Comprehensive Analysis of the Immune and Stromal Compartments of the CNS in EAE Mice Reveal Pathways by Which Chloroquine Suppresses Neuroinflammation
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. EAE Induction and Evaluation
2.3. CQ Treatment
2.4. Isolation of Mononuclear Cells in the CNS of Mice with EAE
2.5. Flow Cytometry
2.6. Isolation of Primary MG and CQ Treatment
2.7. PCRArray and Gene Ontology Analysis
2.8. Co-Culture of MG and T Cells
2.9. Co-Culture of MG and Astrocytes
2.10. Statistical Analyses
3. Results
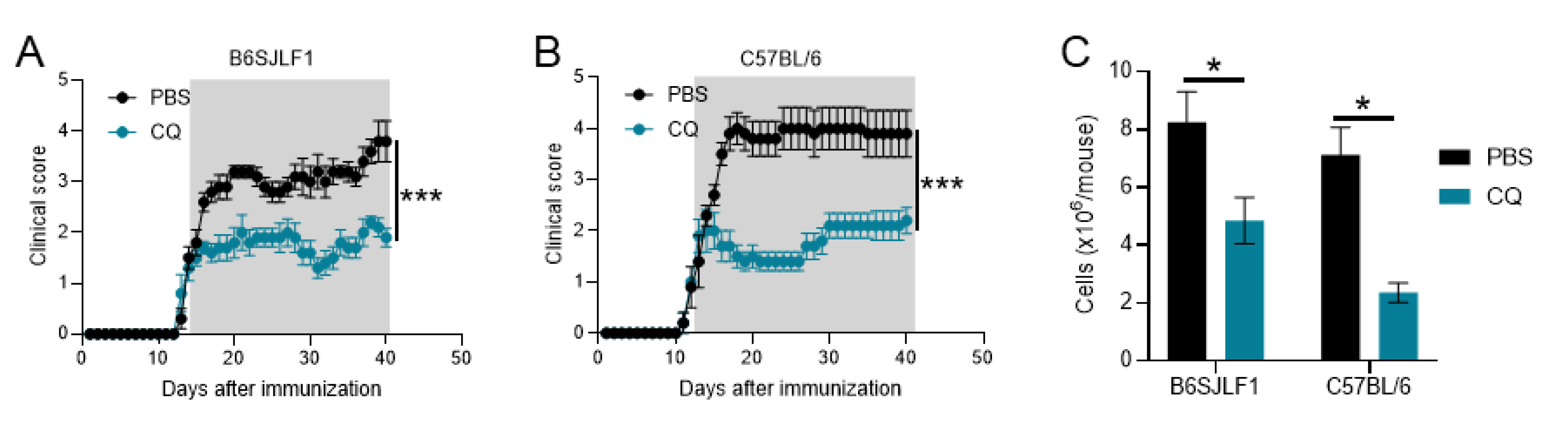
3.1. CQ Reduces Ongoing Inflammation in Relapsing EAE
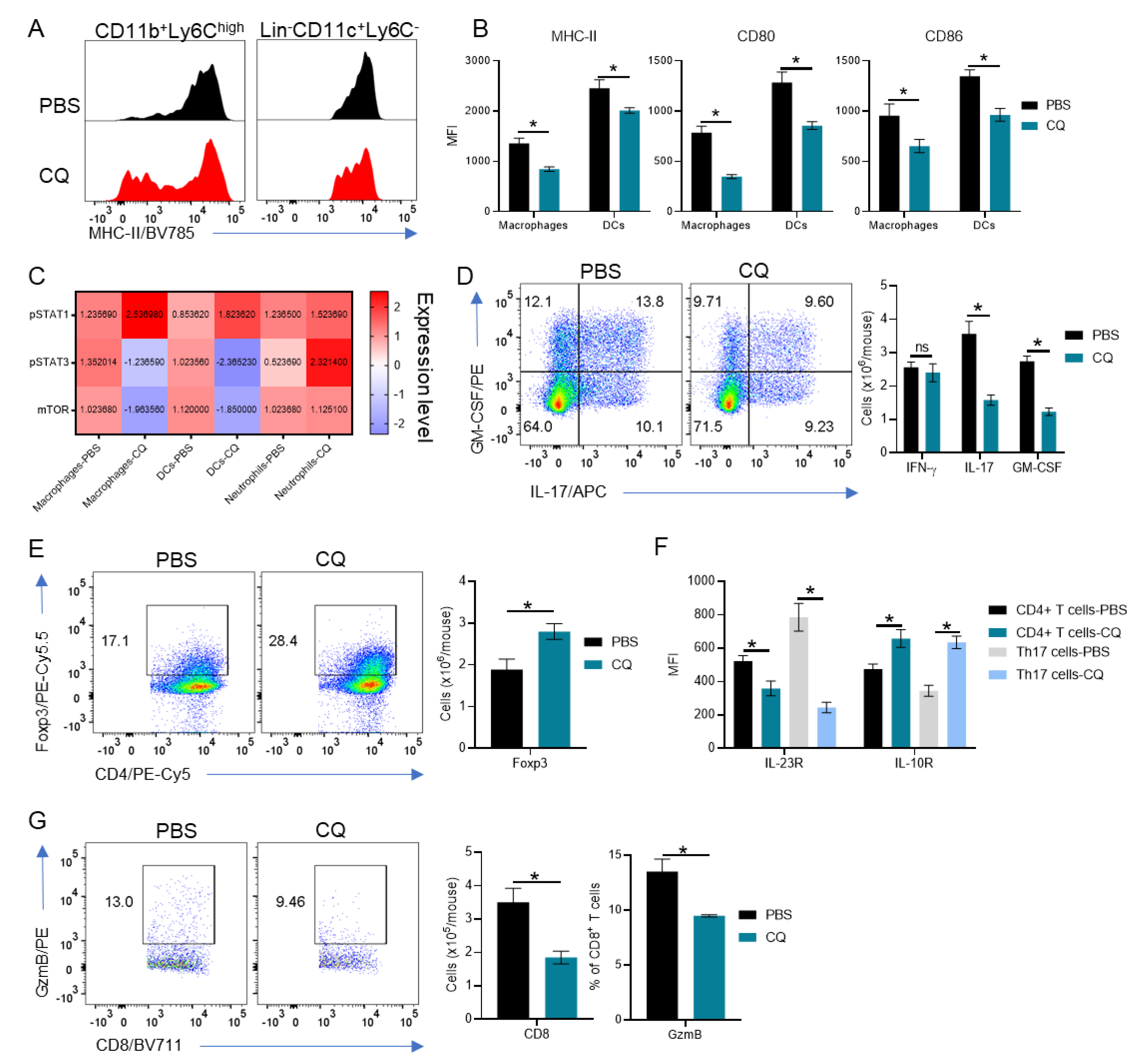
3.2. Peripherally-Derived Leukocytes Are Modulated in CQ-Treated Mice
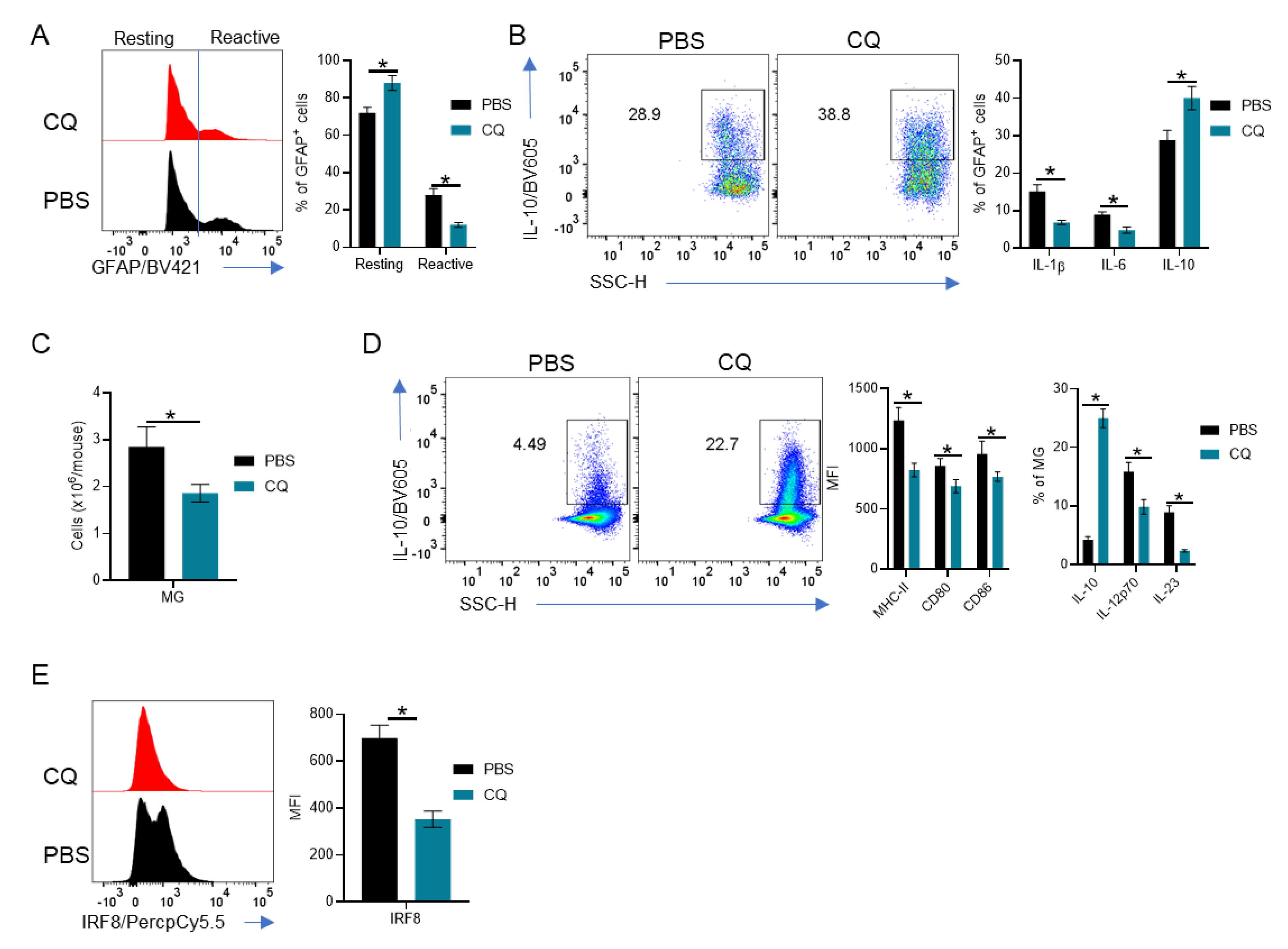
3.3. MG and Astrocytes Are Modulated by CQ
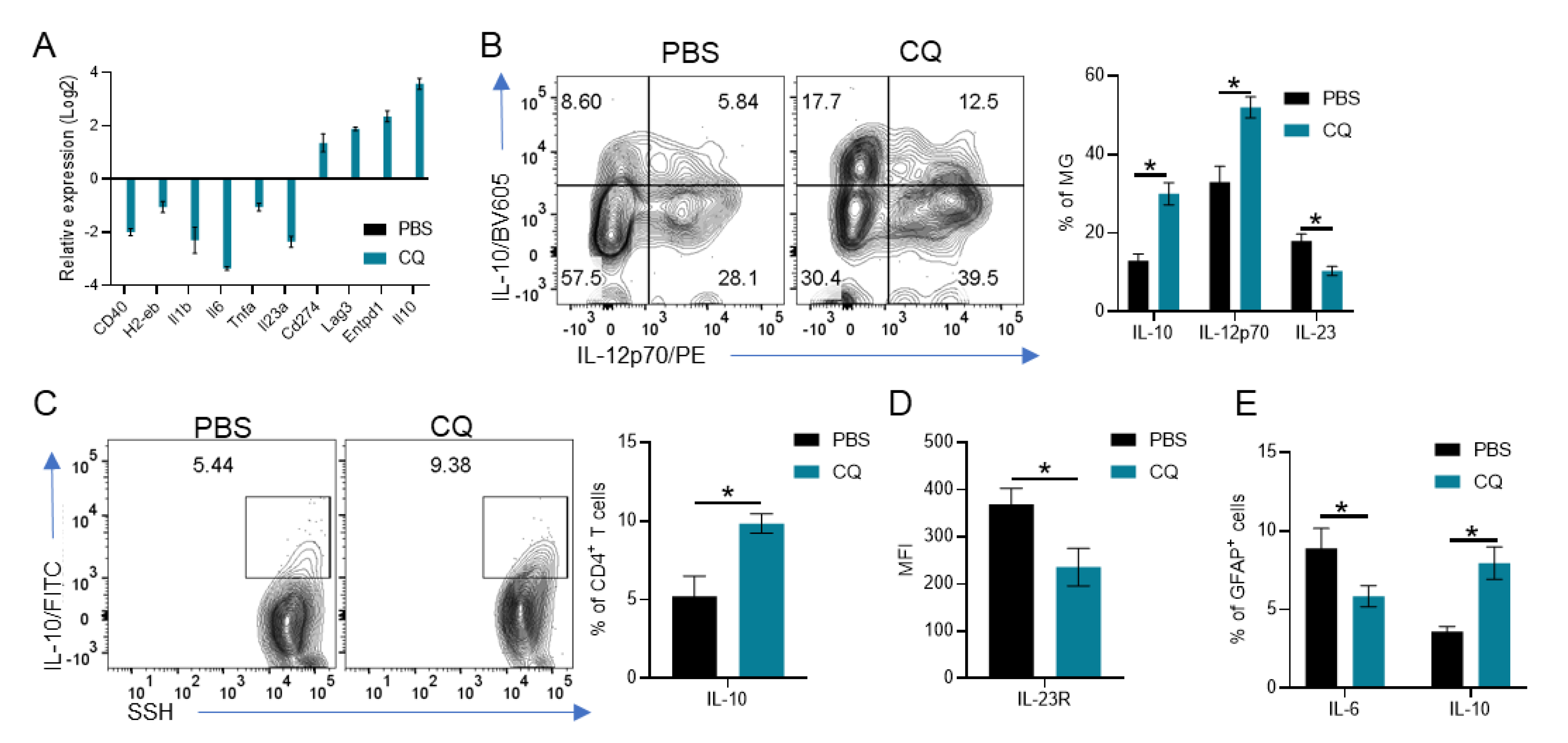
3.4. CQ Induces IL-10 and IL-12p70 in MG Which Enhances IL-10 Production by T Cells and Reduces Astrocyte Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef]
- Baron, J.L.; Madri, J.A.; Ruddle, N.H.; Hashim, G.; Janeway, C.A. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 1993, 177, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Martin, R.; Bergman, C.; Dalal, M.; Ruddle, N.H.; McFarland, H.F. T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity 1993, 15, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Flavell, R.A.; Stockinger, B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 2006, 7, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhenyi, X.; Ying, L.; Hongkun, L.; Xiangdong, G.; Yan, L.; Hongwei, Y.; Lijuan, Z.; Yurong, D.; Zhi, Y. MicroRNA-181c promotes Th17 cell differentiation and mediates experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2018, 70, 305–314. [Google Scholar] [CrossRef]
- Pare, A.; Mailhot, B.; Lévesque, S.A.; Juzwik, C.; Doss, P.M.I.A.; Lécuyer, M.; Prat, A.; Rangachari, M.; Fournier, A.; Lacroix, S. IL-1beta enables CNS access to CCR2(hi) monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1194–E1203. [Google Scholar] [CrossRef]
- El-Behi, M.; Ciric, B.; Hong, D.; Yaping, Y.; Cullimore, M.; Safavi, F.; Guang-Xian, Z.; Dittel, B.N.; Rostami, A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011, 12, 568–575. [Google Scholar] [CrossRef]
- Codarri, L.; Gyülvészi, G.; Tosevski, V.; Hesske, L.; Fontana, A.; Magnenat, L.; Suter, T.; Bechere, B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011, 12, 560–567. [Google Scholar] [CrossRef]
- Komuczki, J.; Tuzlak, S.; Friebel, E.; Hartwig, T.; Spath, S.; Rosenstiel, P.; Waisman, A.; Opitz, L.; Oukka, M.; Schreiner, B.; et al. Fate-Mapping of GM-CSF Expression Identifies a Discrete Subset of Inflammation-Driving T Helper Cells Regulated by Cytokines IL-23 and IL-1beta. Immunity 2019, 50, 1289–1304. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef]
- McIntyre, L.L.; Greilach, S.A.; Othy, S.; Sears-Kraxberger, I.; Wi, B.; Ayala-Angulo, J.; Vu, E.; Pham, Q.; Silva, J.; Dang, K.; et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol. Dis. 2020, 140, 104868. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, Y.; O’Hagan, T.; Dittmer, M.; Penalva, R.; Mayoral, S.R.; Bankhead, P.; Fleville, S.; Eleftheriadis, G.; Chao, Z.; Naughton, M.; et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017, 20, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.L.; Schreiner, B.; McMahon, E.J.; Miller, S.D. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007, 8, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Mundt, S.; Mrdjen, D.; Utz, S.G.; Greter, M.; Schreiner, B.; Becher, B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 2019, 4, eaau8380. [Google Scholar] [CrossRef]
- Chu, F.; Shi, M.; Zheng, C.; Shen, D.; Zhu, J.; Zheng, X.; Cui, L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018, 318, 1–7. [Google Scholar] [CrossRef]
- Tanabe, S.; Saitoh, S.; Miyajima, H.; Itokazu, T.; Yamashita, T. Microglia suppress the secondary progression of autoimmune encephalomyelitis. Glia 2019, 67, 1694–1704. [Google Scholar] [CrossRef]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 2018, 10, e8743. [Google Scholar] [CrossRef]
- Zhang, C.J.; Jiang, M.; Zhou, H.; Liu, W.; Wang, C.; Kang, Z.; Han, B.; Zhang, Q.; Chen, X.; Xiao, J.; et al. TLR-stimulated IRAKM activates caspase-8 inflammasome in microglia and promotes neuroinflammation. J. Clin. Investig. 2018, 128, 5399–5412. [Google Scholar] [CrossRef]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.M.; Takenaka, M.C.; Chao, C.C.; Ardura-Fabregat, A.; Alves de Lima, K.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [PubMed]
- Chrzanowski, U.; Bhattarai, S.; Scheld, M.; Clarner, T.; Fallier-Becker, P.; Beyer, C.; Rohr, S.O.; Schmitz, C.; Hochstrasser, T.; Schweiger, F.; et al. Oligodendrocyte degeneration and concomitant microglia activation directs peripheral immune cells into the forebrain. Neurochem. Int. 2019, 126, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Thome, R.; Lopes, S.C.P.; Costa, F.T.M.; Verinaud, L. Chloroquine: Modes of action of an undervalued drug. Immunol. Lett. 2013, 153, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Choi, J.H.; Byun, M.S.; Jue, D.M. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology 2006, 45, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Liu, X.; Wang, N.; Zhou, H.; Zheng, J. Chloroquine attenuates LPS-mediated macrophage activation through miR-669n-regulated SENP6 protein translation. Am. J. Transl. Res. 2015, 7, 2335–2345. [Google Scholar]
- Thome, R.; Issayama, L.K.; DiGangi, R.; Bombeiro, A.L.; da Costa, T.A.; Ferreira, I.T.; de Oliveira, A.L.R.; Verinaud, L. Dendritic cells treated with chloroquine modulate experimental autoimmune encephalomyelitis. Immunol. Cell Biol. 2014, 92, 124–132. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.R.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.Q.; Liang, X.Y.; Lv, J.D.; Jin, X.; Liu, J.Y.; Mo, S.Q.; Zhang, T.Z.; et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef]
- Verinaud, L.; Issayama, L.K.; Zanucoli, F.; de Carvalho, A.C.; da Costa, T.A.; Di Gangi, R.; Bonfanti, A.P.; Ferreira, I.T.; de Oliveira, A.L.R.; Machado, D.R.S.; et al. Nitric oxide plays a key role in the suppressive activity of tolerogenic dendritic cells. Cell Mol. Immunol. 2015, 12, 384–386. [Google Scholar] [CrossRef][Green Version]
- Thome, R.; Boehm, A.; Ishikawa, L.L.W.; Casella, G.; Munhoz, J.; Ciric, B.; Zhang, G.X.; Rostami, A. Chloroquine reduces Th17 cell differentiation by stimulating T-bet expression in T cells. Cell Mol. Immunol. 2020. [Google Scholar]
- Thome, R.; Bonfanti, A.P.; Rasouli, J.; Mari, E.R.; Zhang, G.X.; Rostami, A.; Verinaud, L. Chloroquine-treated dendritic cells require STAT1 signaling for their tolerogenic activity. Eur. J. Immunol. 2018, 48, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Thome, R.; Moore, J.N.; Mari, E.R.; Rasouli, J.; Hwang, D.; Yoshimura, S.; Ciric, B.; Zhang, G.X.; Rostami, A.M. Induction of Peripheral Tolerance in Ongoing Autoimmune Inflammation Requires Interleukin 27 Signaling in Dendritic Cells. Front. Immunol. 2017, 8, 1392. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Thome, R.; Konno, S.; Mari, E.R.; Rasouli, J.; Hwang, D.; Boehm, A.; Li, Y.; Zhang, G.X.; Ciric, B.; et al. IL-9 Controls Central Nervous System Autoimmunity by Suppressing GM-CSF Production. J. Immunol. 2020, 204, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Thome, R.; Moraes, A.S.; Bombeiro, A.L.; Farias Ados, S.; Francelin, C.; da Costa, T.A.; Di Gangi, R.; dos Santos, L.M.; de Oliveira, A.L.; Verinaud, L. Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS ONE 2013, 8, e65913. [Google Scholar] [CrossRef]
- Skundric, D.S.; Dai, R.; Zakarian, V.L.; Zhou, W. Autoimmune-induced preferential depletion of myelin-associated glycoprotein (MAG) is genetically regulated in relapsing EAE (B6 x SJL) F1 mice. Mol. Neurodegener. 2008, 3, 7. [Google Scholar] [CrossRef]
- Zhang, G.X.; Yu, S.; Gran, B.; Li, J.; Calida, D.; Ventura, E.; Chen, X.; Rostami, A. T cell and antibody responses in remitting-relapsing experimental autoimmune encephalomyelitis in (C57BL/6 x SJL) F1 mice. J. Neuroimmunol. 2004, 148, 1–10. [Google Scholar] [CrossRef]
- Frik, J.; Merl-Pham, J.; Plesnila, N.; Mattugini, N.; Kjell, J.; Kraska, J.; Gómez, R.M.; Hauck, S.M.; Sirko, S.; Götz, M. Cross-talk between monocyte invasion and astrocyte proliferation regulates scarring in brain injury. EMBO Rep. 2018, 19, e45294. [Google Scholar] [CrossRef]
- Anderson, M.A.; Ao, Y.; Sofroniew, M.V. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014, 565, 23–29. [Google Scholar] [CrossRef]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Shibata, K.; Yoshida, K.; Shigetomi, E.; Gachet, C.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y1 Receptor Downregulation. Cell Rep. 2017, 19, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, A.; Løbner, M.; Cédile, O.; Owens, T. Comparison of microglia and infiltrating CD11c(+) cells as antigen presenting cells for T cell proliferation and cytokine response. J. Neuroinflamm. 2014, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ohmori, K.; Fujiwara, M. Immune regulation by brain cells in the central nervous system: Microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology 1992, 76, 209–216. [Google Scholar] [PubMed]
- Hu, J.; He, H.; Yang, Z.; Zhu, G.; Kang, L.; Jing, X.; Lu, H.; Song, W.; Bai, B.; Tang, H. Programmed Death Ligand-1 on Microglia Regulates Th1 Differentiation via Nitric Oxide in Experimental Autoimmune Encephalomyelitis. Neurosci. Bull. 2016, 32, 70–82. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, K.; Sun, Y.; Cao, Y.; Yang, J. Transcriptional mechanism of IRF8 and PU.1 governs microglial activation in neurodegenerative condition. Protein Cell 2019, 10, 87–103. [Google Scholar] [CrossRef]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Masuda, T.; Iwamoto, S.; Mikuriya, S.; Tozaki-Saitoh, H.; Tamura, T.; Tsuda, M.; Inoue, K. Transcription factor IRF1 is responsible for IRF8-mediated IL-1beta expression in reactive microglia. J. Pharmacol. Sci. 2015, 128, 216–220. [Google Scholar] [CrossRef]
- Masuda, T.; Tsuda, M.; Yoshinaga, R.; Tozaki-Saitoh, H.; Ozato, K.; Tamura, T.; Inoue, K. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012, 1, 334–340. [Google Scholar] [CrossRef]
- Jiang, H.; Braunstein, N.S.; Yu, B.; Winchester, R.; Chess, L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc. Natl. Acad. Sci. USA 2001, 98, 6301–6306. [Google Scholar] [CrossRef]
- Leuenberger, T.; Paterka, M.; Reuter, E.; Herz, J.; Niesner, R.A.; Radbruch, H.; Bopp, T.; Zipp, F.; Siffrin, V. The role of CD8+ T cells and their local interaction with CD4+ T cells in myelin oligodendrocyte glycoprotein35-55-induced experimental autoimmune encephalomyelitis. J. Immunol. 2013, 191, 4960–4968. [Google Scholar] [CrossRef]
- Bettini, M.; Rosenthal, K.; Evavold, B.D. Pathogenic MOG-reactive CD8+ T cells require MOG-reactive CD4+ T cells for sustained CNS inflammation during chronic EAE. J. Neuroimmunol. 2009, 213, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Wan, X.; Shahzad, K.A.; Zhang, L.; Song, S.; Jin, X.; Wang, L.; Zhao, C.; Shen, C. Direct modulation of myelin-autoreactive CD4(+) and CD8(+) T cells in EAE mice by a tolerogenic nanoparticle co-carrying myelin peptide-loaded major histocompatibility complexes, CD47 and multiple regulatory molecules. Int. J. Nanomed. 2018, 13, 3731–3750. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Jang, Y.; Kim, W.; Shin, J.; Toh, H.T.; Kim, C.H.; Yoon, H.S.; Leblanc, P.; Kim, K.S. Chloroquine modulates inflammatory autoimmune responses through Nurr1 in autoimmune diseases. Sci. Rep. 2019, 9, 15559. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Chen, Y.; Wang, C.; Yang, C.; Wu, Z.H.; Xue, J.; Ye, L.; Wang, S.; Liu, H.F.; Pan, Q. Chloroquine Autophagic Inhibition Rebalances Th17/Treg-Mediated Immunity and Ameliorates Systemic Lupus Erythematosus. Cell Physiol. Biochem. 2017, 44, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Erny, D.; Hagemeyer, N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 2017, 18, 385–392. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A.; et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef]
- Chu, T.; Tran, T.; Yang, F.; Beech, W.; Cole, G.M.; Frautschy, S.A. Effect of chloroquine and leupeptin on intracellular accumulation of amyloid-beta (A beta) 1-42 peptide in a murine N9 microglial cell line. FEBS Lett. 1998, 436, 439–444. [Google Scholar] [CrossRef][Green Version]
- Koch, M.W.; Zabad, R.; Giuliani, F.; Hader, W., Jr.; Lewkonia, R.; Metz, L.; Wee Yong, V. Hydroxychloroquine reduces microglial activity and attenuates experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2015, 358, 131–137. [Google Scholar] [CrossRef]
- Barateiro, A.; Brites, D.; Fernandes, A. Oligodendrocyte Development and Myelination in Neurodevelopment: Molecular Mechanisms in Health and Disease. Curr. Pharm. Des. 2016, 22, 656–679. [Google Scholar] [CrossRef]
- Domingues, H.S.; Cruz, A.; Chan, J.R.; Relvas, J.B.; Rubinstein, B.; Pinto, I.M. Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia 2018, 66, 5–14. [Google Scholar] [CrossRef]
- Foster, A.Y.; Bujalka, H.; Emery, B. Axoglial interactions in myelin plasticity: Evaluating the relationship between neuronal activity and oligodendrocyte dynamics. Glia 2019, 67, 2038–2049. [Google Scholar] [CrossRef]
- Aharoni, R.; Eilam, R.; Domev, H.; Labunskay, G.; Sela, M.; Arnon, R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc. Natl. Acad. Sci. USA 2005, 102, 19045–19050. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, A.; Boutière, C.; Rico, A.; Audoin, B.; Pelletier, J. How much progress has there been in the second-line treatment of multiple sclerosis: A 2017 update. Rev. Neurol. 2018, 174, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Tyor, W.R. Update on disease-modifying therapies for multiple sclerosis. J. Investig. Med. 2017, 65, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; White, N.J. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clin. Pharmacokinet. 1996, 30, 263–299. [Google Scholar] [CrossRef]
- Gustafsson, L.L.; Walker, O.; Alván, G.; Beermann, B.; Estevez, F.; Gleisner, L.; Lindström, B.; Sjöqvist, F. Disposition of chloroquine in man after single intravenous and oral doses. Br. J. Clin. Pharmacol. 1983, 15, 471–479. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thome, R.; Boehm, A.; Ishikawa, L.L.W.; Casella, G.; Munhoz, J.; Ciric, B.; Zhang, G.-X.; Rostami, A. Comprehensive Analysis of the Immune and Stromal Compartments of the CNS in EAE Mice Reveal Pathways by Which Chloroquine Suppresses Neuroinflammation. Brain Sci. 2020, 10, 348. https://doi.org/10.3390/brainsci10060348
Thome R, Boehm A, Ishikawa LLW, Casella G, Munhoz J, Ciric B, Zhang G-X, Rostami A. Comprehensive Analysis of the Immune and Stromal Compartments of the CNS in EAE Mice Reveal Pathways by Which Chloroquine Suppresses Neuroinflammation. Brain Sciences. 2020; 10(6):348. https://doi.org/10.3390/brainsci10060348
Chicago/Turabian StyleThome, Rodolfo, Alexandra Boehm, Larissa Lumi Watanabe Ishikawa, Giacomo Casella, Jaqueline Munhoz, Bogoljub Ciric, Guang-Xian Zhang, and Abdolmohamad Rostami. 2020. "Comprehensive Analysis of the Immune and Stromal Compartments of the CNS in EAE Mice Reveal Pathways by Which Chloroquine Suppresses Neuroinflammation" Brain Sciences 10, no. 6: 348. https://doi.org/10.3390/brainsci10060348
APA StyleThome, R., Boehm, A., Ishikawa, L. L. W., Casella, G., Munhoz, J., Ciric, B., Zhang, G.-X., & Rostami, A. (2020). Comprehensive Analysis of the Immune and Stromal Compartments of the CNS in EAE Mice Reveal Pathways by Which Chloroquine Suppresses Neuroinflammation. Brain Sciences, 10(6), 348. https://doi.org/10.3390/brainsci10060348




