Do Adolescents Use Substances to Relieve Uncomfortable Sensations? A Preliminary Examination of Negative Reinforcement among Adolescent Cannabis and Alcohol Users
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Interview
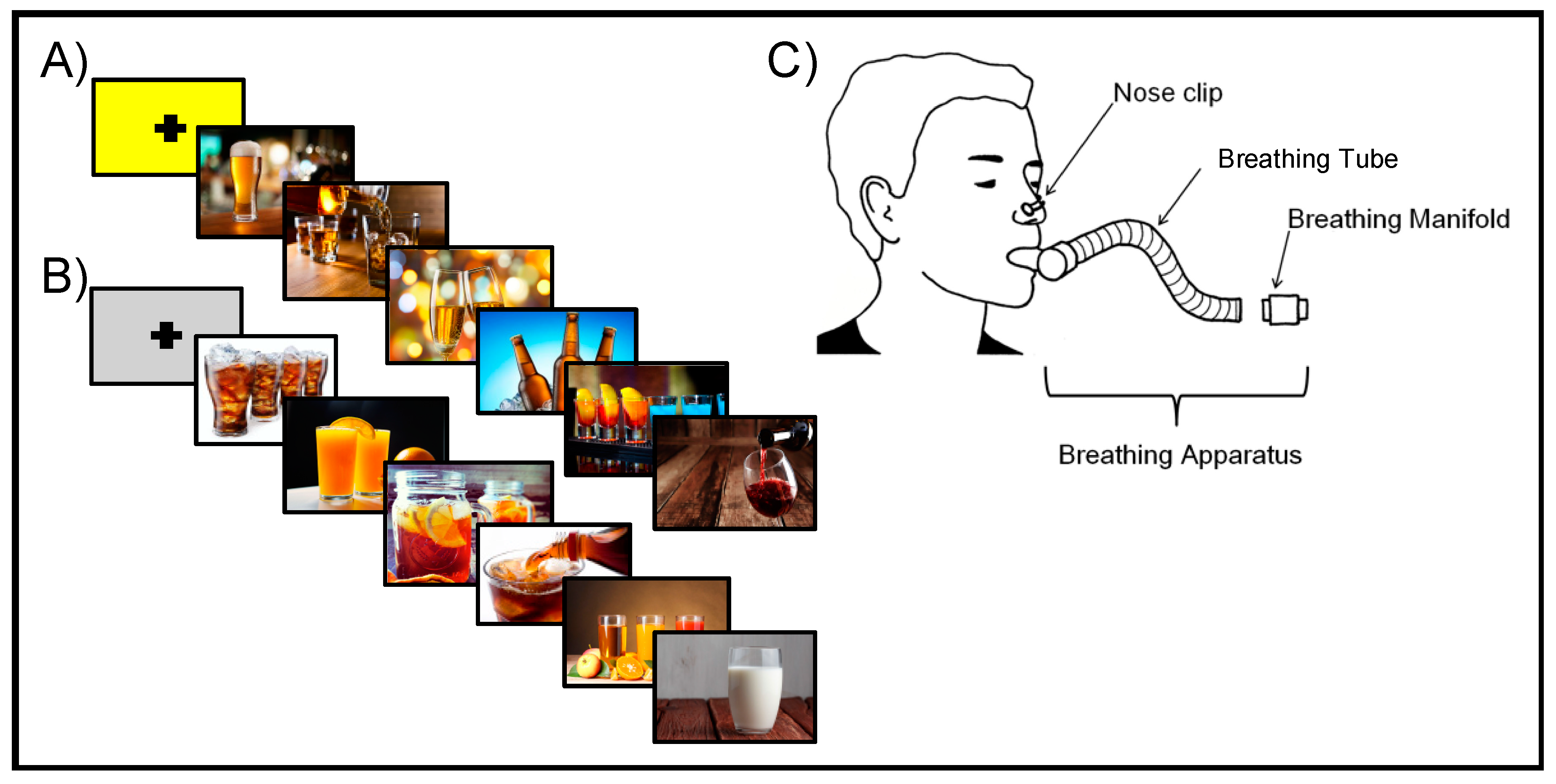
2.3. Neuroimaging Procedures
2.4. Neuroimaging Data Acquisition
2.5. Neuroimaging Data Analysis
2.5.1. Individual-Level Processing
2.5.2. Group-Level Analysis
3. Results
3.1. Subject Characteristics
3.2. Neuroimaging Results
3.2.1. The Group by interoception interaction
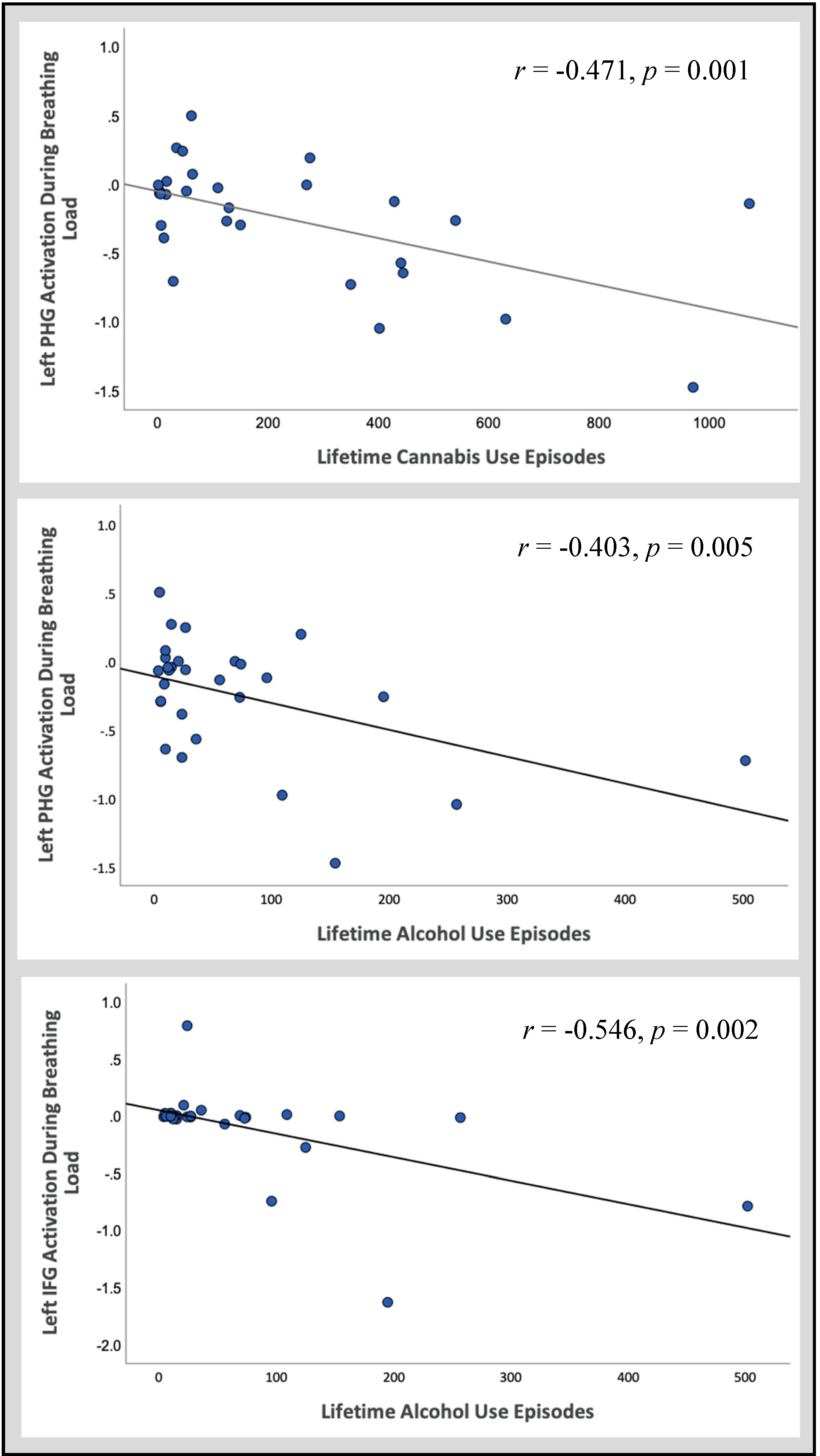
3.2.2. Follow-Up Correlations
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Casey, B.J.; Jones, R.M. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.D.; Miech, R.A.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E.; Patrick, M.E. Monitoring the Future National Survey Results on Drug Use 1975–2019: Overview, Key Findings on Adolescent Drug Use; Education Resources Information Center: Washington, DC, USA, 2020. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health, HHS; HHS Publ. No. PEP19-5068, NSDUH Ser. H-54; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2019. [CrossRef]
- Hasin, D.S. US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology 2018, 43, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Dawson, D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. J. Subst. Abuse 1997, 9, 103–110. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings; NSDUH Series H-48; HHS Publication No. (SMA) 14-4863; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2014.
- London, E.D.; Ernst, M.; Grant, S.; Bonson, K.; Weinstein, A. Orbitofrontal cortex and human drug abuse: Functional imaging. Cereb. Cortex 2000, 10, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; Juavinett, A.L.; May, A.C.; Davenport, P.W.; Paulus, M.P. Do you feel alright? Attenuated neural processing of aversive interoceptive stimuli in current stimulant users. Psychophysiology 2015, 52, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Tranel, D.; Damasio, H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000, 123. [Google Scholar] [CrossRef]
- Nestor, L.J.; Ghahremani, D.G.; Monterosso, J.; London, E.D. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. Neuroimaging 2011, 194. [Google Scholar] [CrossRef]
- Paulus, M.P.; Feinstein, J.S.; Tapert, S.F.; Liu, T.T. Trend detection via temporal difference model predicts inferior prefrontal cortex activation during acquisition of advantageous action selection. Neuroimage 2004. [Google Scholar] [CrossRef]
- Verdejo-Garcia, A.; Chong, T.T.J.; Stout, J.C.; Yücel, M.; London, E.D. Stages of dysfunctional decision-making in addiction. Pharmacol. Biochem. Behav. 2018. [Google Scholar] [CrossRef]
- Sönmez, M.B.; Kahyacı Kılıç, E.; Ateş Çöl, I.; Görgülü, Y.; Köse Çınar, R. Decreased interoceptive awareness in patients with substance use disorders. J. Subst. Use 2017, 22. [Google Scholar] [CrossRef]
- May, A.C.; Stewart, J.L.; Migliorini, R.; Tapert, S.F.; Paulus, M.P. Methamphetamine Dependent Individuals Show Attenuated Brain Response to Pleasant Interoceptive Stimuli. Drug Alcohol Depend. 2013, 131, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.H.; Zvolensky, M.J.; Eifert, G.H. Negative-reinforcement drinking motives mediate the relation between anxiety sensitivity and increased drinking behavior. Pers. Individ. Differ. 2001. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Gramann, K.; Schandry, R. Neural systems connecting interoceptive awareness and feelings. Hum. Brain Mapp. 2007. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Barrett, L.F.; Simmons, W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015. [Google Scholar] [CrossRef]
- Paulus, M.P.; Tapert, S.F.; Schulteis, G. The role of interoception and alliesthesia in addiction. Pharmacol. Biochem. Behav. 2009. [Google Scholar] [CrossRef]
- Verdejo-Garcia, A.; Clark, L.; Dunn, B.D. The role of interoception in addiction: A critical review. Neurosci. Biobehav. Rev. 2012. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Bechara, A. The Insula: A Critical Neural Substrate for Drug Seeking under Conflict and Risk. Wiley Handb. Cogn. 2015. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stewart, J.L. Interoception and drug addiction. Neuropharmacology 2014, 76, 342–350. [Google Scholar] [CrossRef]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Buechel, C.; Gross, J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; Flagan, T.M.; May, A.C.; Reske, M.; Simmons, A.N.; Paulus, M.P. Young adults at risk for stimulant dependence show reward dysfunction during reinforcement-based decision making. Biol. Psychiatry 2013, 73, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Berk, L.; Stewart, J.L.; May, A.C.; Wiers, R.W.; Davenport, P.W.; Paulus, M.P.; Tapert, S.F. Under pressure: Adolescent substance users show exaggerated neural processing of aversive interoceptive stimuli. Addiction 2015, 110, 2025–2036. [Google Scholar] [CrossRef]
- Stewart, J.L.; Butt, M.; May, A.C.; Tapert, S.F.; Paulus, M.P. Insular and cingulate attenuation during decision making is associated with future transition to stimulant use disorder. Addiction 2017, 112, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-García, A.; Bechara, A. A somatic marker theory of addiction. Neuropharmacology 2009. [Google Scholar] [CrossRef] [PubMed]
- Wills, T.A.; Pokhrel, P.; Morehouse, E.; Fenster, B. Behavioral and Emotional Regulation and Adolescent Substance Use Problems: A Test of Moderation Effects in a Dual-Process Model. Psychol. Addict. Behav. 2011, 25, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Payer, D.; Lieberman, M.; London, E. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch. Gen. Psychiatry 2011, 68, 271–282. [Google Scholar] [CrossRef]
- Veinante, P.; Yalcin, I.; Barrot, M. The amygdala between sensation and affect: A role in pain. J. Mol. Psychiatry 2013. [Google Scholar] [CrossRef]
- Morawetz, C.; Bode, S.; Derntl, B.; Heekeren, H.R. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2017. [Google Scholar] [CrossRef]
- Conrod, P.; Nikolaou, K. Annual Research Review: On the developmental neuropsychology of substance use disorders. J. Child Psychol. Psychiatry Allied Discip. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wolitzky-Taylor, K.; McBeth, J.; Guillot, C.R.; Stone, M.D.; Kirkpatrick, M.G.; Zvolensky, M.J.; Buckner, J.D.; Leventhal, A.M. Transdiagnostic processes linking anxiety symptoms and substance use problems among adolescents. J. Addict. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Karoly, H.C.; Schacht, J.P.; Meredith, L.R.; Jacobus, J.; Tapert, S.F.; Gray, K.M.; Squeglia, L.M. Investigating a novel fMRI cannabis cue reactivity task in youth. Addict. Behav. 2019, 89, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Tapert, S.F.; Cheung, E.H.; Brown, G.G.; Frank, L.R.; Paulus, M.P.; Schweinsburg, A.D.; Meloy, M.J.; Brown, S.A. Neural Response to Alcohol Stimuli in Adolescents with Alcohol Use Disorder. Arch. Gen. Psychiatry 2003, 60, 727. [Google Scholar] [CrossRef] [PubMed]
- Lopata, M.; La Fata, J.; Evanich, M.J.; Lourenço, R.V. Effects of flow-resistive loading on mouth occlusion pressure during CO2 rebreathing. Am. Rev. Respir. Dis. 1977. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; Parnass, J.M.; May, A.C.; Davenport, P.W.; Paulus, M.P. Altered frontocingulate activation during aversive interoceptive processing in young adults transitioning to problem stimulant use. Front. Syst. Neurosci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; May, A.C.; Poppa, T.; Davenport, P.W.; Tapert, S.F.; Paulus, M.P. You are the danger: Attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend. 2014, 142, 110–119. [Google Scholar] [CrossRef]
- Schacht, J.P.; Anton, R.F.; Myrick, H. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addict. Biol. 2013. [Google Scholar] [CrossRef]
- Zhou, X.; Zimmermann, K.; Xin, F.; Zhao, W.; Derckx, R.T.; Sassmannshausen, A.; Scheele, D.; Hurlemann, R.; Weber, B.; Kendrick, K.M.; et al. Cue Reactivity in the Ventral Striatum Characterizes Heavy Cannabis Use, Whereas Reactivity in the Dorsal Striatum Mediates Dependent Use. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019. [Google Scholar] [CrossRef]
- Pierucci-Lagha, A.; Gelernter, J.; Feinn, R.; Cubells, J.F.; Pearson, D.; Pollastri, A.; Farrer, L.; Kranzler, H.R. Diagnostic reliability of the semi-structured assessment for drug dependence and alcoholism (SSADDA). Drug Alcohol Depend. 2005. [Google Scholar] [CrossRef]
- Brown, S.A.; Myers, M.G.; Lippke, L.; Tapert, S.F.; Stewart, D.G.; Vik, P.W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J. Stud. Alcohol 1998, 59, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, S.P.; Lynam, D.R. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Pers. Individ. Differ. 2001, 30, 669–689. [Google Scholar] [CrossRef]
- Mehling, W.E.; Price, C.; Daubenmier, J.J.; Acree, M.; Bartmess, E.; Stewart, A. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Pomerleau, O.F.; Fagerström, K.O.; Marks, J.L.; Tate, J.C.; Pomerleau, C.S. Development and validation of a self-rating scale for positive- and negative-reinforcement smoking: The Michigan nicotine reinforcement questionnaire. Nicotine Tob. Res. 2003. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.P.; Flagan, T.; Simmons, A.N.; Gillis, K.; Kotturi, S.; Thom, N.; Johnson, D.C.; Van Orden, K.F.; Davenport, P.W.; Swain, J.L. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLoS ONE 2012, 7, e29394. [Google Scholar] [CrossRef]
- Davenport, P.W.; Vovk, A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol. 2009. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996. [Google Scholar] [CrossRef]
- Eklund, A.; Nichols, T.E.; Knutsson, H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef]
- Luijten, M.; Schellekens, A.F.; Kühn, S.; Machielse, M.W.J.; Sescousse, G. Disruption of Reward Processing in Addiction. JAMA Psychiatry 2017. [Google Scholar] [CrossRef]
- Dager, A.D.; Anderson, B.M.; Rosen, R.; Khadka, S.; Sawyer, B.; Jiantonio-Kelly, R.E.; Austad, C.S.; Raskin, S.A.; Tennen, H.; Wood, R.M.; et al. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 2014. [Google Scholar] [CrossRef]
- Somerville, L.H.; Jones, R.M.; Casey, B.J. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Locke, H.M.; Stenger, V.A.; Fiez, J.A. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cogn. Affect. Behav. Neurosci. 2003, 3, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Nelson, E.E.; Jazbec, S.; McClure, E.B.; Monk, C.S.; Leibenluft, E.; Blair, J.; Pine, D.S. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage 2005, 25, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Newman, J.L.; Longe, O.A.; Deakin, J.F.W. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. J. Neurosci. 2003, 23, 303–307. [Google Scholar] [CrossRef]
- Seymour, B.; Daw, N.; Dayan, P.; Singer, T.; Dolan, R. Differential Encoding of Losses and Gains in the Human Striatum. J. Neurosci. 2007, 27, 4826–4831. [Google Scholar] [CrossRef]
- Hardin, M.G.; Ernst, M. Functional brain imaging of development-related risk and vulnerability for substance use in adolescents. Annu. Rev. Clin. Psychol. 2016, 11, 361–377. [Google Scholar] [CrossRef]
- May, A.C.; Stewart, J.L.; Tapert, S.F.; Paulus, M.P. The effect of age on neural processing of pleasant soft touch stimuli. Front. Behav. Neurosci. 2014, 8, 52. [Google Scholar] [CrossRef][Green Version]
- Aguinaldo, L.D.; Squeglia, L.M.; Gray, K.M.; Coronado, C.; Lees, B.; Tomko, R.L.; Jacobus, J. Behavioral Treatments for Adolescent Cannabis Use Disorder: A Rationale for Cognitive Retraining. Curr. Addict. Rep. 2019. [Google Scholar] [CrossRef]
- Fatseas, M.; Serre, F.; Alexandre, J.M.; Debrabant, R.; Auriacombe, M.; Swendsen, J. Craving and substance use among patients with alcohol, tobacco, cannabis or heroin addiction: A comparison of substance- and person-specific cues. Addiction 2015. [Google Scholar] [CrossRef]
- Karoly, H.C.; Schacht, J.P.; Jacobus, J.; Meredith, L.R.; Taylor, C.T.; Tapert, S.F.; Gray, K.M.; Squeglia, L.M. Preliminary evidence that computerized approach avoidance training is not associated with changes in fMRI cannabis cue reactivity in non-treatment-seeking adolescent cannabis users. Drug Alcohol Depend. 2019. [Google Scholar] [CrossRef]
- Filbey, F.M.; Claus, E.; Audette, A.R.; Niculescu, M.; Banich, M.T.; Tanabe, J.; Du, Y.P.; Hutchison, K.E. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 2008. [Google Scholar] [CrossRef] [PubMed]
- Strang, N.M.; Claus, E.D.; Ramchandani, V.A.; Graff-Guerrero, A.; Boileau, I.; Hendershot, C.S. Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology 2015. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.M.; Smith, A.R.; Hommer, D.W. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage 2008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gowin, J.L.; Stewart, J.L.; May, A.C.; Ball, T.M.; Wittmann, M.; Tapert, S.F.; Paulus, M.P. Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: Losses lose impact. Addiction 2014, 109, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.A.; Chmelka, M.B.; Howard, B.K.; Thompson, R.W. Comorbid alcohol and cannabis use disorders among high-risk youth at intake into residential care. J. Adolesc. Health 2013. [Google Scholar] [CrossRef] [PubMed]

| CAN+ALC-SUDGroup Description | % Meeting Diagnostic Criteria | Diagnostic Criteria Endorsed | |||||
| M(SD) | Min | Max | |||||
| THC Use Disorder | 92.31 | 3.42 (1.38) | 2 | 6 | |||
| Alcohol Use Disorder | 61.54 | 2.63 (.74) | 2 | 4 | |||
| Substance Use | CAN+ALC-SUD Cannabis/Alcohol Substance Use Disorder | CAN+ALC-EXP Cannabis/Alcohol Experimenter | CTL Little to No Substance Use | df | t | p | |
| Lifetime Cannabis Use | 467.85 (288.05) | 39.38 (45.15) | 0.17 (0.514) | 12.48 | −5.31 | <0.001 | |
| Days Since Last THC Use | 18.69 (33.34) | 71.69 (82.25) | 46.11 (160.19) | 20.63 | 2.35 | 0.029 | |
| Lifetime Alcohol Use | 131.92 (131.55) | 17.44 (15.87) | 0.22 (0.73) | 12.28 | −3.12 | 0.009 | |
| Days Since Last Alcohol | 16.46 (11.67) | 45.38 (98.99) | 22.22 (66.12) | 27 | 1.04 | 0.306 | |
| Lifetime Alcohol Binge Episode | 92.83 (71.90) | 7.87 (7.97) | 0.11 (0.47) | 11.22 | −4.07 | 0.002 | |
| Days Since Last Binge | 24.70 (24.83) | 90.93 (135. 77) | 240 (--) | 15.38 | 1.84 | 0.085 | |
| Lifetime Hallucinogen Use | 2.69 (3.88) | 0.13 (0.50) | -- | 12.32 | −2.37 | 0.035 | |
| Days Since Last Hallucinogen | 82.31 (93.58) | 9.81 (39.25) | -- | 15.42 | −2.61 | 0.019 | |
| Lifetime Sedative Use | 0.77 (1.36) | -- | -- | 12.00 | −2.03 | 0.065 | |
| Days Since Last Sedative Use | 179.15 (330.97) | -- | -- | 12.00 | −1.95 | 0.075 | |
| Lifetime Amphetamine Use | 0.31 (1.11) | -- | -- | 12.00 | −1.00 | 0.337 | |
| Days Since Last Amphetamine Use | 14.46 (52.14) | -- | -- | 12 | −1.00 | 0.337 | |
| Lifetime Rx Stimulant Use | 1.92 (5.48) | 0.06 (0.25) | -- | 12.04 | −1.22 | 0.245 | |
| Days Since Last Rx Stimulant Use | 148.23 (297.47) | 17.94 (71.75) | -- | 13.14 | −1.54 | 0.147 | |
| Lifetime Cocaine Use | 0.92 (1.50) | -- | -- | 12.00 | −2.22 | 0.046 | |
| Days Since Last Cocaine Use | 55.00 (91.33) | -- | -- | 12.00 | −2.17 | 0.051 | |
| Lifetime Ecstasy Use | 14.85 (27.65) | -- | -- | 12.00 | −1.94 | 0.077 | |
| Days Since Last Ecstasy Use | 293.62 (333.72) | -- | -- | 12.00 | −3.17 | 0.008 | |
| Lifetime Opiate Use | 0.92 (2.75) | 1.94 (7.49) | -- | 27 | 0.462 | 0.647 | |
| Days Since Last Opiate Use | 139.31 (277.92) | 26.56 (73.13) | -- | 13.35 | −1.42 | 0.178 | |
| Lifetime Inhalant Use | 2.38 (8.30) | -- | -- | 12.00 | −1.04 | 0.321 | |
| Days Since Last Inhalant Use | 106.00 (259.42) | -- | -- | 12.00 | −1.47 | 0.166 | |
| Lifetime Nicotine Use | 232.00 (409.19) | 4.19 (6.73) | 0.56 (2.36) | 12.00 | −2.01 | 0.068 | |
| Days Since Last Nicotine Use | 92.69 (108.66) | 130.69 (157.63) | 21.94 (93.10) | 26.39 | 0.766 | 0.451 | |
| CAN+ALC-SUD Cannabis/Alcohol Substance Use Disorder | CAN+ALC-EXP Cannabis/Alcohol Experimenter | CTL Little to No Substance Use | ||||
|---|---|---|---|---|---|---|
| Demographics | M(SD) | M(SD) | M(SD) | df | F | p |
| Age (in years) | 16.62 (0.51) | 16.69 (0.70) | 16.33 (0.77) | 2,44 | 1.27 | 0.290 |
| Education (in years) | 10.46 (0.78) | 10.47 (0.83) | 10.11 (0.90) | 2,43 | 0.956 | 0.392 |
| WRAT 4 Verbal IQ | 107.31 (14.29) | 106.75 (12.37) | 112.00 (13.82) | 2,44 | 0.770 | 0.469 |
| VAS Ratings | M(SD) | M(SD) | M(SD) | df | F | p |
| Unpleasant | 5.69 (3.29) | 4.63 (2.64) | 5.34 (3.49) | 2,44 | 0.432 | 0.652 |
| Intensity | 4.08 (3.47) | 2.13 (2.77) | 4.41 (2.89) | 2,44 | 2.68 | 0.08 |
| Questionnaires | M(SD) | M(SD) | M(SD) | df | F/t | p |
| MAIA | ||||||
| Noticing | 2.83 (1.52) | 2.75 (1.03) | 2.78 (1.19) | 2,43 | 0.016 | 0.984 |
| Not Distracting | 2.14 (0.50) | 2.37 (1.12) | 2.52 (1.30) | 2,43 | 0.443 | 0.645 |
| Not Worrying | 2.89 (1.43) | 2.81 (1.42) | 2.70 (1.05) | 2,43 | 0.079 | 0.925 |
| Attention Regulation | 3.17 (.95) | 3.45 (0.74) | 3.14 (1.15) | 2,43 | 0.494 | 0.613 |
| Emotional Awareness | 3.18 (1.43) | 3.04 (1.31) | 3.07 (.93) | 2,43 | 0.054 | 0.948 |
| Self-Regulation | 3.10 (1.05) | 3.23 (0.90) | 3.01 (1.05) | 2,43 | 0.207 | 0.814 |
| Body Listening | 1.36 (1.16) | 1.96 (1.44) | 1.79 (1.05) | 2,43 | 0.844 | 0.437 |
| Trusting | 3.47 (1.40) | 3.73 (1.08) | 3.72 (0.92) | 2,43 | 0.231 | 0.795 |
| UPPS | ||||||
| Lack of Premeditation | 2.08 (0.39) | 2.18 (0.49) | 1.89 (0.42) | 2,44 | 1.92 | 0.159 |
| Urgency | 2.30 (0.66) | 2.17 (0.59) | 2.06 (0.51) | 2,44 | 0.672 | 0.516 |
| Sensation Seeking | 3.18 (0.29) | 3.09 (0.48) | 3.03 (0.44) | 2,44 | 0.528 | 0.594 |
| Lack of Perseverance | 2.03 (0.59) | 2.13 (0.58) | 1.83 (0.34) | 2,44 | 1.53 | 0.229 |
| MNRQ | ||||||
| Negative Reinforcement | 2.85 (2.38) | 0.875 (1.63) | -- | 20.52 | 2.55 | 0.019 |
| Positive Reinforcement | 11.38 (2.53) | 7.25 (3.45) | -- | 27 | 3.59 | 0.001 |
| GROUP BY INTEROCEPTIVE CONDITION INTERACTION | |||||||||
| R/L | Voxels | Volume | X | Y | Z | BA | Anticipation | Load | |
| Amygdala | R | 33 | 2112 | 28 | −9 | −30 | 28 | SUD > EXP | EXP > SUD |
| Inferior Frontal Gyrus | L | 28 | 1792 | −13 | 24 | −20 | 11 | -- | EXP = CTL > SUD |
| Posterior Cingulate | R | 25 | 1600 | 13 | −65 | 16 | 31 | -- | EXP = SUD > CTL |
| Parahippocampal Gyrus | L | 21 | 1344 | −24 | −7 | −19 | 35 | CTL > EXP | CTL = EXP > SUD |
| MAIN EFFECT OF INTEROCEPTIVE CONDITION | |||||||||
| R/L | Voxels | Volume | X | Y | Z | BA | Condition Effect | ||
| Cingulate Gyrus | R | 3141 | 201024 | 8 | −6 | 23 | 24 | Load > Anticipation | |
| Fusiform Gyrus | R | 663 | 42432 | 40 | −12 | −24 | 20 | Anticipation > Load | |
| Superior Frontal Gyrus | R | 334 | 21376 | 1 | 4 | 57 | 6 | Load > Anticipation | |
| Cingulate Gyrus | L | 131 | 8384 | −2 | −25 | 37 | 31 | Load > Anticipation | |
| Cuneus | R | 112 | 7168 | 18 | −85 | 28 | 18 | Load > Anticipation | |
| Thalamus | R | 64 | 4096 | 6 | −18 | 4 | Load > Anticipation | ||
| Declive | L | 61 | 3904 | −15 | −63 | −20 | Load > Anticipation | ||
| Middle Frontal Gyrus | L | 48 | 3072 | −36 | 37 | 28 | 9 | Anticipation > Load | |
| Middle Occipital Gyrus | R | 43 | 2752 | 34 | −83 | 9 | 19 | Load > Anticipation | |
| Anterior Cingulate | L | 39 | 2496 | −6 | 31 | 15 | 24 | Load > Anticipation | |
| Precuneus | R | 36 | 2304 | 5 | −43 | 43 | 7 | Load > Anticipation | |
| Precentral Gyrus | R | 29 | 1856 | 18 | −26 | 64 | 4 | Load > Anticipation | |
| Precentral Gyrus | L | 24 | 1536 | −18 | −29 | 63 | 4 | Anticipation > Load | |
| MAIN EFFECT OF CUE STIMULUS TYPE | |||||||||
| R/L | Voxels | Volume | X | Y | Z | BA | Stimulus Effect | ||
| Medial Frontal Gyrus | R | 43 | 2752 | 1 | 44 | 30 | 9 | Substance > Comparison | |
| Anterior Cingulate | L | 23 | 1472 | −1 | 46 | 8 | 32 | Substance > Comparison | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, A.C.; Jacobus, J.; Stewart, J.L.; Simmons, A.N.; Paulus, M.P.; Tapert, S.F. Do Adolescents Use Substances to Relieve Uncomfortable Sensations? A Preliminary Examination of Negative Reinforcement among Adolescent Cannabis and Alcohol Users. Brain Sci. 2020, 10, 214. https://doi.org/10.3390/brainsci10040214
May AC, Jacobus J, Stewart JL, Simmons AN, Paulus MP, Tapert SF. Do Adolescents Use Substances to Relieve Uncomfortable Sensations? A Preliminary Examination of Negative Reinforcement among Adolescent Cannabis and Alcohol Users. Brain Sciences. 2020; 10(4):214. https://doi.org/10.3390/brainsci10040214
Chicago/Turabian StyleMay, April C., Joanna Jacobus, Jennifer L. Stewart, Alan N. Simmons, Martin P. Paulus, and Susan F. Tapert. 2020. "Do Adolescents Use Substances to Relieve Uncomfortable Sensations? A Preliminary Examination of Negative Reinforcement among Adolescent Cannabis and Alcohol Users" Brain Sciences 10, no. 4: 214. https://doi.org/10.3390/brainsci10040214
APA StyleMay, A. C., Jacobus, J., Stewart, J. L., Simmons, A. N., Paulus, M. P., & Tapert, S. F. (2020). Do Adolescents Use Substances to Relieve Uncomfortable Sensations? A Preliminary Examination of Negative Reinforcement among Adolescent Cannabis and Alcohol Users. Brain Sciences, 10(4), 214. https://doi.org/10.3390/brainsci10040214







