Clinical Predictors of 3- and 6-Month Outcome for Mild Traumatic Brain Injury Patients with a Negative Head CT Scan in the Emergency Department: A TRACK-TBI Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design of the TRACK-TBI Pilot Study
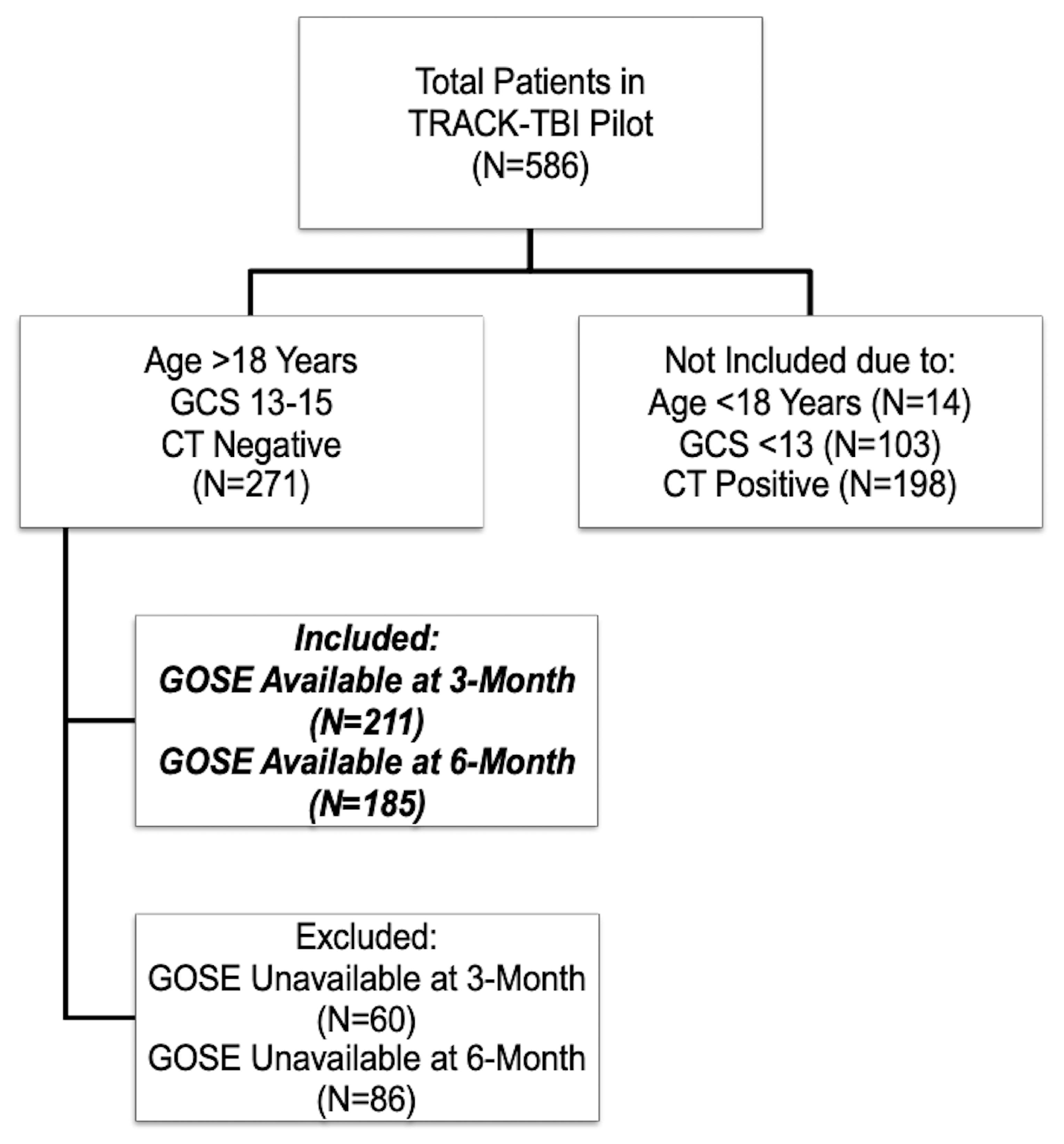
2.2. Participants Included in the Current Analysis
2.3. Outcome Measure
2.4. Statistical Analysis
3. Results
3.1. Univariate Predictors of 3- and 6-Month GOSE
3.2. Multivariable Predictors of 3- and 6-Month GOSE
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
TRACK-TBI INVESTIGATORS (to be indexed under “Collaborators” in PubMed)
References
- Marincowitz, C.; Lecky, F.E.; Townend, W.; Borakati, A.; Fabbri, A.; Sheldon, T.A. The Risk of Deterioration in GCS13-15 Patients with Traumatic Brain Injury Identified by Computed Tomography Imaging: A Systematic Review and Meta-Analysis. J. Neurotrauma 2018, 35, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, C.; Coronado, V.G.; Taylor, C.A.; Xu, L. Epidemiology of Isolated Versus Nonisolated Mild Traumatic Brain Injury Treated in Emergency Departments in the United States, 2006–2012: Sociodemographic Characteristics. J. Head Trauma Rehabil. 2017, 32, E37–E46. [Google Scholar] [CrossRef] [PubMed]
- Selassie, A.W.; Fakhry, S.M.; Ford, D.W. Population-based study of the risk of in-hospital death after traumatic brain injury: The role of sepsis. J. Trauma 2011, 71, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Jain, S.; Giacino, J.T.; Levin, H.; Dikmen, S.; Nelson, L.D.; Vassar, M.J.; Okonkwo, D.O.; Diaz-Arrastia, R.; Robertson, C.S.; et al. Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients After Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA Psychiatry 2019, 76, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.J.; Cassidy, J.D.; Cancelliere, C.; Cote, P.; Hincapie, C.A.; Kristman, V.L.; Holm, L.W.; Borg, J.; Nygren-de Boussard, C.; Hartvigsen, J.; et al. Systematic Review of the Prognosis after Mild Traumatic Brain Injury in Adults: Cognitive, Psychiatric, and Mortality Outcomes: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S152–S173. [Google Scholar] [CrossRef]
- Cnossen, M.C.; Winkler, E.A.; Yue, J.K.; Okonkwo, D.O.; Valadka, A.; Steyerberg, E.W.; Lingsma, H.; Manley, G.T.; the TRACK-TBI Investigators. Development of a Prediction Model for Post-Concussive Symptoms following Mild Traumatic Brain Injury: A TRACK-TBI Pilot Study. J. Neurotrauma 2017, 34, 2396–2409. [Google Scholar] [CrossRef]
- McMahon, P.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T.; et al. Symptomatology and Functional Outcome in Mild Traumatic Brain Injury: Results from the Prospective TRACK-TBI study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef]
- Kraus, J.F.; Hsu, P.; Schafer, K.; Afifi, A.A. Sustained Outcomes Following Mild Traumatic Brain Injury: Results of a Five-Emergency Department Longitudinal Study. Brain Inj. 2014, 28, 1248–1256. [Google Scholar] [CrossRef]
- Ratcliff, J.J.; Adeoye, O.; Lindsell, C.J.; Hart, K.W.; Pancioli, A.; McMullan, J.T.; Yue, J.K.; Nishijima, D.K.; Gordon, W.A.; Valadka, A.B.; et al. ED Disposition of the Glasgow Coma Scale 13 to 15 Traumatic Brain Injury Patient: Analysis of the Transforming Research and Clinical Knowledge in TBI study. Am. J. Emerg. Med. 2014, 32, 844–850. [Google Scholar] [CrossRef]
- Hsia, R.Y.; Markowitz, A.J.; Lin, F.; Guo, J.; Madhok, D.Y.; Manley, G.T. Ten-Year Trends in Traumatic Brain Injury: A Retrospective Cohort Study of California Emergency Department and Hospital Revisits and Readmissions. BMJ Open 2018, 8, e022297. [Google Scholar] [CrossRef]
- Ganti, L.; Conroy, L.M.; Bodhit, A.; Daneshvar, Y.; Patel, P.S.; Ayala, S.; Kuchibhotla, S.; Hatchitt, K.; Pulvino, C.; Peters, K.R.; et al. Understanding Why Patients Return to the Emergency Department after Mild Traumatic Brain Injury within 72 Hours. West J. Emerg. Med. 2015, 16, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Pandor, A.; Goodacre, S.; Harnan, S.; Holmes, M.; Pickering, A.; Fitzgerald, P.; Rees, A.; Stevenson, M. Diagnostic Management Strategies for Adults and Children with Minor Head Injury: A Systematic Review and an Economic Evaluation. Health Technol. Assess. 2011, 15, 1–202. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Vassar, M.J.; Lingsma, H.F.; Cooper, S.R.; Okonkwo, D.O.; Valadka, A.B.; Gordon, W.A.; Maas, A.I.; Mukherjee, P.; Yuh, E.L.; et al. Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot: Multicenter Implementation of the Common Data Elements for Traumatic Brain Injury. J. Neurotrauma 2013, 30, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Si, B.; Dumkrieger, G.; Wu, T.; Zafonte, R.; Valadka, A.B.; Okonkwo, D.O.; Manley, G.T.; Wang, L.; Dodick, D.W.; Schwedt, T.J. Sub-classifying Patients with Mild Traumatic Brain Injury: A Clustering Approach Based on Baseline Clinical Characteristics and 90-day and 180-day Outcomes. PLoS ONE 2018, 13, e0198741. [Google Scholar] [CrossRef]
- Barbosa, R.R.; Jawa, R.; Watters, J.M.; Knight, J.C.; Kerwin, A.J.; Winston, E.S.; Barraco, R.D.; Tucker, B.; Bardes, J.M.; Rowell, S.E. Evaluation and Management of Mild Traumatic Brain Injury: An Eastern Association for the Surgery of Trauma Practice Management Guideline. J. Trauma Acute Care Surg. 2012, 73, S307–S314. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Gardner, A.J.; Brubacher, J.R.; Panenka, W.J.; Li, J.J.; Iverson, G.L. Systematic Review of Multivariable Prognostic Models for Mild Traumatic Brain Injury. J. Neurotrauma 2015, 32, 517–526. [Google Scholar] [CrossRef]
- Tavender, E.J.; Bosch, M.; Green, S.; O’Connor, D.; Pitt, V.; Phillips, K.; Bragge, P.; Gruen, R.L. Quality and Consistency of Guidelines for the Management of Mild Traumatic Brain Injury in the Emergency Department. Acad. Emerg. Med. 2011, 18, 880–889. [Google Scholar] [CrossRef]
- Bossers, S.M.; Pol, K.M.; Ophuis, E.O.; Jacobs, B.; Visser, M.C.; Loer, S.A.; Boer, C.; van der Naalt, J.; Schober, P. Discrepancy between the Initial Assessment of Injury Severity and Post Hoc Determination of Injury Severity in Patients with Apparently Mild Traumatic Brain Injury: A Retrospective Multicenter Cohort Analysis. Eur. J. Trauma Emerg. Surg. 2018, 44, 889–896. [Google Scholar] [CrossRef]
- Theadom, A.; Starkey, N.; Barker-Collo, S.; Jones, K.; Ameratunga, S.; Feigin, V.; BIONIC4you Research Group. Population-Based Cohort Study of the Impacts of Mild Traumatic Brain Injury in Adults Four Years Post-Injury. PLoS ONE 2018, 13, e0191655. [Google Scholar] [CrossRef]
- van der Naalt, J.; Timmerman, M.E.; de Koning, M.E.; van der Horn, H.J.; Scheenen, M.E.; Jacobs, B.; Hageman, G.; Yilmaz, T.; Roks, G.; Spikman, J.M. Early Predictors of Outcome after Mild Traumatic Brain Injury (UPFRONT): An Observational Cohort Study. Lancet Neurol. 2017, 16, 532–540. [Google Scholar] [CrossRef]
- de Koning, M.E.; Scheenen, M.E.; van der Horn, H.J.; Hageman, G.; Roks, G.; Spikman, J.M.; van der Naalt, J. Non-Hospitalized Patients with Mild Traumatic Brain Injury: The Forgotten Minority. J. Neurotrauma 2017, 34, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Duhaime, A.C.; Gean, A.D.; Haacke, E.M.; Hicks, R.; Wintermark, M.; Mukherjee, P.; Brody, D.; Latour, L.; Riedy, G.; Common Data Elements Neuroimaging Working Group Members; et al. Common Data Elements in Radiologic Imaging of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.; Harrison-Felix, C.L.; Menon, D.; Adelson, P.D.; Balkin, T.; Bullock, R.; Engel, D.C.; Gordon, W.; Orman, J.L.; Lew, H.L.; et al. Common Data Elements for Traumatic Brain Injury: Recommendations from the Interagency Working Group on Demographics and Clinical Assessment. Arch. Phys. Med. Rehabil. 2010, 91, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Diaz-Arrastia, R.; Brophy, M.; Engel, D.; Goodman, C.; Gwinn, K.; Veenstra, T.D.; Ling, G.; Ottens, A.K.; Tortella, F.; et al. Common Data Elements for Traumatic Brain Injury: Recommendations from the Biospecimens and Biomarkers Working Group. Arch. Phys. Med. Rehabil. 2010, 91, 1667–1672. [Google Scholar] [CrossRef]
- Wilde, E.A.; Whiteneck, G.G.; Bogner, J.; Bushnik, T.; Cifu, D.X.; Dikmen, S.; French, L.; Giacino, J.T.; Hart, T.; Malec, J.F.; et al. Recommendations for the Use of Common Outcome Measures in Traumatic Brain Injury Research. Arch. Phys. Med. Rehabil. 2010, 91, 1650–1660.e17. [Google Scholar] [CrossRef]
- Stulemeijer, M.; van der Werf, S.; Borm, G.F.; Vos, P.E. Early Prediction of Favourable Recovery 6 Months after Mild Traumatic Brain Injury. J. Neurol. Neurosurg. Psychiatry 2008, 79, 936–942. [Google Scholar] [CrossRef]
- Jacobs, B.; Beems, T.; Stulemeijer, M.; van Vugt, A.B.; van der Vliet, T.M.; Borm, G.F.; Vos, P.E. Outcome prediction in mild traumatic brain injury: Age and clinical variables are stronger predictors than CT abnormalities. J. Neurotrauma 2010, 27, 655–668. [Google Scholar] [CrossRef]
- Teasdale, G.M.; Pettigrew, L.E.; Wilson, J.T.; Murray, G.; Jennett, B. Analyzing Outcome of Treatment of Severe Head Injury: A Review and Update on Advancing the Use of the Glasgow Outcome Scale. J. Neurotrauma 1998, 15, 587–597. [Google Scholar] [CrossRef]
- Wilson, J.T.; Pettigrew, L.E.; Teasdale, G.M. Structured Interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for Their Use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef]
- Nelson, L.D.; Ranson, J.; Ferguson, A.R.; Giacino, J.; Okonkwo, D.O.; Valadka, A.; Manley, G.; McCrea, M. Validating Multidimensional Outcome Assessment Using the TBI Common Data Elements: An Analysis of the TRACK-TBI Pilot Sample. J. Neurotrauma 2017, 34, 3158–3172. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 20 March 2020).
- Chiang, C.C.; Guo, S.E.; Huang, K.C.; Lee, B.O.; Fan, J.Y. Trajectories And Associated Factors of Quality of Life, Global Outcome, and Post-Concussion Symptoms in the First Year Following Mild Traumatic Brain Injury. Qual. Life Res. 2016, 25, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Voormolen, D.C.; Cnossen, M.C.; Polinder, S.; von Steinbuechel, N.; Vos, P.E.; Haagsma, J.A. Divergent Classification Methods of Post-Concussion Syndrome after Mild Traumatic Brain Injury: Prevalence Rates, Risk Factors, and Functional Outcome. J. Neurotrauma 2018, 35, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- McCauley, S.R.; Wilde, E.A.; Barnes, A.; Hanten, G.; Hunter, J.V.; Levin, H.S.; Smith, D.H. Patterns of Early Emotional and Neuropsychological Sequelae after Mild Traumatic Brain Injury. J. Neurotrauma 2014, 31, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lasprilla, J.C.; Rosenthal, M.; Deluca, J.; Komaroff, E.; Sherer, M.; Cifu, D.; Hanks, R. Traumatic Brain Injury and Functional Outcomes: Does Minority Status Matter? Brain Inj. 2007, 21, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lasprilla, J.C.; Rosenthal, M.; Deluca, J.; Cifu, D.X.; Hanks, R.; Komaroff, E. Functional Outcomes from Inpatient Rehabilitation after Traumatic Brain Injury: How do Hispanics Fare? Arch. Phys. Med. Rehabil. 2007, 88, 11–18. [Google Scholar] [CrossRef]
- Shafi, S.; Marquez de la Plata, C.; Diaz-Arrastia, R.; Shipman, K.; Carlile, M.; Frankel, H.; Parks, J.; Gentilello, L.M. Racial Disparities in Long-Term Functional Outcome After Traumatic Brain Injury. J. Trauma 2007, 63, 1263–1268. [Google Scholar] [CrossRef]
- Marquez de la Plata, C.; Hewlitt, M.; de Oliveira, A.; Hudak, A.; Harper, C.; Shafi, S.; Diaz-Arrastia, R. Ethnic Differences in Rehabilitation Placement and Outcome after TBI. J. Head Trauma Rehabil. 2007, 22, 113–121. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Buki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Levin, H.S.; Diaz-Arrastia, R.R. Diagnosis, Prognosis, and Clinical Management of Mild Traumatic Brain Injury. Lancet Neurol. 2015, 14, 506–517. [Google Scholar] [CrossRef]
- Lingsma, H.F.; Yue, J.K.; Maas, A.I.; Steyerberg, E.W.; Manley, G.T.; the TRACK-TBI Investigators. Outcome Prediction after Mild and Complicated Mild Traumatic Brain Injury: External Validation of Existing Models and Identification of New Predictors Using The TRACK-TBI Pilot Study. J. Neurotrauma 2015, 32, 83–94. [Google Scholar] [CrossRef]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Adelson, P.D.; Keim, S.M.; Viscusi, C.; et al. The Effect of Combined Out-of-Hospital Hypotension and Hypoxia on Mortality in Major Traumatic Brain Injury. Ann. Emerg. Med. 2017, 69, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Sherrill, D.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Viscusi, C.; Mullins, T.; et al. Mortality and Prehospital Blood Pressure in Patients with Major Traumatic Brain Injury: Implications for the Hypotension Threshold. JAMA Surg. 2017, 152, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Fearnside, M.R.; Cook, R.J.; McDougall, P.; McNeil, R.J. The Westmead Head Injury Project Outcome in Severe Head Injury. A Comparative Analysis of Pre-Hospital, Clinical and CT variables. Br. J. Neurosurg. 1993, 7, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Abraham, K.J.; West, R.H. Factors Affecting Outcome in the Resuscitation of Severely Injured Patients. Aust. N. Z. J. Surg. 1993, 63, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Jagoda, A.S.; Bazarian, J.J.; Bruns, J.J.; Cantrill, S.V.; Gean, A.D.; Howard, P.K.; Ghajar, J.; Riggio, S.; Wright, D.W.; Wears, R.L.; et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann. Emerg. Med. 2008, 52, 714–748. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, A.R.; Li, X.; McCauley, S.R.; Wilde, E.A.; Barnes, A.; Hanten, G.; Mendez, D.; McCarthy, J.J.; Levin, H.S. Prevalence and Predictors of Poor Recovery from Mild Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.; Machamer, J.; Temkin, N. Mild Traumatic Brain Injury: Longitudinal Study of Cognition, Functional Status, and Post-Traumatic Symptoms. J. Neurotrauma 2017, 34, 1524–1530. [Google Scholar] [CrossRef]
- McCrea, M.A.; Nelson, L.D.; Guskiewicz, K. Diagnosis and Management of Acute Concussion. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 271–286. [Google Scholar] [CrossRef]
- Hiploylee, C.; Dufort, P.A.; Davis, H.S.; Wennberg, R.A.; Tartaglia, M.C.; Mikulis, D.; Hazrati, L.N.; Tator, C.H. Longitudinal Study of Postconcussion Syndrome: Not Everyone Recovers. J. Neurotrauma 2017, 34, 1511–1523. [Google Scholar] [CrossRef]
- Seabury, S.A.; Gaudette, E.; Goldman, D.P.; Markowitz, A.J.; Brooks, J.; McCrea, M.A.; Okonkwo, D.O.; Manley, G.T.; the TRACK-TBI Investigators. Assessment of Follow-up Care after Emergency Department Presentation for Mild Traumatic Brain Injury and Concussion: Results from the TRACK-TBI Study. JAMA Netw. Open 2018, 1, e180210. [Google Scholar] [CrossRef]
- de Koning, M.E.; Scheenen, M.E.; van der Horn, H.J.; Hageman, G.; Roks, G.; Yilmaz, T.; Spikman, J.M.; van der Naalt, J. Outpatient Follow-Up After Mild Traumatic Brain Injury: Results of the UPFRONT-study. Brain Inj. 2017, 31, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Janak, J.C.; Cooper, D.B.; Bowles, A.O.; Alamgir, A.H.; Cooper, S.P.; Gabriel, K.P.; Perez, A.; Orman, J.A. Completion of Multidisciplinary Treatment for Persistent Postconcussive Symptoms Is Associated With Reduced Symptom Burden. J. Head Trauma Rehabil. 2017, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nygren-de Boussard, C.; Holm, L.W.; Cancelliere, C.; Godbolt, A.K.; Boyle, E.; Stalnacke, B.M.; Hincapie, C.A.; Cassidy, J.D.; Borg, J. Nonsurgical Interventions after Mild Traumatic Brain Injury: A Systematic Review. Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S257–S264. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, J.; Willmott, C.; Rothwell, A.; Cameron, P.; Ayton, G.; Nelms, R.; Curran, C.; Ng, K. Impact of Early Intervention on Outcome after Mild Traumatic Brain Injury in Children. Pediatrics 2001, 108, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J.J.; McClung, J.; Cheng, Y.T.; Flesher, W.; Schneider, S.M. Emergency Department Management of Mild Traumatic Brain Injury in the USA. Emerg. Med. J. 2005, 22, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Seichepine, D.; Tschoe, C.; Fritts, N.G.; Alosco, M.L.; Berkowitz, O.; Burke, P.; Howland, J.; Olshaker, J.; Cantu, R.C.; et al. Concussion Care Practices and Utilization of Evidence-Based Guidelines in the Evaluation and Management of Concussion: A Survey of New England Emergency Departments. J. Neurotrauma 2017, 34, 861–868. [Google Scholar] [CrossRef]
- Einstadter, D.; Cebul, R.D.; Franta, P.R. Effect of a Nurse Case Manager on Postdischarge Follow-Up. J. Gen. Intern. Med. 1996, 11, 684–688. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Langenbahn, D.M.; Braden, C.; Malec, J.F.; Kalmar, K.; Fraas, M.; Felicetti, T.; Laatsch, L.; Harley, J.P.; Bergquist, T.; et al. Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature from 2003 through 2008. Arch. Phys. Med. Rehabil. 2011, 92, 519–530. [Google Scholar] [CrossRef]
- Ponsford, J.L.; Downing, M.G.; Olver, J.; Ponsford, M.; Acher, R.; Carty, M.; Spitz, G. Longitudinal Follow-Up of Patients with Traumatic Brain Injury: Outcome at Two, Five, and Ten Years Post-Injury. J. Neurotrauma 2014, 31, 64–77. [Google Scholar] [CrossRef]
- Shames, J.; Treger, I.; Ring, H.; Giaquinto, S. Return to Work Following Traumatic Brain Injury: Trends and Challenges. Disabil. Rehabil. 2007, 29, 1387–1395. [Google Scholar] [CrossRef]
- Tiersky, L.A.; Anselmi, V.; Johnston, M.V.; Kurtyka, J.; Roosen, E.; Schwartz, T.; Deluca, J. A Trial of Neuropsychologic Rehabilitation in Mild-Spectrum Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2005, 86, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Nahmani, M.; Turrigiano, G.G. Adult Cortical Plasticity Following Injury: Recapitulation of Critical Period Mechanisms? Neuroscience 2014, 283, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Recovery after Brain Injury: Mechanisms and Principles. Front. Hum. Neurosci. 2013, 7, 887. [Google Scholar] [CrossRef]
- Gardner, R.C.; Yaffe, K. Epidemiology of Mild Traumatic Brain Injury and Neurodegenerative Disease. Mol. Cell Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hou, S.W.; Lee, C.C.; Hsu, C.Y.; Huang, Y.S.; Su, Y.C. Increased Risk of Dementia in Patients with Mild Traumatic Brain Injury: A Nationwide Cohort Study. PLoS ONE 2013, 8, e62422. [Google Scholar] [CrossRef] [PubMed]
- Transforming Research and Clinical Knowledge in TBI. Available online: https://tracktbi.ucsf.edu/ (accessed on 20 March 2020).
- Hicks, R.; Giacino, J.; Harrison-Felix, C.; Manley, G.; Valadka, A.; Wilde, E.A. Progress in Developing Common Data Elements For Traumatic Brain Injury Research: Version Two—The End of the Beginning. J. Neurotrauma 2013, 30, 1852–1861. [Google Scholar] [CrossRef]
- Yurgil, K.A.; Barbauskas, D.A.; Vasterling, J.J.; Nievergelt, C.M.; Larson, G.E.; Schork, N.J.; Litz, B.T.; Nash, W.P.; Baker, D.G.; Marine Resiliency Study Team. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry 2014, 71, 149–157. [Google Scholar] [CrossRef]
- Combs, H.L.; Berry, D.T.; Pape, T.; Babcock-Parziale, J.; Smith, B.; Schleenbaker, R.; Shandera-Ochsner, A.; Harp, J.P.; High, W.M., Jr. The Effects of Mild Traumatic Brain Injury, Post-Traumatic Stress Disorder, and Combined Mild Traumatic Brain Injury/Post-Traumatic Stress Disorder on Returning Veterans. J. Neurotrauma 2015, 32, 956–966. [Google Scholar] [CrossRef]
- Cooper, D.B.; Vanderploeg, R.D.; Armistead-Jehle, P.; Lewis, J.D.; Bowles, A.O. Factors associated with neurocognitive performance in OIF/OEF servicemembers with postconcussive complaints in postdeployment clinical settings. J. Rehabil. Res. Dev. 2014, 51, 1023–1034. [Google Scholar] [CrossRef]
- Elliott, T.R.; Hsiao, Y.Y.; Kimbrel, N.A.; Meyer, E.; DeBeer, B.B.; Gulliver, S.B.; Kwok, O.M.; Morissette, S.B. Resilience and Traumatic Brain Injury among Iraq/Afghanistan War Veterans: Differential Patterns of Adjustment and Quality of Life. J. Clin. Psychol. 2017, 73, 1160–1178. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Yuh, E.L.; Korley, F.K.; Sun, X.; Puffer, R.C.; Deng, H.; Choy, W.; Chandra, A.; Taylor, S.R.; Ferguson, A.R.; et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: A prospective multicentre study. Lancet Neurol. 2019, 18, 953–961. [Google Scholar] [CrossRef]
| 3 Months, n = 211 | 6 Months, n = 185 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | GOSE < 8 (n = 127) | GOSE = 8 (n = 84) | p-Value | GOSE < 8 (n = 121) | GOSE = 8 (n = 64) | p-Value | |||||
| Age | Years (med, IQR) | 40 | 26–54 | 36 | 26–50 | 0.30 | 40 | 26–52 | 35 | 24–51 | 0.37 |
| Gender | Male | 84 | 59% | 59 | 41% | 0.55 | 79 | 63% | 46 | 37% | 0.41 |
| Female | 43 | 63% | 25 | 37% | 42 | 70% | 18 | 30% | |||
| Race | Caucasian | 83 | 54% | 72 | 46% | 0.02 | 80 | 60% | 54 | 40% | 0.07 |
| African American or African | 18 | 75% | 6 | 25% | 15 | 75% | 5 | 25% | |||
| Asian | 12 | 86% | 2 | 14% | 12 | 92% | 1 | 8% | |||
| American Indian or Alaskan | 2 | 100% | 0 | 0% | 2 | 100% | 0 | 0% | |||
| Hawaiian or Pacific Islander | 2 | 100% | 0 | 0% | 4 | 100% | 0 | 0% | |||
| More than One Race | 6 | 60% | 4 | 40% | 7 | 64% | 4 | 36% | |||
| Not Available | 1 | 100% | 0 | 0% | 1 | 100% | 0 | 0% | |||
| Ethnicity | Non-Hispanic | 96 | 58% | 70 | 42% | 0.23 | 91 | 61% | 57 | 39% | 0.03 |
| Hispanic | 31 | 69% | 14 | 31% | 30 | 81% | 7 | 19% | |||
| Years of Education | Years (med, IQR) | 13 | 12–16 | 15 | 12.5–16 | 0.006 | 14 | 12–16 | 15 | 13–16.5 | 0.002 |
| Psychiatric History | No | 72 | 53% | 63 | 47% | <0.01 | 63 | 56% | 50 | 44% | <0.001 |
| Yes | 55 | 72% | 21 | 28% | 58 | 81% | 14 | 19% | |||
| Injury Mechanism | Fall/Accident/Other | 101 | 56% | 78 | 44% | 0.01 | 95 | 62% | 59 | 38% | 0.02 |
| Assault | 26 | 81% | 6 | 19% | 26 | 84% | 5 | 16% | |||
| Glasgow Coma Scale | 13 | 5 | 71% | 2 | 29% | 0.81 | 5 | 100% | 0 | 0% | 0.22 |
| 14 | 28 | 62% | 17 | 38% | 24 | 60% | 16 | 40% | |||
| 15 | 94 | 59% | 65 | 41% | 92 | 66% | 48 | 34% | |||
| Anticoagulant Use | No | 120 | 60% | 79 | 40% | >0.99 | 112 | 65% | 61 | 35% | 0.55 |
| Yes | 7 | 58% | 5 | 42% | 9 | 75% | 3 | 25% | |||
| Loss of consciousness | None | 39 | 61% | 25 | 39% | 0.58 | 31 | 60% | 21 | 40% | 0.75 |
| < 30 min | 54 | 57% | 41 | 43% | 59 | 69% | 27 | 31% | |||
| ≥ 30 min | 4 | 50% | 4 | 50% | 7 | 64% | 4 | 36% | |||
| Unknown | 30 | 68% | 14 | 32% | 24 | 67% | 12 | 33% | |||
| Post-traumatic amnesia | None | 48 | 61% | 31 | 39% | 0.66 | 45 | 63% | 26 | 37% | 0.61 |
| < 30 min | 41 | 56% | 32 | 44% | 39 | 66% | 13 | 34% | |||
| ≥ 30 min | 21 | 60% | 14 | 40% | 20 | 61% | 13 | 39% | |||
| Unknown | 17 | 71% | 7 | 29% | 17 | 77% | 5 | 23% | |||
| Pre-hospital hypotension (SBP < 90) | No | 96 | 58% | 69 | 42% | 0.47 | 83 | 60% | 56 | 40% | 0.15 |
| Yes | 6 | 75% | 2 | 25% | 7 | 88% | 1 | 13% | |||
| Unknown | 25 | 66% | 13 | 34% | 31 | 82% | 7 | 18% | |||
| Pre-hospital hypoxia (SpO2 < 90) | No | 96 | 58% | 70 | 42% | 0.08 | 85 | 60% | 56 | 40% | 0.28 |
| Yes | 5 | 100% | 0 | 0% | 3 | 100% | 0 | 0% | |||
| Unknown | 26 | 65% | 14 | 35% | 33 | 80% | 8 | 20% | |||
| Urine toxicology screen | Negative | 120 | 61% | 78 | 39% | 0.77 | 113 | 65% | 62 | 35% | 0.5 |
| Positive | 7 | 54% | 6 | 46% | 8 | 80% | 2 | 20% | |||
| Blood alcohol level | Negative | 27 | 60% | 18 | 40% | 0.66 | 23 | 62% | 14 | 38% | 0.41 |
| Positive | 16 | 70% | 7 | 30% | 18 | 78% | 5 | 22% | |||
| Not obtained | 84 | 59% | 59 | 41% | 80 | 64% | 45 | 36% | |||
| ED hypotension | No | 125 | 60% | 83 | 40% | >0.99 | 119 | 65% | 64 | 35% | 0.55 |
| Yes | 1 | 50% | 1 | 50% | 2 | 100% | 0 | 0% | |||
| Unknown | 1 | 100% | 0 | 0% | 0 | 0% | 0 | 0% | |||
| ED hypoxia | No | 120 | 60% | 81 | 40% | >0.99 | 116 | 65% | 62 | 35% | >0.99 |
| Yes | 5 | 63% | 3 | 38% | 4 | 67% | 2 | 33% | |||
| Unknown | 2 | 100% | 0 | 0% | 1 | 100% | 0 | 0% | |||
| Risk Factors | GOSE < 8 vs. GOSE = 8 at 3 Months Postinjury | GOSE < 8 vs. GOSE = 8 at 6 Months Postinjury | ||
|---|---|---|---|---|
| AOR (95% CI) | P-Value | AOR (95% CI) | P-Value | |
| Years of Education | 0.88 (0.78–0.998) | 0.046 | 0.85 (0.74–0.98) | 0.026 |
| Psychiatric History | ||||
| No | 1 | 0.015 | 1 | 0.001 |
| Yes | 2.30 (1.18–4.49) | 3.75 (1.73–8.12) | ||
| Injury Mechanism | ||||
| Fall/Accident/Other | 1 | 0.025 | 1 | 0.054 |
| Assault | 3.53 (1.17–10.63) | 3.41 (0.98–11.85) | ||
| Race | ||||
| White | 1 | 0.006 | 1 | 0.025 |
| Black | 2.53 (0.82–7.83) | 1.77 (0.48–6.51) | ||
| Asian/Other | 12.40 (2.66–57.77) | 23.99 (2.93–196.84) | ||
| More than one race | 0.88 (0.20–3.84) | 1.03 (0.22–4.70) | ||
| Ethnicity | ||||
| Non-Hispanic | 1 | 0.124 | 1 | 0.014 |
| Hispanic | 1.84 (0.85–4.02) | 3.48 (1.29–9.37) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhok, D.Y.; Yue, J.K.; Sun, X.; Suen, C.G.; Coss, N.A.; Jain, S.; Manley, G.T.; the TRACK-TBI Investigators. Clinical Predictors of 3- and 6-Month Outcome for Mild Traumatic Brain Injury Patients with a Negative Head CT Scan in the Emergency Department: A TRACK-TBI Pilot Study. Brain Sci. 2020, 10, 269. https://doi.org/10.3390/brainsci10050269
Madhok DY, Yue JK, Sun X, Suen CG, Coss NA, Jain S, Manley GT, the TRACK-TBI Investigators. Clinical Predictors of 3- and 6-Month Outcome for Mild Traumatic Brain Injury Patients with a Negative Head CT Scan in the Emergency Department: A TRACK-TBI Pilot Study. Brain Sciences. 2020; 10(5):269. https://doi.org/10.3390/brainsci10050269
Chicago/Turabian StyleMadhok, Debbie Y., John K. Yue, Xiaoying Sun, Catherine G. Suen, Nathan A. Coss, Sonia Jain, Geoffrey T. Manley, and the TRACK-TBI Investigators. 2020. "Clinical Predictors of 3- and 6-Month Outcome for Mild Traumatic Brain Injury Patients with a Negative Head CT Scan in the Emergency Department: A TRACK-TBI Pilot Study" Brain Sciences 10, no. 5: 269. https://doi.org/10.3390/brainsci10050269
APA StyleMadhok, D. Y., Yue, J. K., Sun, X., Suen, C. G., Coss, N. A., Jain, S., Manley, G. T., & the TRACK-TBI Investigators. (2020). Clinical Predictors of 3- and 6-Month Outcome for Mild Traumatic Brain Injury Patients with a Negative Head CT Scan in the Emergency Department: A TRACK-TBI Pilot Study. Brain Sciences, 10(5), 269. https://doi.org/10.3390/brainsci10050269






