Stress-Induced Increase in Cortisol Negatively Affects the Consolidation of Contextual Elements of Episodic Memories
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Materials
2.3. Salivary Sampling and Biochemical Analysis
2.4. Procedure
3. Results
3.1. Measures
3.2. Outliers
3.3. Analyses
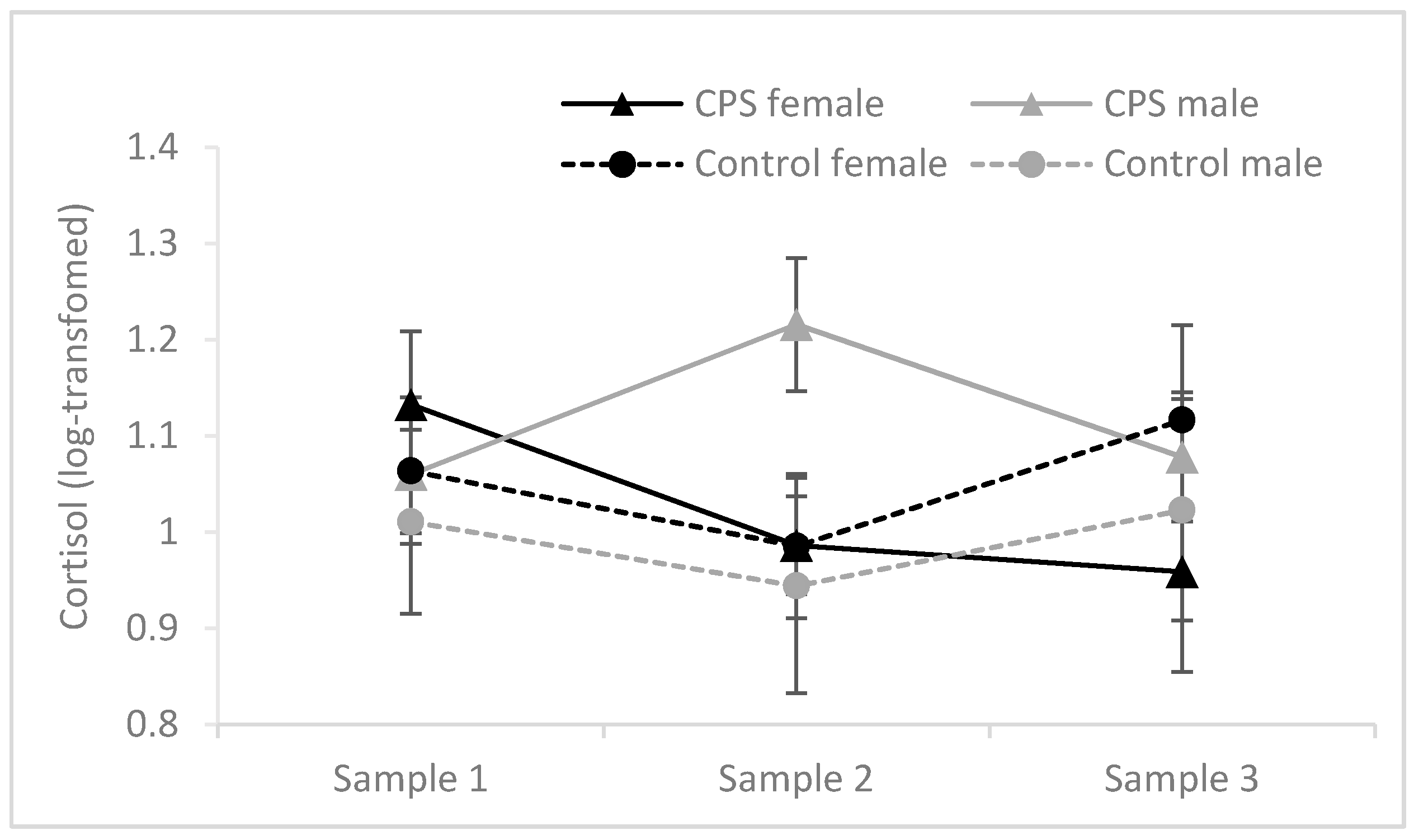
3.3.1. Cortisol Responses
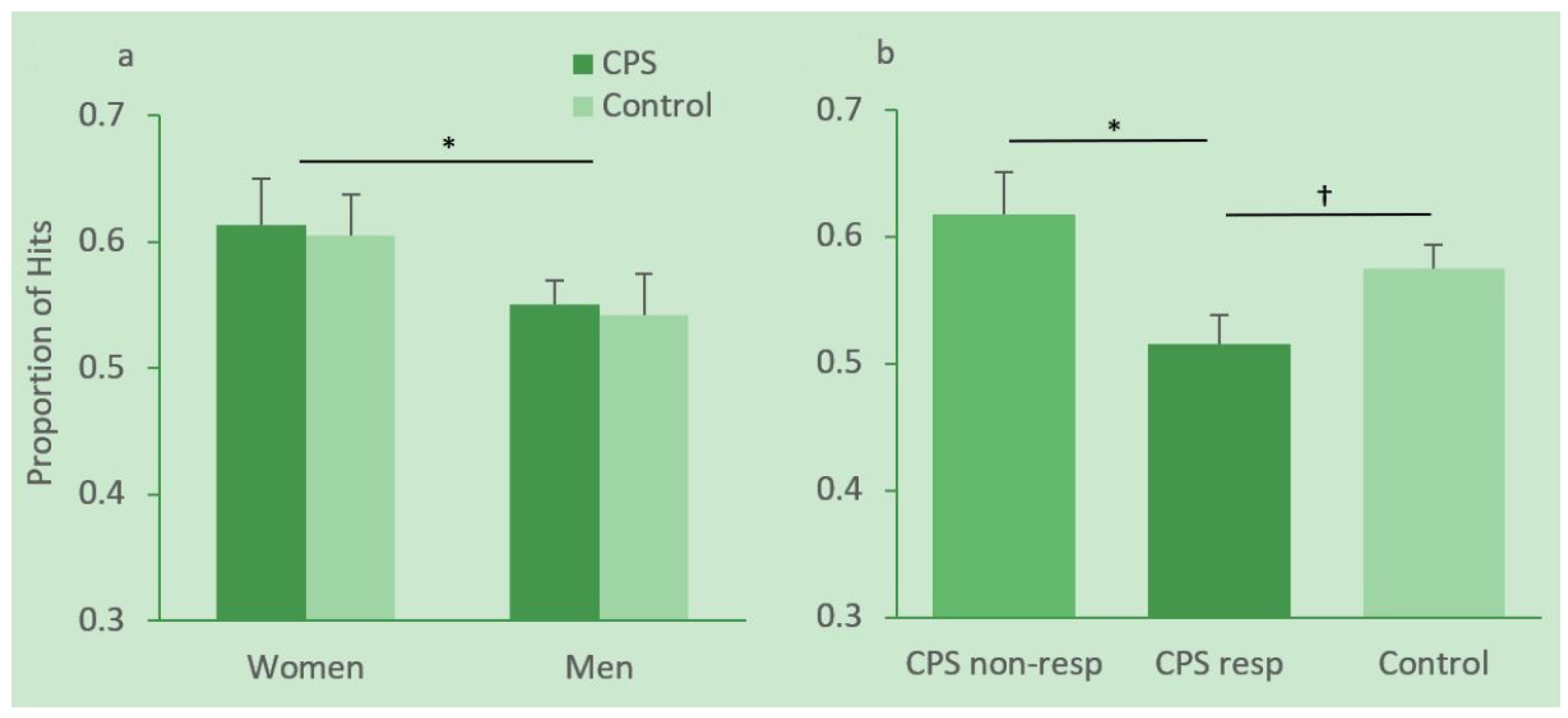
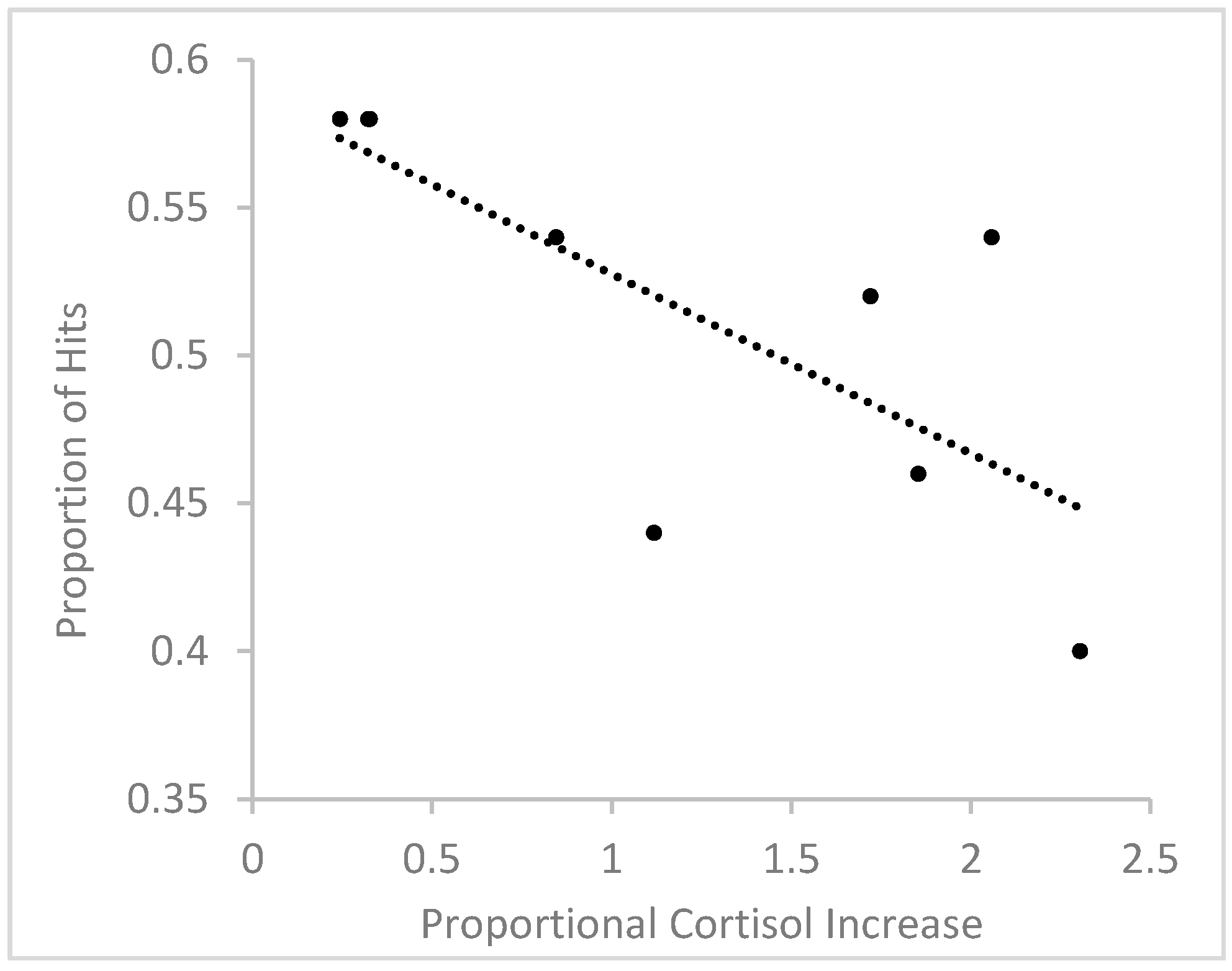
3.3.2. Memory Performance
3.3.3. Subjective Ratings of Pain and Stress and Hormonal Contraceptive use
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Seckl, J.R.; Dickson, K.L.; Yates, C.; Fink, G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res. 1991, 561, 332–337. [Google Scholar] [CrossRef]
- Watzka, M.; Beyenburg, S.; Blümcke, I.; Elger, C.E.; Bidlingmaier, F.; Stoffel-Wagner, B. Expression of mineralocorticoid and glucocorticoid receptor mRNA in the human hippocampus. Neurosci. Lett. 2000, 290, 121–124. [Google Scholar] [CrossRef]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2013, 112, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Joëls, M.; Roozendaal, B.; Wolf, O.T.; Oitzl, M.S. Stress effects on memory: An update and integration. Neurosci. Biobehav. Rev. 2012, 36, 1740–1749. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; McCullough, A.M.; Yonelinas, A.P. The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol. Bull. 2017, 143, 636–675. [Google Scholar] [CrossRef]
- Payne, J.; Jackson, E.; Ryan, L.; Hoscheidt, S.; Jacobs, J.; Nadel, L. The impact of stress on neutral and emotional aspects of episodic memory. Memory 2006, 14, 1–16. [Google Scholar] [CrossRef]
- Payne, J.D.; Jackson, E.D.; Hoscheidt, S.; Ryan, L.; Jacobs, W.J.; Nadel, L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn. Mem. 2007, 14, 861–868. [Google Scholar] [CrossRef]
- Wiemers, U.S.; Sauvage, M.M.; Schoofs, D.; Hamacher-Dang, T.C.; Wolf, O.T. What we remember from a stressful episode. Psychoneuroendocrinology 2013, 38, 2268–2277. [Google Scholar] [CrossRef]
- Gagnon, S.A.; Wagner, A.D. Acute stress and episodic memory retrieval: Neurobiological mechanisms and behavioral consequences. Ann. N. Y. Acad. Sci. 2016, 1369, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Wolf, O.T. Stress and memory retrieval: Mechanisms and consequences. Curr. Opin. Behav. Sci. 2017, 14, 40–46. [Google Scholar] [CrossRef]
- Cahill, L.; Gorski, L.; Le, K. Enhanced Human Memory Consolidation with Post-Learning Stress: Interaction with the Degree of Arousal at Encoding. Learn. Mem. 2003, 10, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Drexler, S.M.; Wolf, O.T. Stress and Memory Consolidation. In Eye Movement Research; Springer: Berlin/Heidelberg, Germany, 2017; pp. 285–300. [Google Scholar]
- Sazma, M.; Shields, G.S.; Yonelinas, A.P. The effects of post-encoding stress and glucocorticoids on episodic memory in humans and rodents. Brain Cogn. 2018, 133, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Yonelinas, A.P.; Parks, C.M.; Koen, J.D.; Jorgenson, J.; Mendoza, S.P. The effects of post-encoding stress on recognition memory: Examining the impact of skydiving in young men and women. Stress 2010, 14, 136–144. [Google Scholar] [CrossRef]
- McCullough, A.M.; Yonelinas, A.P. Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiol. Learn. Mem. 2013, 106, 11–17. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.M.; Ritchey, M.; Ranganath, C.; Yonelinas, A.P. Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiol. Learn. Mem. 2015, 123, 1–10. [Google Scholar] [CrossRef]
- Horner, A.J.; Doeller, C.F. Plasticity of hippocampal memories in humans. Curr. Opin. Neurobiol. 2017, 43, 102–109. [Google Scholar] [CrossRef]
- Diana, R.; Yonelinas, A.P.; Ranganath, C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends Cogn. Sci. 2007, 11, 379–386. [Google Scholar] [CrossRef]
- Ranganath, C. Binding Items and Contexts. Curr. Dir. Psychol. Sci. 2010, 19, 131–137. [Google Scholar] [CrossRef]
- Roediger, H.L.; McDermott, K.B. Creating false memories: Remembering words not presented in lists. J. Exp. Psychol. Learn. Mem. Cogn. 1995, 21, 803. [Google Scholar] [CrossRef]
- Pardilla-Delgado, E.; Alger, S.E.; Cunningham, T.J.; Kinealy, B.P.; Payne, J.D. Effects of post-encoding stress on performance in the DRM false memory paradigm. Learn. Mem. 2016, 23, 46–50. [Google Scholar] [CrossRef]
- Nadel, L.; Payne, J.D. The relationship between episodic memory and context: Clues from memory errors made while under stress. Physiol. Res. 2002, 51, 3. [Google Scholar]
- Smeets, T.; Otgaar, H.P.; Candel, I.; Wolf, O.T. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology 2008, 33, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Laxmi, T.R.; Chattarji, S. Functional Connectivity from the Amygdala to the Hippocampus Grows Stronger after Stress. J. Neurosci. 2013, 33, 7234–7244. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M.; Fernandez, G.; Roozendaal, B. Stress and emotional memory: A matter of timing. Trends Cogn. Sci. 2011, 15, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Murawski, N.J.; Cincotta, C.; McKissick, O.; Finkelstein, A.; Hamidi, A.B.; Merfeld, E.; Doucette, E.; Grella, S.; Shpokayte, M.; et al. Artificially Enhancing and Suppressing Hippocampus-Mediated Memories. Curr. Boil. 2019, 29, 1885–1894. [Google Scholar] [CrossRef]
- Smeets, T.; Sijstermans, K.; Gijsen, C.; Peters, M.; Jelicic, M.; Merckelbach, H. Acute consolidation stress enhances reality monitoring in healthy young adults. Stress 2008, 11, 235–245. [Google Scholar] [CrossRef]
- Simons, J.; Garrison, J.R.; Johnson, M.K. Brain Mechanisms of Reality Monitoring. Trends Cogn. Sci. 2017, 21, 462–473. [Google Scholar] [CrossRef]
- Packard, M.G. Anxiety, cognition, and habit: A multiple memory systems perspective. Brain Res. 2009, 1293, 121–128. [Google Scholar] [CrossRef]
- Goldfarb, E.V.; Mendelevich, Y.; Phelps, E.A. Acute Stress Time-dependently Modulates Multiple Memory Systems. J. Cogn. Neurosci. 2017, 29, 1877–1894. [Google Scholar] [CrossRef]
- Hupbach, A.; Fieman, R. Moderate stress enhances immediate and delayed retrieval of educationally relevant material in healthy young men. Behav. Neurosci. 2012, 126, 819–825. [Google Scholar] [CrossRef][Green Version]
- Hupbach, A.; Dorskind, J.M. Stress selectively affects the reactivated components of a declarative memory. Behav. Neurosci. 2014, 128, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, A.D.; Yonelinas, A.P. Precision, binding, and the hippocampus: Precisely what are we talking about? Neuropsychologia 2020, 138, 107341. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.K.; Moses, S.N.; Riggs, L.; Ryan, J.D. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front. Hum. Neurosci. 2012, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.J.; Winocur, G.; Moscovitch, M. The hippocampus and related neocortical structures in memory transformation. Neurosci. Lett. 2018, 680, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Hannula, D.E.; Ranganath, C. The Eyes Have It: Hippocampal Activity Predicts Expression of Memory in Eye Movements. Neuron 2009, 63, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Haddad, L.; Schachinger, H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 2008, 33, 890–895. [Google Scholar] [CrossRef]
- Miller, R.; Plessow, F.; Kirschbaum, C.; Stalder, T. Classification Criteria for Distinguishing Cortisol Responders From Nonresponders to Psychosocial Stress. Psychosom. Med. 2013, 75, 832–840. [Google Scholar] [CrossRef]
- Jiang, A.; Tran, T.T.; Madison, F.N.; Bakker, A. Acute stress-induced cortisol elevation during memory consolidation enhances pattern separation. Learn. Mem. 2019, 26, 121–127. [Google Scholar] [CrossRef]
- Buchanan, T.W.; Tranel, D.; Adolphs, R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn. Mem. 2006, 13, 382–387. [Google Scholar] [CrossRef]
- Stock, L.-M.; Merz, C.J. Memory retrieval of everyday information under stress. Neurobiol. Learn. Mem. 2018, 152, 32–38. [Google Scholar] [CrossRef]
- Smeets, T.; Van Ruitenbeek, P.; Hartogsveld, B.; Quaedflieg, C.W.E.M. Stress-induced reliance on habitual behavior is moderated by cortisol reactivity. Brain Cogn. 2019, 133, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Kudielka, B.M.; Gaab, J.; Schommer, N.C.; Hellhammer, D.H. Impact of Gender, Menstrual Cycle Phase, and Oral Contraceptives on the Activity of the Hypothalamus-Pituitary-Adrenal Axis. Psychosom. Med. 1999, 61, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Segal, S.K.; Worden, I.V.; Yim, I.S.; Cahill, L. Hormonal contraception use alters stress responses and emotional memory. Boil. Psychol. 2012, 92, 257–266. [Google Scholar] [CrossRef]
- Wolf, O.T.; Schommer, N.C.; Hellhammer, D.H.; McEwen, B.S.; Kirschbaum, C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 2001, 26, 711–720. [Google Scholar] [CrossRef]
- Mordecai, K.L.; Rubin, L.H.; Eatough, E.; Sundermann, E.; Drogos, L.; Savarese, A.M.; Maki, P. Cortisol reactivity and emotional memory after psychosocial stress in oral contraceptive users. J. Neurosci. Res. 2017, 95, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Leal, S.; Yassa, M.A.; Payne, J.D. Post-encoding stress enhances mnemonic discrimination of negative stimuli. Learn. Mem. 2018, 25, 611–619. [Google Scholar] [CrossRef]
- Sekeres, M.J.; Bonasia, K.; St-Laurent, M.; Pishdadian, S.; Winocur, G.; Grady, C.; Moscovitch, M. Recovering and preventing loss of detailed memory: Differential rates of forgetting for detail types in episodic memory. Learn. Mem. 2016, 23, 72–82. [Google Scholar] [CrossRef]
- Sazma, M.; McCullough, A.M.; Shields, G.S.; Yonelinas, A.P. Using acute stress to improve episodic memory: The critical role of contextual binding. Neurobiol. Learn. Mem. 2019, 158, 1–8. [Google Scholar] [CrossRef]
- Gisquet-Verrier, P.; Riccio, D.C. Memory integration: An alternative to the consolidation/reconsolidation hypothesis. Prog. Neurobiol. 2018, 171, 15–31. [Google Scholar] [CrossRef]
- Asperholm, M.; Högman, N.; Rafi, J.; Herlitz, A. What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychol. Bull. 2019, 145, 785–821. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabia, M.; Hupbach, A. Stress-Induced Increase in Cortisol Negatively Affects the Consolidation of Contextual Elements of Episodic Memories. Brain Sci. 2020, 10, 358. https://doi.org/10.3390/brainsci10060358
Sabia M, Hupbach A. Stress-Induced Increase in Cortisol Negatively Affects the Consolidation of Contextual Elements of Episodic Memories. Brain Sciences. 2020; 10(6):358. https://doi.org/10.3390/brainsci10060358
Chicago/Turabian StyleSabia, Matthew, and Almut Hupbach. 2020. "Stress-Induced Increase in Cortisol Negatively Affects the Consolidation of Contextual Elements of Episodic Memories" Brain Sciences 10, no. 6: 358. https://doi.org/10.3390/brainsci10060358
APA StyleSabia, M., & Hupbach, A. (2020). Stress-Induced Increase in Cortisol Negatively Affects the Consolidation of Contextual Elements of Episodic Memories. Brain Sciences, 10(6), 358. https://doi.org/10.3390/brainsci10060358




