Sensory Profiles of Children with Autism Spectrum Disorder with and without Feeding Problems: A Comparative Study in Sicilian Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Measures
2.4. Statistical Analysis
2.5. Ethics Committee Approval
3. Results
3.1. Comparisons Between ASD-W and ASD-WO Subgroups
3.2. ASD-WO Intra-Group Analysis
3.3. ASD-W Intra-Group Analysis
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watling, R.; Deitz, J.; White, O. Comparison of sensory profile scores of young children with and without autism spectrum disorders. Am. J. Occup. Ther. 2001, 55, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Tomchek, S.D.; Dunn, W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.A. Sensory processing in children with autism spectrum disorders and impact on functioning. Pediatr. Clin. N. Am. 2012, 59, 203–214. [Google Scholar] [CrossRef]
- Brockevelt, B.L.; Nissen, R.; Schweinle, W.E.; Kurtz, E.; Larson, K.J. A comparison of the Sensory Profile scores of children with autism and an age- and gender-matched sample. S. D. J. Med. 2013, 66, 463–465. [Google Scholar]
- Green, D.; Chandler, S.; Charman, T.; Simonoff, E.; Baird, G. Brief Report: DSM-5 Sensory behaviours in children with and without an Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 3597–3606. [Google Scholar] [CrossRef]
- McCormick, C.; Hepburn, S.; Young, G.S.; Rogers, S.J. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: A longitudinal study. Autism 2016, 20, 572–579. [Google Scholar] [CrossRef]
- Miller, L.J.; Anzalone, M.E.; Lane, S.; Cermak, S.A.; Osten, E. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Ausderau, K.; Sideris, J.; Furlong, M.; Little, L.; Bulluck, J.C.; Baranek, G.T. National survey of sensory features in children with ASD: Factor structure of the sensory experience questionnaire (3.0). J. Autism Dev. Disord. 2014, 44, 915–925. [Google Scholar] [CrossRef]
- Baranek, G.T.; David, F.J.; Poe, M.D.; Stone, W.L.; Watson, L.R. Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 2006, 47, 591–601. [Google Scholar] [CrossRef]
- Lane, A.E.; Young, R.L.; Baker, A.E.; Angley, M.T. Sensory processing subtypes in autism: Association with adaptive behavior. J. Autism Dev. Disord. 2010, 40, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.; Saulnier, C.; Fein, D.; Kinsbourne, M. Sensory and attention abnormalities in autistic spectrum disorders. Autism 2006, 10, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Ausderau, K.K.; Furlong, M.; Sideris, J.; Bulluk, J.; Little, L.M.; Watson, L.R.; Boyd, B.A.; Belger, A.; Dickie, V.A.; Baranek, G.T. Sensory subtypes in children with autism spectrum disorder: Latent profile transition analysis using a national survey of sensory features. J. Child Psychol. Psychiatry 2014, 55, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Ashburner, J.; Ziviani, J.; Rodger, S. Sensory processing and classroom emotional, behavioral, and educational outcomes in children with autism spectrum disorder. Am. J. Occup. Ther. 2008, 62, 564–573. [Google Scholar] [CrossRef]
- Wiggins, L.D.; Robins, D.L.; Bakeman, R.; Adamson, L.B. Brief report: Sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. J. Autism Dev. Disord. 2009, 39, 1087–1091. [Google Scholar] [CrossRef]
- Fernández-Andrés, M.I.; Pastor-Cerezuela, G.; Sanz-Cervera, P.; Tárraga-Mínguez, R. A comparative study of sensory processing in children with and without autism spectrum disorder in the home and classroom environments. Res. Dev. Disabil. 2015, 38, 202–212. [Google Scholar] [CrossRef]
- Sanz-Cervera, P.; Pastor-Cerezuela, G.; González-Sala, F.; Tárraga-Mínguez, R.; Fernández-Andrés, M.I. Sensory processing in children with autism spectrum disorder and/or attention deficit/hyperactivity disorder in the home and classroom context. Front. Psychol. 2017, 8, 1772. [Google Scholar] [CrossRef]
- Sanz-Cervera, P.; Pastor-Cerezuela, G.; Fernández-Andrés, M.I.; Tárraga-Mínguez, R. Sensory processing in children with autism spectrum disorder: Relationship with non-verbal IQ, autism severity and attention deficit/hyperactivity disorder symptomatology. Res. Dev. Disabil. 2015, 45, 188–201. [Google Scholar] [CrossRef]
- Kargas, N.; López, B.; Reddy, V.; Morris, P. The relationship between auditory processing and restricted, repetitive behaviors in adults with autism spectrum disorders. J. Autism Dev. Disord. 2015, 45, 658–668. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Balikçi, Ö.S.; Çiyiltepe, M. Feeding problems of children with autism. Int. J. Soc. Sci. 2017, 3, 870–880. [Google Scholar] [CrossRef][Green Version]
- Smith, B.; Rogers, S.L.; Blisset, J.; Ludlow, A.K. The relationship between sensory sensitivity, food fussiness and food preferences in children with neurodevelopmental disorders. Appetite 2020, 150, 104643. [Google Scholar] [CrossRef] [PubMed]
- Kodak, T.; Piazza, C.C. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Twachtman-Reilly, J.; Amaral, S.C.; Zebrowsk, P.P. Addressing feeding disorders in children on the autism spectrum in school- based settings: Physiological and behavioral issues. Lang. Speech Hear. Serv. 2008, 39, 261–272. [Google Scholar] [CrossRef]
- Panerai, S.; Suraniti, G.S.; Catania, V.; Carmeci, R.; Elia, M.; Ferri, R. Improvements in mealtime behaviors of children with special needs following a day-center-based behavioral intervention for feeding problems. Riv. Psichiatr. 2018, 53, 299–308. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef]
- Zobel-Lachiusa, J.; Andrianopoulos, M.V.; Mailloux, Z.; Cermak, S.A. Sensory differences and mealtime behavior in children with Autism. Am. J. Occup. Ther. 2015, 69, 6905185050p1–6905185050p8. [Google Scholar] [CrossRef]
- Nadon, G.; Feldman, D.E.; Dunn, W.; Gisel, E. Association of sensory processing and eating problems in children with autism spectrum disorders. Autism Res. Treat. 2011, 2011, 541926. [Google Scholar] [CrossRef]
- Chistol, L.T.; Bandini, L.G.; Must, A.; Phillips, S.; Cermak, S.A.; Curtin, C. Sensory sensitivity and food selectivity in children with autism spectrum disorder. J. Autism Dev. Disord. 2018, 48, 583–591. [Google Scholar] [CrossRef]
- Dunn, W. The Sensory Profile: User’s Manual; Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Al-Heizan, M.O.; AlAbdulwahab, S.S.; Kachanathu, S.J.; Natho, M. Sensory processing dysfunction among Saudi children with and without autism. J. Phys. Ther. Sci. 2015, 27, 1313–1316. [Google Scholar] [CrossRef]
- Lukens, C.T.; Linscheid, T.R. Development and validation of an inventory to assess mealtime behavior problems in children with autism. J. Autism Dev. Disord. 2008, 38, 342–352. [Google Scholar] [CrossRef] [PubMed]
- DeMand, A.; Johnson, C.; Foldes, E. Psychometric Properties of the Brief Autism Mealtime Behaviors Inventory. J. Autism Dev. Disord. 2015, 45, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the children’s eating behaviour questionnaire. J. Child Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, D.N.; Miller, L.J.; Shyu, V. Development and validation of the short sensory profile (SSP). In The Sensory Profile: Examiner’s Manual; Dunn, W., Ed.; The Psychological Corporation: San Antonio, TX, USA, 1999; pp. 59–73. [Google Scholar]
- Little, L.M.; Freuler, A.C.; Houser, M.B.; Guckian, L.; Carbine, K.; David, F.J.; Baranek, G.T. Psychometric validation of the sensory experiences questionnaire. Am. J. Occup. Ther. 2011, 65, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; Watson, L.R.; Boyd, B.A.; Poe, M.D.; David, F.J.; McGuire, L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Dev. Psychopathol. 2013, 25, 307–320. [Google Scholar] [CrossRef]
- Baranek, G.T.; Boyd, B.A.; Poe, M.D.; David, F.J.; Watson, L.R. Hyperresponsive sensory patterns in young children with autism, developmental delay, and typical development. Am. J. Ment. Retard. 2007, 112, 233–245. [Google Scholar] [CrossRef]
- Schoen, S.A.; Miller, L.J.; Green, K.E. Pilot study of the sensory over-responsivity scales: Assessment and inventory. Am. J. Occup. Ther. 2008, 62, 393–406. [Google Scholar] [CrossRef]
- Ledford, J.R.; Gast, D.L. Feeding problems in children with autism spectrum disorders: A review. Focus Autism Dev. Disabil. 2006, 21, 153–166. [Google Scholar] [CrossRef]
- Sharp, W.G.; Berry, R.C.; McCracken, C.; Nuhu, N.; Marvel, E.; Saulnier, C.A.; Klin, A.; Jones, W.; Jaquess, D.L. Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. J. Autism Dev. Disord. 2013, 43, 2159–2173. [Google Scholar] [CrossRef]
- O’Donnell, S.; Deitz, J.; Kartin, D.; Nalty, T.; Dawson, G. Sensory processing, problem behavior, adaptive behavior, and cognition in preschool children with autism spectrum disorders. Am. J. Occup. Ther. 2012, 66, 586–594. [Google Scholar] [CrossRef]
- Boudjarane, M.A.; Grandgeorge, M.; Marianowski, R.; Misery, L.; Lemonnier, E. Perception of odors and tastes in autism spectrum disorders: A systematic review of assessments. Autism Res. 2017, 10, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Bennetto, L.; Kuschner, E.S.; Hyman, S.L. Olfaction and taste processing in autism. Biol. Psychiatry 2007, 62, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.H.; Stevenson, R.A.; Wallace, M.T. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015, 134, 140–160. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.A.; Ingeholm, J.E.; Wohltjen, S.; Collins, M.; Riddell, C.D.; Gotts, S.J.; Kenworthy, L.; Wallace, G.L.; Simmons, W.K.; Martin, A. Neural correlates of taste reactivity in autism spectrum disorder. NeuroImage Clin. 2018, 19, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Wodka, E.L.; Mostofsky, S.H.; Puts, N.A.J. Autism spectrum disorder in the scope of tactile processing. Dev. Cogn. Neurosci. 2018, 29, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Koehler, L.; Fournel, A.; Albertowski, K.; Roessner, V.; Gerber, J.; Hummel, C.; Hummel, T.; Bensafi, M. Impaired odor perception in autism spectrum disorder is associated with decreased activity in olfactory cortex. Chem. Senses 2018, 43, 627–634. [Google Scholar] [CrossRef]
- Lane, A.E.; Molloy, C.A.; Bishop, S.L. Classification of children with autism spectrum disorder by sensory subtype: A case for sensory-based phenotypes. Autism Res. 2014, 7, 322–333. [Google Scholar] [CrossRef]
| TitleSample Features | ASD-W | ASD-WO | z = | p ≤ | Effect Size 3 r |
|---|---|---|---|---|---|
| N = | 37 | 74 | |||
| Males/Females | 30/7 | 56/18 | NS 1 | ||
| Severity level 3/2/1 | 27/8/2 | 45/22/7 | NS 1 | ||
| Chronological age, months | 60 (44–76) | 63.5 (45.2–74.0) | NS 2 | ||
| BAMBI 18 total scores | 41 (38–46) | 26.5 (22.25–29.75) | −8.44 | 0.00001 2 | 0.8 |
| BAMBI 18 subdomains | |||||
| Food Selectivity | 14 (12–16) | 9 (7–11) | −7.1 | 0.00001 2 | 0.67 |
| Disruptive Behaviors | 13 (10–16) | 7.5 (6–9) | −6.48 | 0.00001 2 | 0.61 |
| Food Refusal | 7 (5–9) | 4 (3–4.75) | −7.02 | 0.00001 2 | 0.67 |
| Mealtime Rigidity | 8 (6–11) | 4 (3–7) | −5.69 | 0.00001 2 | 0.54 |
| CEBQ | 93 (84–105) | 10 (7–16) | −8.54 | 0.00001 2 | 0.81 |
| CEBQ subdomains | |||||
| Food Responsiveness | 12 (7–16) | 10 (7–16) | −0.424 | NS 2 | |
| Emotional Over-eating | 5 (4–8) | 6 (4–7) | 0.237 | NS 2 | |
| Enjoyment of food | 13 (10–16) | 16 (13–17) | 2.939 | 0.038 2 | 0.28 |
| Desire to Drink | 7 (5–9) | 6 (5–8) | −0.88 | NS 2 | |
| Satiety responsiveness | 12 (10–15) | 11 (8–13) | −2.36 | 0.018 2 | 0.22 |
| Slowness in Eating | 12 (10–15) | 9 (8–12) | −2.13 | 0.033 2 | 0.2 |
| Emotional Under-eating | 10 (6–12) | 8 (6–12) | −1.84 | NS 2 | |
| Food Fussiness | 21 (18–26) | 16 (12–19) | −4.58 | 0.00001 2 | 0.435 |
| MeasuresTitle | ASD-W break//N = 37 | ASD-WO N = 74 | z = | p ≤ 1 | Effect Size r = 2 |
|---|---|---|---|---|---|
| SEQ Total scores | 51 (41–62) | 46.5 (39–57) | −1.94 | 0.026 | 0.18 |
| SEQ subsections | |||||
| HY | 21 (18–28) | 19 (16–23) | −2.49 | 0.006 | 0.24 |
| HY-S | 11 (8–15) | 10 (7–12) | −1.75 | 0.04 | 0.17 |
| HY-NS | 11 (10–13) | 10 (7.25–12) | −2.52 | 0.006 | 0.24 |
| HO | 29 (23–34) | 29 (22–34) | −1.03 | NS | |
| HO-S | 8 (5–9) | 7 (5–9) | −1.13 | NS | |
| HO-NS | 22 (18–24) | 20 (17–25.75) | −0.89 | NS | |
| SSP Total scores | 132 (113–149) | 156 (140.25–165.5) | 4.5 | 0.00001 | 0.43 |
| SSP subsections | |||||
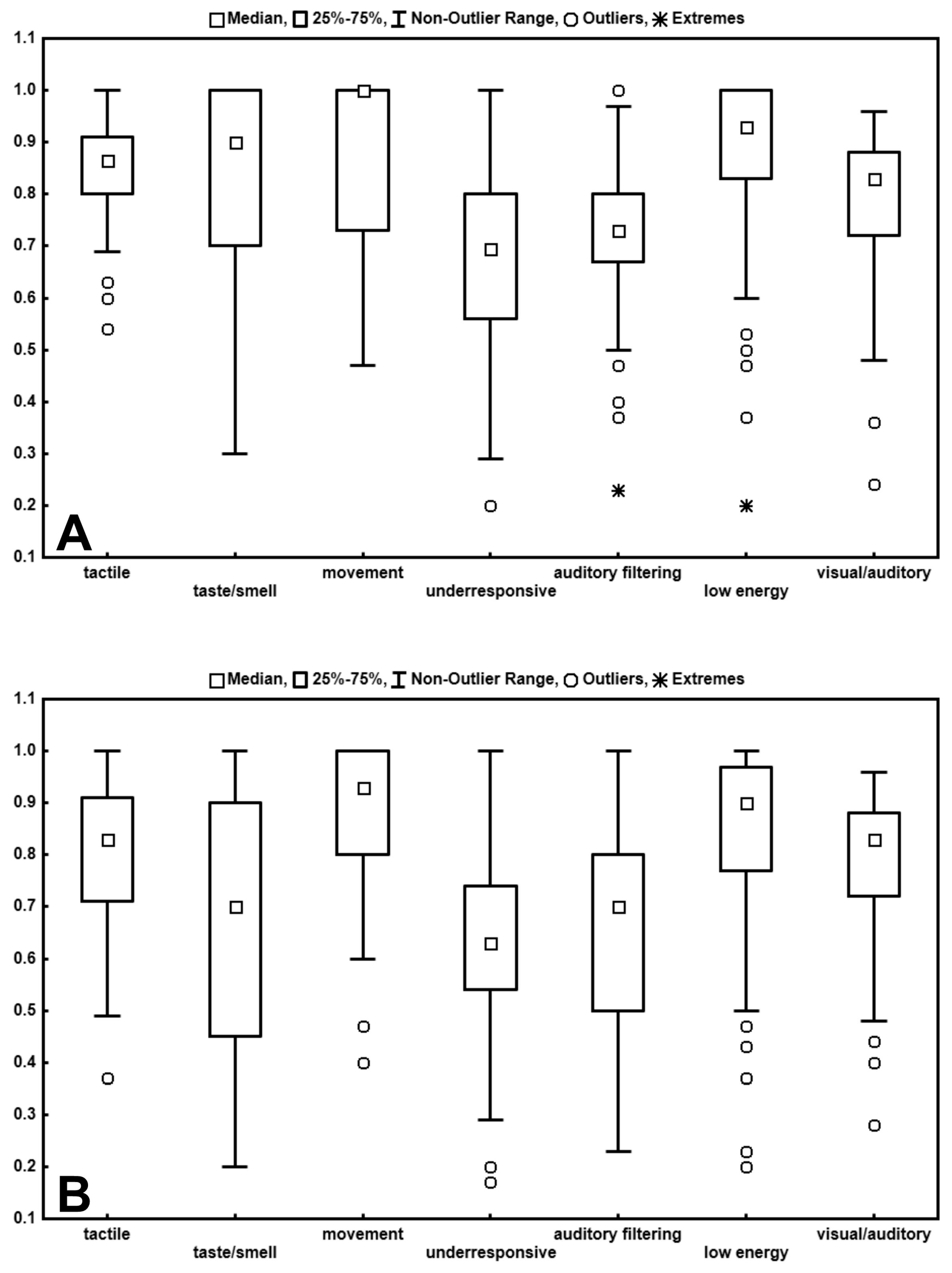
| Tactile sensitivity | 27 (23–31) | 30.5 (28–33) | 2.99 | 0.0014 | 0.28 |
| Taste/Smell sensitivity | 9 (7–13) | 18 (14–20) | 5.42 | 0.00001 | 0.515 |
| Movement sensitivity | 13 (10–15) | 15 (11.25–15) | 1.87 | 0.03 | 0.18 |
| Under-responsive/Seeks sensation | 21 (17–22) | 24.5 (19.25–27.75) | 3.43 | 0.0003 | 0.33 |
| Auditory filtering | 16 (14–22) | 22 (20–24) | 3.79 | 0.00008 | 0.36 |
| Low energy/Weak | 26 (20–28) | 28 (25.25–30) | 2.77 | 0.028 | 0.26 |
| Visual/Auditory sensitivity | 20 (17–22) | 21 (18–23) | 1.004 | NS |
| SSP Performance CategoriesTitle | All ASD (N = 111) | ASD-W (N = 37) | ASD-WO (N = 74) | ASD-W vs. ASD-WO p ≤ 1 | Cramer’s V Effect Size | Tomchek and Dunn, 2007 [3] | Nadon et al., 2011 [29] | AL-Heizan et al., 2015 [32] |
|---|---|---|---|---|---|---|---|---|
| SSP Total scores | ||||||||
| Definite Difference | 43.2 | 73 | 28.4 | 0.00001 3 | 0.46 | 83.6 | 65.3 2 | 84.8 |
| Probable Difference | 15.3 | 10.8 | 17.6 | 11.4 | 21.1 | 8.7 | ||
| Typical Performance | 41.4 | 16.2 | 54 | 5 | 13.7 | 6.5 | ||
| SSP subsections: | ||||||||
| Tactile sensitivity | ||||||||
| Definite Difference | 28.8 | 48.6 | 19 | 0.000015 4 | 0.33 | 60.9 | 36.8 | 60.9 |
| Probable Difference | 22.5 | 21.6 | 23 | 18.5 | 24.2 | 21.7 | ||
| Typical Performance | 48.7 | 29.7 | 58 | 20.6 | 37.9 | 17.4 | ||
| Taste/Smell sensitivity | ||||||||
| Definite Difference | 28.8 | 62.2 | 12.2 | 0.00001 5 | 0.54 | 54.1 | 48.4 | 52.2 |
| Probable Difference | 18.9 | 16.2 | 20.2 | 13.9 | 15.8 | 19.6 | ||
| Typical Performance | 52.3 | 21.6 | 67.6 | 32 | 34.7 | 28.26 | ||
| Movement sensitivity | ||||||||
| Definite Difference | 14.4 | 24.3 | 9.5 | 0.0013 6 | 0.26 | 23.1 | 28.4 | 50 |
| Probable Difference | 17.1 | 8.1 | 21.6 | 21 | 20 | 15.2 | ||
| Typical Derformance | 68.5 | 67.6 | 68.9 | 55.9 | 51.6 | 34.8 | ||
| Under-responsive/Seeks sensation | ||||||||
| Definite Difference | 54.1 | 78.4 | 41.9 | 0.00001 7 | 0.38 | 86.1 | 67.4 | 89.1 |
| Probable Difference | 22.5 | 13.5 | 27 | 7.5 | 16.8 | 2.2 | ||
| Typical Derformance | 23.4 | 8 | 31.1 | 6.4 | 15.8 | 8.7 | ||
| Auditory Filtering | ||||||||
| Definite Difference | 39.6 | 70.3 | 24.3 | 0.00001 8 | 0.47 | 77.6 | 55.8 | 73.9 |
| Probable Difference | 23.5 | 8.1 | 31.1 | 14.6 | 24.2 | 8.7 | ||
| Typical Derformance | 36.9 | 21.6 | 44.6 | 7.8 | 20 | 17.4 | ||
| Low Energy/Weak | ||||||||
| Definite difference | 26.1 | 37.8 | 20.2 | 0.0035 9 | 0.24 | 23.1 | 43.2 | 58.7 |
| Probable difference | 7.2 | 10.8 | 5.4 | 18.9 | 12.6 | 10.9 | ||
| Typical performance | 66.7 | 51.3 | 74.3 | 58 | 44.2 | 30.4 | ||
| Visual/Auditory sensitivity | ||||||||
| Definite difference | 10.8 | 13.5 | 9.5 | NS | 43.8 | 22.1 | 34.8 | |
| Probable difference | 23.4 | 27 | 21.6 | 25.3 | 31.6 | 19.6 | ||
| Typical performance | 65.8 | 59.4 | 68.9 | 31 | 46.3 | 45.7 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panerai, S.; Ferri, R.; Catania, V.; Zingale, M.; Ruccella, D.; Gelardi, D.; Fasciana, D.; Elia, M. Sensory Profiles of Children with Autism Spectrum Disorder with and without Feeding Problems: A Comparative Study in Sicilian Subjects. Brain Sci. 2020, 10, 336. https://doi.org/10.3390/brainsci10060336
Panerai S, Ferri R, Catania V, Zingale M, Ruccella D, Gelardi D, Fasciana D, Elia M. Sensory Profiles of Children with Autism Spectrum Disorder with and without Feeding Problems: A Comparative Study in Sicilian Subjects. Brain Sciences. 2020; 10(6):336. https://doi.org/10.3390/brainsci10060336
Chicago/Turabian StylePanerai, Simonetta, Raffaele Ferri, Valentina Catania, Marinella Zingale, Daniela Ruccella, Donatella Gelardi, Daniela Fasciana, and Maurizio Elia. 2020. "Sensory Profiles of Children with Autism Spectrum Disorder with and without Feeding Problems: A Comparative Study in Sicilian Subjects" Brain Sciences 10, no. 6: 336. https://doi.org/10.3390/brainsci10060336
APA StylePanerai, S., Ferri, R., Catania, V., Zingale, M., Ruccella, D., Gelardi, D., Fasciana, D., & Elia, M. (2020). Sensory Profiles of Children with Autism Spectrum Disorder with and without Feeding Problems: A Comparative Study in Sicilian Subjects. Brain Sciences, 10(6), 336. https://doi.org/10.3390/brainsci10060336







