Early Mortality of Brain Infarction Patients and Red Blood Cell Distribution Width
Abstract
1. Introduction
2. Methods
2.1. Design and Subjects
2.2. Statistical Methods
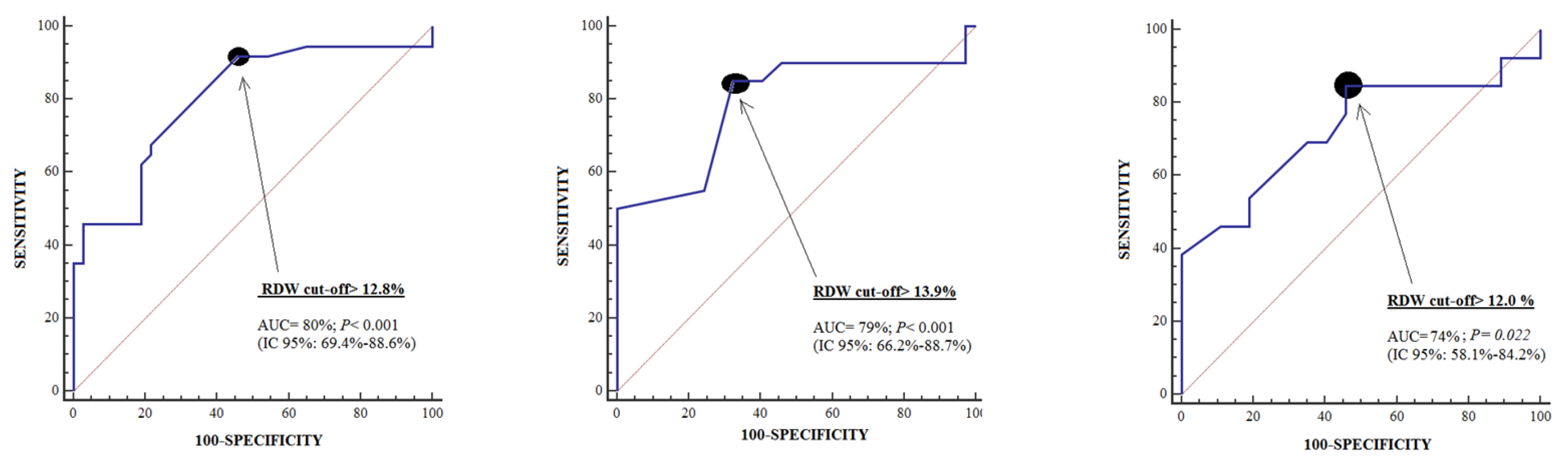
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aslan, D.; Gümrük, F.; Gürgey, A.; Altay, C. Importance of RDW value in differential diagnosis of hypochrome anemias. Am. J. Hematol. 2002, 69, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Abrahan, L.L., 4th; Ramos, J.D.A.; Cunanan, E.L.; Tiongson, M.D.A.; Punzalan, F.E.R. Red Cell Distribution Width and Mortality in Patients with Acute Coronary Syndrome: A Meta-Analysis on Prognosis. Cardiol. Res. 2018, 9, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Fontana, V.; Spadaro, S.; Villois, P.; Righy Shinotsuka, C.; Fogagnolo, A.; Nobile, L.; Vincent, J.L.; Creteur, J.; Taccone, F.S. Can red blood cell distribution width predict outcome after cardiac arrest? Minerva Anestesiol. 2018, 84, 693–702. [Google Scholar]
- Salvatori, M.; Formiga, F.; Moreno-Gónzalez, R.; Chivite, D.; Migone De Amicis, M.; Cappellini, M.D.; Corbella, X. Red blood cell distribution width as a prognostic factor of mortality in elderly patients firstly hospitalized due to heart failure. Kardiol. Pol. 2019, 77, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.; Awad, H.; Hu, Z.D. Prognostic value of admission red blood cell distribution width in acute pancreatitis: A systematic review. Ann. Transl. Med. 2017, 5, 342. [Google Scholar] [CrossRef]
- Milas, G.P.; Karageorgiou, V.; Cholongitas, E. Red cell distribution width to platelet ratio for liver fibrosis: A systematic review and meta-analysis of diagnostic accuracy. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 877–891. [Google Scholar] [CrossRef]
- Hu, Z.D.; Lippi, G.; Montagnana, M. Diagnostic and prognostic value of red blood cell distribution width in sepsis: A narrative review. Clin. Biochem. 2020, 77, 1–6. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Solé-Violán, J.; Ferreres, J.; Labarta, L.; Díaz, C.; González, O.; García, D.; Jiménez, A.; et al. Red Blood Cell Distribution Width during the First Week Is Associated with Severity and Mortality in Septic Patients. PLoS ONE 2014, 9, e105436. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J. Red blood cell distribution width as a prognostic biomarker for mortality in traumatic brain injury. Int. J. Clin. Exp. Med. 2015, 8, 19172–19175. [Google Scholar]
- Song, S.Y.; Hua, C.; Dornbors, D., 3rd; Kang, R.J.; Zhao, X.X.; Du, X.; He, W.; Ding, Y.C.; Meng, R. Baseline Red Blood Cell Distribution Width as a Predictor of Stroke Occurrence and Outcome: A Comprehensive Meta-Analysis of 31 Studies. Front. Neurol. 2019, 10, 1237. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Q.; Wu, X.; Ju, X.; Wang, L.; Hu, S. Correlation between the red blood cell distribution width and prognosis in elderly patients with cerebral infarction and severe hemiplegia. Chin. J. Cerebrovasc. Dis. 2015, 6, 287–291. [Google Scholar]
- Fan, L.; Gui, L.; Chai, E.Q.; Wei, C.J. Routine hematological parameters are associated with short- and long-term prognosis of patients with ischemic stroke. J. Clin. Lab. Anal. 2018, 32, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.D.; Song, T.J.; Park, J.H.; Lee, H.S.; Nam, C.M.; Nam, H.S.; Heo, J.H. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb. Haemost. 2012, 108, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P.; Hryniewiecki, T.; Kusmierczyk, M.; Szymanski, P. Red cell distribution width is a prognostic marker of perioperative stroke in patients undergoing cardiac valve surgery. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessement of coma and impaired conciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Riaño-Ruiz, M.; Jiménez, A. Serum malondialdehyde levels in patients with malignant middle cerebral artery infarction are associated with mortality. PLoS ONE 2015, 10, e0125893. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Sabatel, R.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Riaño-Ruiz, M.; García-Marín, V. Serum malondialdehyde levels and mortality in patients with spontaneous intracerebral haemorrhage. World Neurosurg. 2018, 113, e542–e547. [Google Scholar] [CrossRef]
- Zangari, R.; Zanier, E.R.; Torgano, G.; Bersano, A.; Beretta, S.; Beghi, E.; Casolla, B.; Checcarelli, N.; Lanfranconi, S.; Maino, A.; et al. Early ficolin-1 is a sensitive prognostic marker for functional outcome in ischemic stroke. J. Neuroinflammation 2016, 13, 16. [Google Scholar] [CrossRef]
- Di Napoli, M.; Slevin, M.; Popa-Wagner, A.; Singh, P.; Lattanzi, S.; Divani, A.A. Monomeric C-Reactive Protein and Cerebral Hemorrhage: From Bench to Bedside. Front. Immunol. 2018, 9, 1921. [Google Scholar] [CrossRef]
- Lattanzi, S.; Di Napoli, M.; Ricci, S.; Divani, A.A. Matrix Metalloproteinases in Acute Intracerebral Hemorrhage. Neurotherapeutics 2020. [Google Scholar] [CrossRef] [PubMed]
- Watters, O.; O’Connor, J.J. A role for tumor necrosis factor-α in ischemia and ischemic preconditioning. J. Neuroinflammation 2011, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Kagansky, N.; Levy, S.; Knobler, H. The Role of Hyperglycemia in Acute Stroke. Arch. Neurol. 2001, 58, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Liu, X.; Li, F.; Wang, J.; Sun, H.; Feng, A.; Sun, Y.; Sun, H.; Yang, F.; Zhao, J.; et al. Blood Pressure Variability at Different Time Periods Within First 24 Hours After Admission and Outcomes of Acute Ischemic Stroke. J. Clin. Hypertens. 2020, 22, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Divani, A.A.; Liu, X.; Di Napoli, M.; Lattanzi, S.; Ziai, W.; James, M.L.; Jafarli, A.; Jafari, M.; Saver, J.L.; Hemphill, J.C.; et al. Blood Pressure Variability Predicts Poor In-Hospital Outcome in Spontaneous Intracerebral Hemorrhage. Stroke 2019, 50, 2023–2029. [Google Scholar] [CrossRef]
- Ghaffari, S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal. 2008, 10, 1923–1940. [Google Scholar] [CrossRef]
- Scharte, M.; Fink, M.P. Red blood cell physiology in critical illness. Crit. Care Med. 2003, 31, S651–S657. [Google Scholar] [CrossRef]
- Pierce, C.N.; Larson, D.F. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 2005, 20, 83–90. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Red blood cell distribution width: A marker of anisocytosis potentially associated with atrial fibrillation. World J. Cardiol. 2019, 11, 292–304. [Google Scholar] [CrossRef]
- Weymann, A.; Ali-Hasan-Al-Saegh, S.; Sabashnikov, A.; Popov, A.F.; Mirhosseini, S.J.; Liu, T.; Lotfaliani, M.; Sá, M.P.B.O.; Baker, W.L.L.; Yavuz, S.; et al. Prediction of New-Onset and Recurrent Atrial Fibrillation by Complete Blood Count Tests: A Comprehensive Systematic Review with Meta-Analysis. Med. Sci. Monit. Basic Res. 2017, 23, 79–222. [Google Scholar] [CrossRef]
- Weymann, A.; Ali-Hasan-Al-Saegh, S.; Popov, A.F.; Sabashnikov, A.; Mirhosseini, S.J.; Liu, T.; Tse, G.; Lotfaliani, M.; Ghanei, A.; Testa, L.; et al. Haematological indices as predictors of atrial fibrillation following isolated coronary artery bypass grafting, valvular surgery, or combined procedures: A systematic review with meta-analysis. Kardiol. Pol. 2018, 76, 107–118. [Google Scholar] [CrossRef] [PubMed]
| Survivors (n = 37) | Non-Survivors (n = 37) | p-Value | |
|---|---|---|---|
| Age (years): median (p 25–75) | 60 (47–68) | 61 (53–70) | 0.50 |
| Female: n (%) | 14 (37.8) | 14 (37.8) | 0.99 |
| Heart failure: n (%) | 1 (2.7) | 1 (2.7) | 0.99 |
| Diabetes mellitus: n (%) | 5 (13.5) | 9 (24.3) | 0.37 |
| COPD: n (%) | 1 (2.7) | 1 (2.7) | 0.99 |
| Chronic renal failure: n (%) | 2 (5.4) | 2 (5.4) | 0.99 |
| Arterial hypertension: n (%) | 21 (56.8) | 19 (51.4) | 0.82 |
| GCS score: median (p 25–75) | 8 (6–8) | 6 (3–7) | 0.01 |
| APACHE-II score: median (p 25–75) | 20 (16–25) | 22 (19–27) | 0.07 |
| Lactic acid (mmol/L): median (p 25–75) | 1.20 (0.90–1.70) | 1.60 (1.01–2.88) | 0.03 |
| Temperature (°C): median (p 25–75) | 36.4 (36.0–37.0) | 36.9 (36.0–37.2) | 0.10 |
| Bilirubin (mg/dL): median (p 25–75) | 0.60 (0.42–0.80) | 0.65 (0.35–1.13) | 0.85 |
| Glycemia (g/dL): median (p 25–75) | 127 (102–170) | 136 (113–161) | 0.50 |
| Creatinine (mg/dL): median (p 25–75) | 0.80 (0.65–1.10) | 1.00 (0.70–1.20) | 0.21 |
| Sodium (mEq/L): median (p 25–75) | 139 (136–143) | 140 (138–143) | 0.50 |
| PaO2 (mmHg): median (p 25–75) | 144 (104–285) | 115 (94–267) | 0.40 |
| PaO2/FIO2 ratio: median (p 25–75) | 293 (204–366) | 248 (188–320) | 0.18 |
| INR: median (p 25–75) | 1.06 (1.00–1.20) | 1.15 (1.01–1.31) | 0.05 |
| aPTT (seconds): median (p 25–75) | 28 (25–30) | 27 (26–32) | 0.99 |
| Platelets: median × 103/mm3 (p 25–75) | 200 (170–267) | 173 (134–212) | 0.02 |
| Fibrinogen (mg/dL): median (p 25–75) | 445 (415–526) | 419 (339–612) | 0.90 |
| Leukocytes: median × 103/mm3 (p 25–75) | 12.2 (9.5–17.0) | 13.8 (9.3–17.7) | 0.40 |
| Hemoglobin (g/dL): median (p 25–75) | 12.2 (11.4–14.5) | 12.5 (11.0–14.8) | 0.97 |
| Thrombolysis: n (%) | 12 (32.4) | 12 (32.4) | 0.99 |
| Hemorrhagic transformation: n (%) | 8 (21.6) | 8 (21.6) | 0.99 |
| Volume infarction (mL): median (p25–75) | 181 (105–235) | 190 (65–288) | 0.72 |
| Midline shift (mm): median (p 25–75) | 6.5 (2.8–11.2) | 10.0 (4.0–15.0) | 0.41 |
| Decompressive craniectomy: n (%) | 9 (24.3) | 7 (18.9) | 0.78 |
| Parameters | Survivors | Non-Survivors | p-Value |
|---|---|---|---|
| Day 1 | (n = 37) | (n = 37) | |
| RDW: median % (percentile 25–75) | 12.7 (11.2–13.2) | 13.9 (13.0–17.0) | <0.001 |
| Malondialdehyde: median nmol/mL (percentile 25–75) | 1.76 (1.39–2.24) | 2.99 (2.08–4.17) | <0.001 |
| TNF-alpha median pg/mL (percentile 25–75) | 9.8 (9.2–11.3) | 15.5 (13.2–16.7) | <0.001 |
| Day 4 | (n = 37) | (n = 20) | |
| RDW: median % (percentile 25–75) | 12.0 (10.3–14.5) | 15.1 (14.0–17.1) | <0.001 |
| Malondialdehyde: median nmol/mL (percentile 25–75) | 1.64 (1.37–1.90) | 2.95 (2.50–3.19) | <0.001 |
| TNF-alpha median pg/mL (percentile 25–75) | 9.8 (9.1–10.9) | 14.9 (13.3–16.2) | <0.001 |
| Day 8 | (n = 37) | (n = 13) | |
| RDW: median % (percentile 25–75) | 11.5 (9.9–14.0) | 14.9 (12.7–16.9) | 0.02 |
| Malondialdehyde: median nmol/mL (percentile 25–75) | 1.46 (1.19–1.92) | 2.71 (2.52–2.88) | <0.001 |
| TNF-alpha: median pg/mL (percentile 25–75) | 9.3 (8.9–10.4) | 14.8 (13.5–17.2) | <0.001 |
| Day 1 | Day 4 | Day 8 | |
|---|---|---|---|
| Cut-off of RDW in % | >12.8 | >13.9 | >12.0 |
| Specificity (95% confidence interval) | 54% (37%–71%) | 68% (50%–82%) | 54% (37%–71%) |
| Sensitivity (95% confidence interval) | 92% (78%–98%) | 85% (62%–97%) | 85% (55%–98%) |
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Platelet count (each 1000/mm3) | 0.995 | 0.987–1.003 | 0.22 |
| Lactic acid (mmol/L) | 1.148 | 0.642–2.053 | 0.64 |
| Glasgow Coma Scale (points) | 0.661 | 0.480–0.910 | 0.01 |
| RDW (%) | 1.695 | 1.230–2.335 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente, L.; Martín, M.M.; Abreu-González, P.; Pérez-Cejas, A.; González-Rivero, A.F.; Ramos-Gómez, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; et al. Early Mortality of Brain Infarction Patients and Red Blood Cell Distribution Width. Brain Sci. 2020, 10, 196. https://doi.org/10.3390/brainsci10040196
Lorente L, Martín MM, Abreu-González P, Pérez-Cejas A, González-Rivero AF, Ramos-Gómez L, Argueso M, Solé-Violán J, Cáceres JJ, Jiménez A, et al. Early Mortality of Brain Infarction Patients and Red Blood Cell Distribution Width. Brain Sciences. 2020; 10(4):196. https://doi.org/10.3390/brainsci10040196
Chicago/Turabian StyleLorente, Leonardo, María M. Martín, Pedro Abreu-González, Antonia Pérez-Cejas, Agustín F. González-Rivero, Luis Ramos-Gómez, Mónica Argueso, Jordi Solé-Violán, Juan J. Cáceres, Alejandro Jiménez, and et al. 2020. "Early Mortality of Brain Infarction Patients and Red Blood Cell Distribution Width" Brain Sciences 10, no. 4: 196. https://doi.org/10.3390/brainsci10040196
APA StyleLorente, L., Martín, M. M., Abreu-González, P., Pérez-Cejas, A., González-Rivero, A. F., Ramos-Gómez, L., Argueso, M., Solé-Violán, J., Cáceres, J. J., Jiménez, A., & García-Marín, V. (2020). Early Mortality of Brain Infarction Patients and Red Blood Cell Distribution Width. Brain Sciences, 10(4), 196. https://doi.org/10.3390/brainsci10040196





