Acute Effects of High-Definition Transcranial Direct Current Stimulation on Foot Muscle Strength, Passive Ankle Kinesthesia, and Static Balance: A Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Protocol
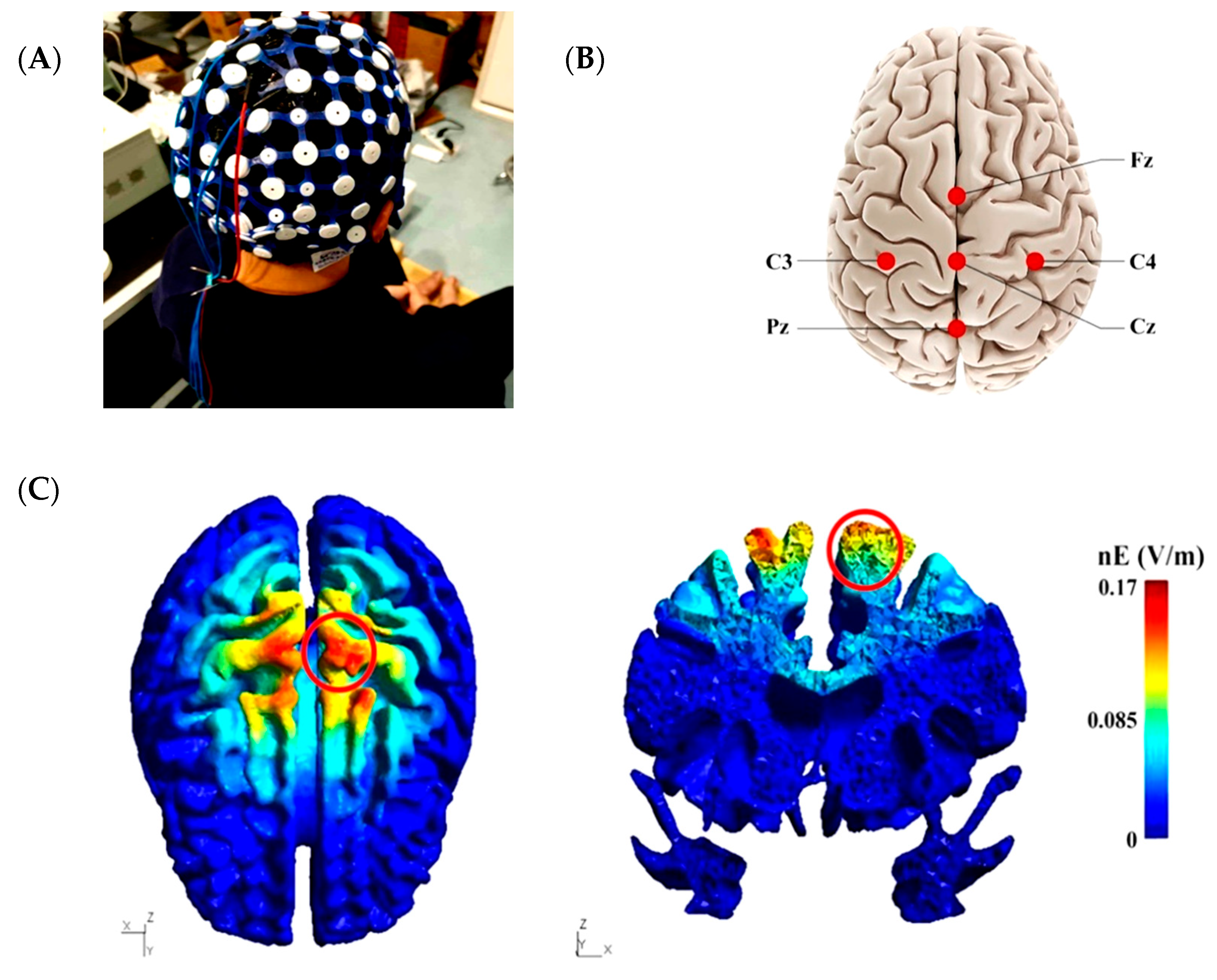
2.3. High-Definition Transcranial Direct Current Stimulation Intervention
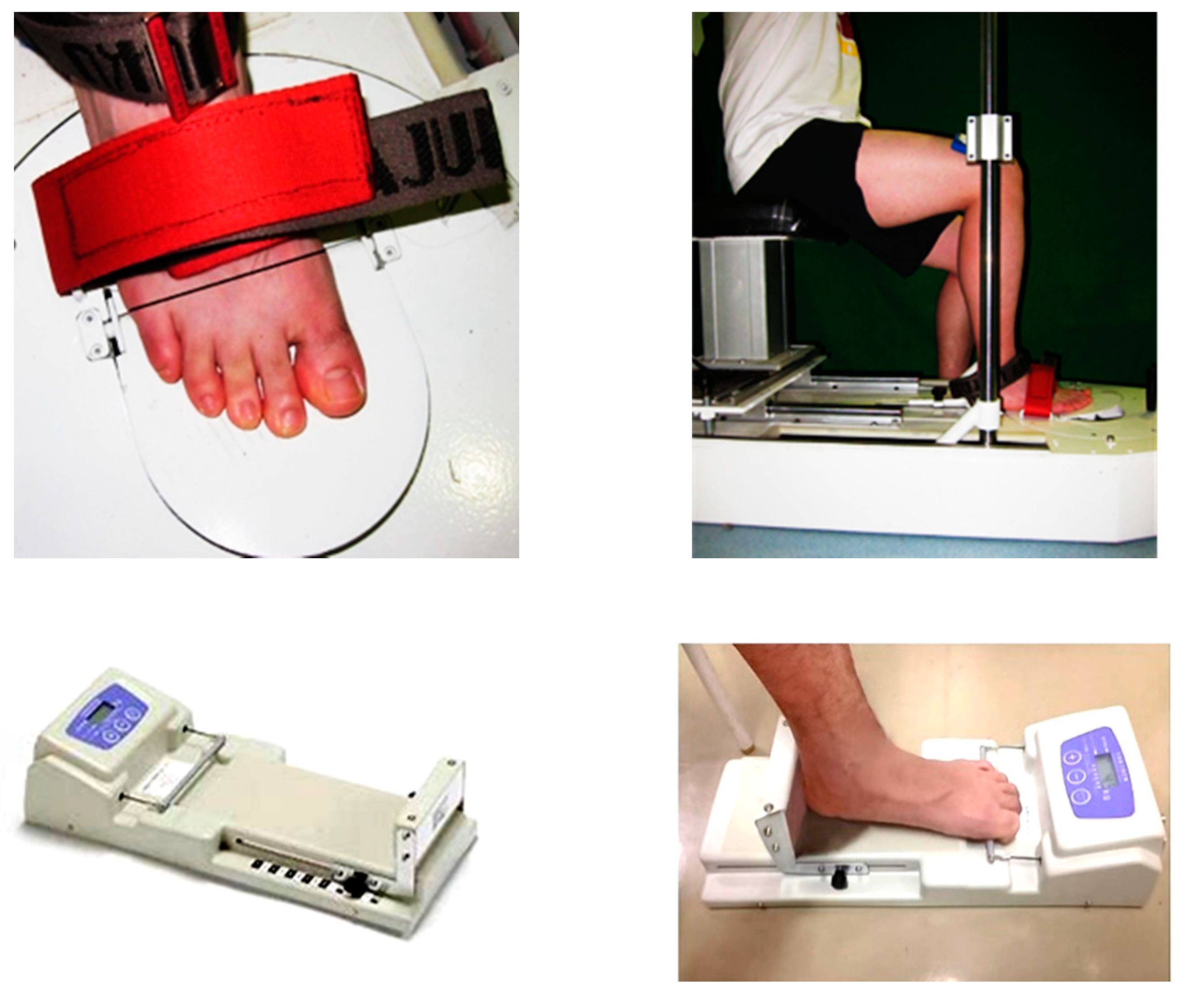
2.4. Data Collection
2.4.1. Passive Ankle Kinesthesia
2.4.2. Metatarsophalangeal Joint Flexor Strength
2.4.3. Toe Flexor Strength
2.4.4. Static Balance Ability
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2015, 49, 290. [Google Scholar] [CrossRef]
- McKeon, P.O.; Fourchet, F. Freeing the foot: Integrating the foot core system into rehabilitation for lower extremity injuries. Clin. Sports Med. 2015, 34, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Lephart, S.M.; Pincivero, D.M.; Giraldo, J.L.; Fu, F.H. The role of proprioception in the management and rehabilitation of athletic injuries. Am. J. Sports Med. 1997, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ko, J.; Zhang, S.; Brown, C.N.; Simpson, K.J. Biomechanics of ankle giving way: A case report of accidental ankle giving way during the drop landing test. J. Sport Health Sci. 2019, 8, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lowrey, C.R.; Strzalkowski, N.D.J.; Bent, L.R. Skin sensory information from the dorsum of the foot and ankle is necessary for kinesthesia at the ankle joint. Neurosci. Lett. 2010, 485, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.T.H.; Sze, L.K.Y.; Mok, N.W.; Ng, G.Y.F. Intrinsic foot muscle volume in experienced runners with and without chronic plantar fasciitis. J. Sci. Med. Sport. 2016, 19, 713–715. [Google Scholar] [CrossRef]
- Lee, E.; Cho, J.; Lee, S. Short-foot exercise promotes quantitative somatosensory function in ankle instability: A randomized controlled trial. Med. Sci. Monit. 2019, 25, 618–626. [Google Scholar] [CrossRef]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central nervous system adaptation after ligamentous injury: A summary of theories, evidence, and clinical interpretation. Sports Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- De Moura, M.C.D.S.; Hazime, F.A.; Marotti Aparicio, L.V.; Grecco, L.A.C.; Brunoni, A.R.; Hasue, R.H. Effects of transcranial direct current stimulation (tDCS) on balance improvement: A systematic review and meta-analysis. Somatosens. Mot. Res. 2019, 36, 122–135. [Google Scholar] [CrossRef]
- Lattari, E.; Oliveira, B.R.R.; Monteiro Júnior, R.S.; Marques Neto, S.R.; Oliveira, A.J.; Maranhão Neto, G.A.; Machado, S.; Budde, H. Acute effects of single dose transcranial direct current stimulation on muscle strength: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0209513. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Unal, G.; Andrade, S.M.; Moreira, A.; Altimari, L.R.; Brunoni, A.R.; Perrey, S.; Mauger, A.R.; Bikson, M.; Okano, A.H. Effect of transcranial direct current stimulation on exercise performance: A systematic review and meta-analysis. Brain Stimul. 2019, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Angius, L.; Pascual-Leone, A.; Santarnecchi, E. Brain stimulation and physical performance. Prog. Brain Res. 2018, 240, 317–339. [Google Scholar] [PubMed]
- Vargas, V.Z.; Baptista, A.F.; Pereira, G.; Pochini, A.C.; Ejnisman, B.; Santos, M.B.; João, S.; Hazime, F.A. Modulation of isometric quadriceps strength in soccer players with transcranial direct current stimulation: A crossover study. J. Strength Cond. Res. 2018, 32, 1336–1341. [Google Scholar] [CrossRef]
- Tanaka, S.; Hanakawa, T.; Honda, M.; Watanabe, K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp. Brain Res. 2009, 196, 459–465. [Google Scholar] [CrossRef]
- Zhou, J.; Lo, O.-Y.; Lipsitz, L.A.; Zhang, J.; Fang, J.; Manor, B. Transcranial direct current stimulation enhances foot sole somatosensation when standing in older adults. Exp. Brain Res. 2018, 236, 795–802. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207. [Google Scholar] [CrossRef]
- Reckow, J.; Rahman-Filipiak, A.; Garcia, S.; Schlaefflin, S.; Calhoun, O.; DaSilva, A.F.; Bikson, M.; Hampstead, B.M. Tolerability and blinding of 4x1 high-definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps. Brain Stimul. 2018, 11, 991–997. [Google Scholar] [CrossRef]
- Fu, W.; Fang, Y.; Gu, Y.; Huang, L.; Li, L.; Liu, Y. Shoe cushioning reduces impact and muscle activation during landings from unexpected, but not self-initiated, drops. J. Sci. Med. Sport 2017, 20, 915–920. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ishii, D.; Ichiba, N.; Yozu, A.; Kohno, Y. Cathodal tDCS on the motor area decreases the tactile threshold of the distal pulp of the hallux. Neurosci. Lett. 2020, 719, 133887. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, A.F.; Truong, D.Q.; DosSantos, M.F.; Toback, R.L.; Datta, A.; Bikson, M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front. Neuroanat. 2015, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Borckardt, J.J.; Bikson, M.; Frohman, H.; Reeves, S.T.; Datta, A.; Bansal, V.; Madan, A.; Barth, K.; George, M.S. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J. Pain 2012, 13, 112–120. [Google Scholar] [CrossRef]
- Villamar, M.F.; Volz, M.S.; Bikson, M.; Datta, A.; Dasilva, A.F.; Fregni, F. Technique and considerations in the use of 4x1 ring high-definition transcranial direct current stimulation (HD-tDCS). J. Vis. Exp. 2013, 77, e50309. [Google Scholar] [CrossRef]
- Cole, L.; Giuffre, A.; Ciechanski, P.; Carlson, H.L.; Zewdie, E.; Kuo, H.-C.; Kirton, A. Effects of high-definition and conventional transcranial direct-current stimulation on motor learning in children. Front. Neurosci. 2018, 12, 787. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Sun, W.; Song, Q.; Yu, B.; Zhang, C.; Mao, D. Test-retest reliability of a new device for assessing ankle joint threshold to detect passive movement in healthy adults. J. Sports Sci. 2015, 33, 1667–1674. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, X.; Deng, L.; Zhang, S.; Cui, K.; Fu, W. Relationships between foot morphology and foot muscle strength in healthy adults. Int. J. Environ. Res. Public Health 2020, 17, 1274. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.; Liu, Y. Does habitual rear-foot strike pattern with modern running shoes affect the muscle strength of the longitudinal arch? Isoki. Exer. Sci. 2019, 27, 213–218. [Google Scholar] [CrossRef]
- Kurihara, T.; Yamauchi, J.; Otsuka, M.; Tottori, N.; Hashimoto, T.; Isaka, T. Maximum toe flexor muscle strength and quantitative analysis of human plantar intrinsic and extrinsic muscles by a magnetic resonance imaging technique. J. Foot Ankle Res. 2014, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Koyama, K. Influence of ankle braces on the maximum strength of plantar and toe flexor muscles. Int. J. Sports Med. 2015, 36, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Chugh, S. Effect of transcranial direct current stimulation on cortico-muscular coherence and standing postural steadiness. In Proceedings of the 2nd IASTED International Conference on Assistive Technologies; ACTA Press: Innsbruck, Austria, 2012. [Google Scholar]
- Saruco, E.; Di Rienzo, F.; Nunez-Nagy, S.; Rubio-Gonzalez, M.A.; Jackson, P.L.; Collet, C.; Saimpont, A.; Guillot, A. Anodal tDCS over the primary motor cortex improves motor imagery benefits on postural control: A pilot study. Sci. Rep. 2017, 7, 480. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yamaguchi, T.; Tatemoto, T.; Kondo, K.; Otaka, Y.; Tanaka, S. Transcranial Direct Current Stimulation Does Not Affect Lower Extremity Muscle Strength Training in Healthy Individuals: A Triple-Blind, Sham-Controlled Study. Front. Neurosci. 2017, 11, 179. [Google Scholar] [CrossRef]
- Flood, A.; Waddington, G.; Keegan, R.J.; Thompson, K.G.; Cathcart, S. The effects of elevated pain inhibition on endurance exercise performance. PeerJ 2017, 5, e3028. [Google Scholar] [CrossRef][Green Version]
- Grimaldi, G.; Manto, M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann. Biomed. Eng. 2013, 41, 2437–2447. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.W.; Chang, W.H.; Kim, Y.H.; Kim, K.; Im, C.H. Inconsistent outcomes of transcranial direct current stimulation may originate from anatomical differences among individuals: Electric field simulation using individual MRI data. Neurosci. Lett. 2014, 564, 6–10. [Google Scholar] [CrossRef]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef]
- Fonteneau, C.; Mondino, M.; Arns, M.; Baeken, C.; Bikson, M.; Brunoni, A.R.; Burke, M.J.; Neuvonen, T.; Padberg, F.; Pascual-Leone, A.; et al. Sham tDCS: A hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 2019, 12, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Nikolin, S.; Martin, D.; Loo, C.K.; Boonstra, T.W. Effects of TDCS dosage on working memory in healthy participants. Brain Stimul. 2018, 11, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Dagan, M.; Herman, T.; Harrison, R.; Zhou, J.; Giladi, N.; Ruffini, G.; Manor, B.; Hausdorff, J.M. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov. Disord. 2018, 33, 642–646. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, H.; Moran, A. Does motor simulation theory explain the cognitive mechanisms underlying motor imagery? A critical review. Front. Hum. Neurosci. 2017, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, M.; Piccardi, L.; Giancola, M.; Nori, R.; D’Amico, S.; Olivetti Belardinelli, M. The format of mental imagery: From a critical review to an integrated embodied representation approach. Cogn. Process. 2019, 20, 277–289. [Google Scholar] [CrossRef] [PubMed]
| Variables | HD-tDCS | Sham | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| PF (°) | 1.29 ± 0.46 | 1.19 ± 0.45 | 1.38 ± 0.52 | 1.35 ± 0.39 |
| DF (°) | 1.48 ± 0.65 | 1.36 ± 0.42 | 1.44 ± 0.53 | 1.28 ± 0.32 |
| INV (°) | 2.73 ± 1.31 | 2.33 ± 1.15 | 2.77 ± 1.23 | 2.44 ± 1.22 |
| EV (°) | 2.43 ± 0.61 | 2.17 ± 0.95 | 2.37 ± 0.82 | 2.22 ± 0.79 |
| MPJ flexor strength (N/kg) | 1.56 ± 0.53 | 1.64 ± 0.38 | 1.43 ± 0.50 | 1.57 ± 0.49 |
| Flexor strength of the first toe (N/kg) | 1.45 ± 0.58 | 1.61 ± 0.67 | 1.46 ± 0.58 | 1.43 ± 0.49 |
| Flexor strength of the other four toes (N/kg) | 1.25 ± 0.41 | 1.30 ± 0.39 | 1.19 ± 0.41 | 1.24 ± 0.44 |
| Flexor strength of the all five toes (N/kg) | 2.84 ± 0.57 | 2.80 ± 0.63 | 2.62 ± 0.54 | 2.74 ± 0.56 |
| Posture Conditions | Variables | HD-tDCS | Sham | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| TL_EO | ML average CoG sway velocity (mm/s) | 6.53 ± 1.12 | 6.60 ± 1.03 | 6.40 ± 1.06 | 6.60 ± 1.09 |
| AP average CoG sway velocity (mm/s) | 8.48 ± 1.45 | 8.41 ± 1.23 | 8.43 ± 1.40 | 8.72 ± 1.76 | |
| TL_EC | ML average CoG sway velocity (mm/s) | 6.64 ± 0.82 | 7.03 ± 0.97 | 6.40 ± 1.17 | 6.46 ± 1.36 |
| AP average CoG sway velocity (mm/s) | 9.40 ± 1.54 | 9.28 ± 1.43 | 9.19 ± 2.02 | 9.01 ± 1.75 | |
| OL_EO | ML average CoG sway velocity (mm/s) | 31.63 ± 7.28 | 30.89 ± 7.80 | 33.42 ± 12.31 | 31.38 ± 10.52 |
| AP average CoG sway velocity (mm/s) | 29.04 ± 4.65 | 28.75 ± 5.28 | 33.63 ± 11.35 | 29.34 ± 6.55 | |
| OL_EC | ML average CoG sway velocity (mm/s) | 65.43 ± 15.80 | 59.56 ± 14.70 | 65.94 ± 17.23 | 59.52 ± 18.56 |
| AP average CoG sway velocity (mm/s) | 67.73 ± 14.45 | 60.20 ± 13.39 | 71.79 ± 17.05 | 58.73 ± 13.64 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Wang, B.; Zhang, X.; Zhou, J.; Fu, W. Acute Effects of High-Definition Transcranial Direct Current Stimulation on Foot Muscle Strength, Passive Ankle Kinesthesia, and Static Balance: A Pilot Study. Brain Sci. 2020, 10, 246. https://doi.org/10.3390/brainsci10040246
Xiao S, Wang B, Zhang X, Zhou J, Fu W. Acute Effects of High-Definition Transcranial Direct Current Stimulation on Foot Muscle Strength, Passive Ankle Kinesthesia, and Static Balance: A Pilot Study. Brain Sciences. 2020; 10(4):246. https://doi.org/10.3390/brainsci10040246
Chicago/Turabian StyleXiao, Songlin, Baofeng Wang, Xini Zhang, Junhong Zhou, and Weijie Fu. 2020. "Acute Effects of High-Definition Transcranial Direct Current Stimulation on Foot Muscle Strength, Passive Ankle Kinesthesia, and Static Balance: A Pilot Study" Brain Sciences 10, no. 4: 246. https://doi.org/10.3390/brainsci10040246
APA StyleXiao, S., Wang, B., Zhang, X., Zhou, J., & Fu, W. (2020). Acute Effects of High-Definition Transcranial Direct Current Stimulation on Foot Muscle Strength, Passive Ankle Kinesthesia, and Static Balance: A Pilot Study. Brain Sciences, 10(4), 246. https://doi.org/10.3390/brainsci10040246





