0.587 |
| Bitter | −2.444 | 1.360 | −0.222 | −1.797 | 0.078 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masala, C.; Loy, F.; Piras, R.; Liscia, A.; Fadda, L.; Moat, A.; Solla, P.; Defazio, G. Effect of Olfactory and Gustatory Dysfunction and Motor Symptoms on Body Weight in Patients with Parkinson’s Disease. Brain Sci. 2020, 10, 218. https://doi.org/10.3390/brainsci10040218
Masala C, Loy F, Piras R, Liscia A, Fadda L, Moat A, Solla P, Defazio G. Effect of Olfactory and Gustatory Dysfunction and Motor Symptoms on Body Weight in Patients with Parkinson’s Disease. Brain Sciences. 2020; 10(4):218. https://doi.org/10.3390/brainsci10040218
Chicago/Turabian StyleMasala, Carla, Francesco Loy, Raffaella Piras, Anna Liscia, Laura Fadda, Alan Moat, Paolo Solla, and Giovanni Defazio. 2020. "Effect of Olfactory and Gustatory Dysfunction and Motor Symptoms on Body Weight in Patients with Parkinson’s Disease" Brain Sciences 10, no. 4: 218. https://doi.org/10.3390/brainsci10040218
APA StyleMasala, C., Loy, F., Piras, R., Liscia, A., Fadda, L., Moat, A., Solla, P., & Defazio, G. (2020). Effect of Olfactory and Gustatory Dysfunction and Motor Symptoms on Body Weight in Patients with Parkinson’s Disease. Brain Sciences, 10(4), 218. https://doi.org/10.3390/brainsci10040218






