Purinergic Signaling and Related Biomarkers in Depression
Abstract
1. Introduction
2. The Adenosine Receptor Antagonist, Caffeine, and Depression
3. Adenosine and Depression
4. ATP and Depression
5. Genetic Studies
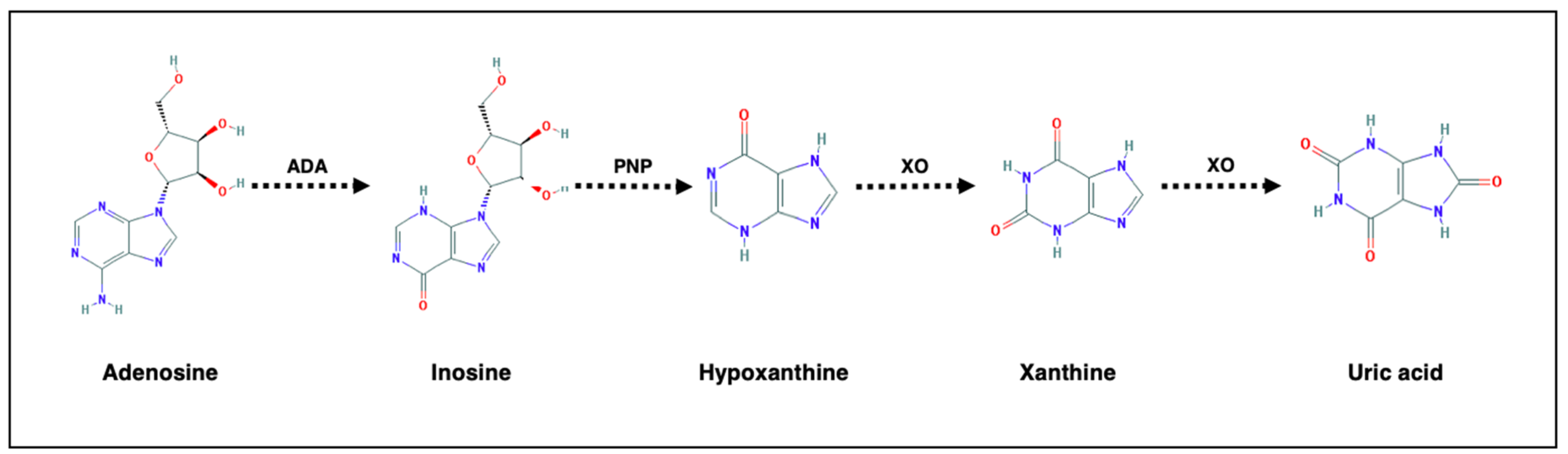
6. Purinergic Metabolism and Biomarkers
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Drury, A.N.; Szent-Györgyi, A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929, 68, 213–237. [Google Scholar] [CrossRef]
- Burnstock, G.; Campbell, G.; Satchell, D.; Smythe, A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 1970, 40, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 2008, 7, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purine and purinergic receptors. Brain Neurosci. Adv. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Burnstock, G. A basis for distinguishing two types of purinergic receptor. In Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach; Straub, R.W., Bolis, L., Eds.; Raven Press: New York, NY, USA, 1978; pp. 107–118. [Google Scholar]
- Bynoe, M.S.; Viret, C.; Yan, A.; Kim, D.G. Adenosine receptor signaling: A key to opening the blood-brain door. Fluids Barriers CNS 2015, 12, 20. [Google Scholar] [CrossRef]
- Diez, R.; Richardson, M.J.E.; Wall, M.J. Reducing Extracellular Ca(2+) Induces Adenosine Release via Equilibrative Nucleoside Transporters to Provide Negative Feedback Control of Activity in the Hippocampus. Front. Neural Circuits 2017, 11, 75. [Google Scholar] [CrossRef]
- Boison, D.; Chen, J.F.; Fredholm, B.B. Adenosine signaling and function in glial cells. Cell Death Differ. 2010, 17, 1071–1082. [Google Scholar] [CrossRef]
- Domenici, M.R.; Ferrante, A.; Martire, A.; Chiodi, V.; Pepponi, R.; Tebano, M.T.; Popoli, P. Adenosine A(2A) receptor as potential therapeutic target in neuropsychiatric disorders. Pharmacol. Res. 2019, 147, 104338. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.M.; Chu, S.F.; Peng, Y.; Chen, N.H. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Cheffer, A.; Castillo, A.R.G.; Corrêa-Velloso, J.; Gonçalves, M.C.B.; Naaldijk, Y.; Nascimento, I.C.; Burnstock, G.; Ulrich, H. Purinergic system in psychiatric diseases. Mol. Psychiatry 2018, 23, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.V.; Kaster, M.P.; Tomé, A.R.; Agostinho, P.M.; Cunha, R.A. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim. Biophys. Acta 2011, 1808, 1380–1399. [Google Scholar] [CrossRef] [PubMed]
- De Lera Ruiz, M.; Lim, Y.H.; Zheng, J. Adenosine A2A receptor as a drug discovery target. J. Med. Chem. 2014, 57, 3623–3650. [Google Scholar] [CrossRef] [PubMed]
- Van Calker, D.; Biber, K.; Domschke, K.; Serchov, T. The role of adenosine receptors in mood and anxiety disorders. J. Neurochem. 2019, 151, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Abbracchio, M.; Burnstock, G. Purinoceptors: Are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 1994, 64, 445–475. [Google Scholar] [CrossRef]
- Puchałowicz, K.; Tarnowski, M.; Baranowska-Bosiacka, I.; Chlubek, D.; Dziedziejko, V. P2X and P2Y receptors—role in the pathophysiology of the nervous system. Int. J. Mol. Sci. 2014, 15, 23672–23704. [Google Scholar] [CrossRef]
- Franke, H.; Illes, P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol. Ther. 2006, 109, 297–324. [Google Scholar] [CrossRef]
- Ortiz, R.; Ulrich, H.; Zarate, C.A., Jr.; Machado-Vieira, R. Purinergic system dysfunction in mood disorders: A key target for developing improved therapeutics. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 57, 117–131. [Google Scholar] [CrossRef]
- Cunha, R.A.; Ferré, S.; Vaugeois, J.M.; Chen, J.F. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr. Pharm. Des. 2008, 14, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Díaz-Ríos, M.; Salamone, J.D.; Prediger, R.D. New Developments on the Adenosine Mechanisms of the Central Effects of Caffeine and Their Implications for Neuropsychiatric Disorders. J. Caffeine Adenosine Res. 2018, 8, 121–131. [Google Scholar] [CrossRef] [PubMed]
- López-Cruz, L.; Salamone, J.D.; Correa, M. Caffeine and Selective Adenosine Receptor Antagonists as New Therapeutic Tools for the Motivational Symptoms of Depression. Front. Pharmacol. 2018, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kobayashi, M.; Kanda, T. Involvement of adenosine A2A receptors in depression and anxiety. Int. Rev. Neurobiol. 2014, 119, 373–393. [Google Scholar]
- Wang, L.; Shen, X.; Wu, Y.; Zhang, D. Coffee and caffeine consumption and depression: A meta-analysis of observational studies. Aust. N. Z. J. Psychiatry 2016, 50, 228–242. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Castellano, S.; Pajak, A.; Galvano, F. Coffee, tea, caffeine and risk of depression: A systematic review and dose-response meta-analysis of observational studies. Mol. Nutr. Food Res. 2016, 60, 223–234. [Google Scholar] [CrossRef]
- Lucas, M.; O’Reilly, E.J.; Pan, A.; Mirzaei, F.; Willett, W.C.; Okereke, O.I.; Ascherio, A. Coffee, caffeine, and risk of completed suicide: Results from three prospective cohorts of American adults. World J. Biol. Psychiatry 2014, 15, 377–386. [Google Scholar] [CrossRef]
- Park, H.; Suh, B.S.; Lee, K. Relationship between daily coffee intake and suicidal ideation. J. Affect. Disord. 2019, 256, 468–472. [Google Scholar] [CrossRef]
- Tanskanen, A.; Tuomilehto, J.; Viinamäki, H.; Vartiainen, E.; Lehtonen, J.; Puska, P. Heavy coffee drinking and the risk of suicide. Eur. J. Epidemiol. 2000, 16, 789–791. [Google Scholar] [CrossRef]
- Pedrazza, E.L.; Rico, E.P.; Senger, M.R.; Pedrazza, L.; Zimmermann, F.F.; Sarkis, J.J.; Bogo, M.R.; Bonan, C.D. Ecto-nucleotidase pathway is altered by different treatments with fluoxetine and nortriptyline. Eur. J. Pharmacol. 2008, 583, 18–25. [Google Scholar] [CrossRef]
- Wilot, L.C.; Da Silva, R.S.; Ferreira, O.J.; Bonan, C.D.; Sarkis, J.J.; Rocha, E.; Battastini, A.M. Chronic treatment with lithium increases the ecto-nucleotidase activities in rat hippocampal synatosomes. Neurosci. Lett. 2004, 368, 167–170. [Google Scholar] [CrossRef]
- Serchov, T.; Clement, H.W.; Schwarz, M.K.; Iasevoli, F.; Tosh, D.K.; Idzko, M.; Jacobson, K.A.; de Bartolomeis, A.; Normann, C.; Biber, K.; et al. Increased Signaling via Adenosine A1 Receptors, Sleep Deprivation, Imipramine, and Ketamine Inhibit Depressive-like Behavior via Induction of Homer1a. Neuron 2015, 87, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.E.; Alves, P.; Canas, P.M.; Valadas, J.S.; Shmidt, T.; Batalha, V.L.; Ferreira, D.G.; Ribeiro, J.A.; Bader, M.; Cunha, R.A.; et al. Overexpression of Adenosine A2A Receptors in Rats: Effects on Depression, Locomotion, and Anxiety. Front. Psychiatry 2014, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Crema, L.M.; Pettenuzzo, L.F.; Schlabitz, M.; Diehl, L.; Hoppe, J.; Mestriner, R.; Laureano, D.; Salbego, C.; Dalmaz, C.; Vendite, D. The effect of unpredictable chronic mild stress on depressive-like behavior and on hippocampal A1 and striatal A2A adenosine receptors. Physiol. Behav. 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Early-life hyperthermic seizures upregulate adenosine A(2A) receptors in the cortex and promote depressive-like behavior in adult rats. Epilepsy Behav. 2018, 86, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kaster, M.P.; Machado, N.J.; Silva, H.B.; Nunes, A.; Ardais, A.P.; Santana, M.; Baqi, Y.; Müller, C.E.; Rodrigues, A.L.; Porciúncula, L.O.; et al. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc. Natl. Acad. Sci. USA 2015, 112, 7833–7838. [Google Scholar] [CrossRef]
- Rial, D.; Lemos, C.; Pinheiro, H.; Duarte, J.M.; Gonçalves, F.Q.; Real, J.I.; Prediger, R.D.; Gonçalves, N.; Gomes, C.A.; Canas, P.M.; et al. Depression as a Glial-Based Synaptic Dysfunction. Front. Cell Neurosci. 2016, 9, 521. [Google Scholar] [CrossRef]
- El Yacoubi, M.; Ledent, C.; Parmentier, M.; Bertorelli, R.; Ongini, E.; Costentin, J.; Vaugeois, J.M. Adenosine A2A receptor antagonists are potential antidepressants: Evidence based on pharmacology and A2A receptor knockout mice. Br. J. Pharmacol. 2001, 134, 68–77. [Google Scholar] [CrossRef]
- El Yacoubi, M.; Costentin, J.; Vaugeois, J.M. Adenosine A2A receptors and depression. Neurology 2003, 61, S82–S87. [Google Scholar] [CrossRef]
- Dziubina, A.; Szmyd, K.; Zygmunt, M.; Sapa, J.; Dudek, M.; Filipek, B.; Drabczyńska, A.; Załuski, M.; Pytka, K.; Kieć-Kononowicz, K. Evaluation of antidepressant-like and anxiolytic-like activity of purinedione-derivatives with affinity for adenosine A(2A) receptors in mice. Pharmacol. Rep. 2016, 68, 1285–1292. [Google Scholar] [CrossRef]
- Padilla, K.M.; Quintanar-Setephano, A.; López-Vallejo, F.; Berumen, L.C.; Miledi, R.; García-Alcocer, G. Behavioral changes induced through adenosine A2A receptor ligands in a rat depression model induced by olfactory bulbectomy. Brain Behav. 2018, 8, e00952. [Google Scholar] [CrossRef] [PubMed]
- Poleszak, E.; Szopa, A.; Bogatko, K.; Wyska, E.; Wośko, S.; Świąder, K.; Doboszewska, U.; Wlaź, A.; Wróbel, A.; Wlaź, P.; et al. Antidepressant-Like Activity of Typical Antidepressant Drugs in the Forced Swim Test and Tail Suspension Test in Mice Is Augmented by DMPX, an Adenosine A(2A) Receptor Antagonist. Neurotox. Res. 2019, 35, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Randall, P.A.; Nunes, E.J.; Janniere, S.L.; Stopper, C.M.; Farrar, A.M.; Sager, T.N.; Baqi, Y.; Hockemeyer, J.; Müller, C.E.; Salamone, J.D. Stimulant effects of adenosine antagonists on operant behavior: Differential actions of selective A2A and A1 antagonists. Psychopharmacology (Berl) 2011, 216, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Voelker, R. Add-on Drug Approved for “Off” Episodes of Parkinson Disease. JAMA 2019, 322, 1246. [Google Scholar] [CrossRef]
- Yamada, K.; Kobayashi, M.; Shiozaki, S.; Ohta, T.; Mori, A.; Jenner, P.; Kanda, T. Antidepressant activity of the adenosine A2A receptor antagonist, istradefylline (KW-6002) on learned helplessness in rats. Psychopharmacology (Berl) 2014, 231, 2839–2849. [Google Scholar] [CrossRef]
- Yamada, K.; Kobayashi, M.; Mori, A.; Jenner, P.; Kanda, T. Antidepressant-like activity of the adenosine A(2A) receptor antagonist, istradefylline (KW-6002), in the forced swim test and the tail suspension test in rodents. Pharmacol. Biochem. Behav. 2013, 114–115, 23–30. [Google Scholar] [CrossRef]
- Okada, M.; Nutt, D.J.; Murakami, T.; Zhu, G.; Kamata, A.; Kawata, Y.; Kaneko, S. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J. Neurosci. 2001, 21, 628–640. [Google Scholar] [CrossRef]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-Derived Neurotrophic Factor and Major Depressive Disorder: Evidence from Meta-Analyses. Front. Psychiatry 2018, 8, 308. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hirayama, Y.; Fujishita, K.; Shibata, K.; Shinozaki, Y.; Shigetomi, E.; Takeda, A.; Le, H.P.N.; Hayashi, H.; Hiasa, M.; et al. Anti-Depressant Fluoxetine Reveals its Therapeutic Effect Via Astrocytes. EBioMedicine 2018, 32, 72–83. [Google Scholar] [CrossRef]
- Hines, D.J.; Schmitt, L.I.; Hines, R.M.; Moss, S.J.; Haydon, P.G. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatry 2013, 3, e212. [Google Scholar] [CrossRef]
- Oliveros, A.; Cho, C.H.; Cui, A.; Choi, S.; Lindberg, D.; Hinton, D.; Jang, M.H.; Choi, D.S. Adenosine A(2A) receptor and ERK-driven impulsivity potentiates hippocampal neuroblast proliferation. Transl. Psychiatry 2017, 7, e1095. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Clerici, M.; Carrà, G. Purinergic system and suicidal behavior: Exploring the link between adenosine A2A receptors and depressive/impulsive features. Mol. Psychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Krügel, U. Purinergic receptors in psychiatric disorders. Neuropharmacology 2016, 104, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.E.; Roncalho, A.L.; Glaser, T.; Ulrich, H.; Wegener, G.; Joca, S. P2X7 Receptor Signaling in Stress and Depression. Int. J. Mol. Sci. 2019, 20, 2778. [Google Scholar] [CrossRef]
- Sperlagh, B.; Csolle, C.; Ando, R.D.; Goloncser, F.; Kittel, A.; Baranyi, M. The role of purinergic signaling in depressive disorders. Neuropsychopharmacol. Hung. 2012, 14, 231–238. [Google Scholar]
- Rybakowski, J.; Potok, E.; Strzyzewski, W. Erythrocyte membrane adenosine triphosphatase activities in patients with endogenous depression and healthy subjects. Eur. J. Clin. Investig. 1981, 11, 61–64. [Google Scholar] [CrossRef]
- Diniz, C.; Rodrigues, M.; Casarotto, P.C.; Pereira, V.S.; Crestani, C.C.; Joca, S.R.L. Monoamine involvement in the antidepressant-like effect induced by P2 blockade. Brain Res. 2017, 1676, 19–27. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.P.; Wang, Q.; Wu, Q.; Hu, H.H.; Zhang, M.; Fang, Y.Y.; Zhang, J.; Li, S.J.; Xiong, W.C.; et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef]
- Jun, M.; Xiaolong, Q.; Chaojuan, Y.; Ruiyuan, P.; Shukun, W.; Junbing, W.; Li, H.; Hong, C.; Jinbo, C.; Rong, W.; et al. Calhm2 governs astrocytic ATP releasing in the development of depression-like behaviors. Mol. Psychiatry 2018, 23, 883–891. [Google Scholar] [CrossRef]
- Wei, L.; Syed Mortadza, S.A.; Yan, J.; Zhang, L.; Wang, L.; Yin, Y.; Li, C.; Chalon, S.; Emond, P.; Belzung, C.; et al. ATP-activated P2X7 receptor in the pathophysiology of mood disorders and as an emerging target for the development of novel antidepressant therapeutics. Neurosci. Biobehav. Rev. 2018, 87, 192–205. [Google Scholar] [CrossRef]
- Rech, J.C.; Bhattacharya, A.; Letavic, M.A.; Savall, B.M. The evolution of P2X7 antagonists with a focus on CNS indications. Bioorg. Med. Chem. Lett. 2016, 26, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ota, K.T.; Li, X.Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.M.; Bratcher, N.A.; Harris, R.R.; Jarvis, M.F.; Decker, M.W.; Rueter, L.E. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: Relevance for neuropsychiatric disorders. Behav. Brain Res. 2009, 198, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Boucher, A.A.; Arnold, J.C.; Hunt, G.E.; Spiro, A.; Spencer, J.; Brown, C.; McGregor, I.S.; Bennett, M.R.; Kassiou, M. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience 2011, 189, 170–177. [Google Scholar] [CrossRef]
- Csölle, C.; Baranyi, M.; Zsilla, G.; Kittel, A.; Goloncser, F.; Illes, P.; Papp, E.; Vizi, E.S.; Sperlagh, B. Neurochemical Changes in the Mouse Hippocampus Underlying the Antidepressant Effect of Genetic Deletion of P2X7 Receptors. PLoS ONE 2013, 8, e66547. [Google Scholar] [CrossRef]
- Guan, S.; Shen, Y.; Ge, H.; Xiong, W.; He, L.; Liu, L.; Yin, C.; Wei, X.; Gao, Y. Dihydromyricetin Alleviates Diabetic Neuropathic Pain and Depression Comorbidity Symptoms by Inhibiting P2X7 Receptor. Front. Psychiatry 2019, 10, 770. [Google Scholar] [CrossRef]
- Fan, Y.; Kong, H.; Ye, X.; Ding, J.; Hu, G. ATP-sensitive potassium channels: Uncovering novel targets for treating depression. Brain Struct. Funct. 2016, 221, 3111–3122. [Google Scholar] [CrossRef]
- Ostadhadi, S.; Akbarian, R.; Norouzi-Javidan, A.; Nikoui, V.; Zolfaghari, S.; Chamanara, M.; Dehpour, A.R. Possible involvement of ATP-sensitive potassium channels in the antidepressant-like effects of gabapentin in mouse forced swimming test. Can. J. Physiol. Pharmacol. 2017, 95, 795–802. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhao, Z.; Yang, D.D.; Cao, L.L.; Zhang, L.; Ji, J.; Gu, J.; Huang, J.Y.; Sun, X.L. Activation of ATP-sensitive potassium channel by iptakalim normalizes stress-induced HPA axis disorder and depressive behaviour by alleviating inflammation and oxidative stress in mouse hypothalamus. Brain Res. Bull. 2017, 130, 146–155. [Google Scholar] [CrossRef]
- Huin, V.; Dhaenens, C.M.; Homa, M.; Carvalho, K.; Buée, L.; Sablonnière, B. Neurogenetics of the Human Adenosine Receptor Genes: Genetic Structures and Involvement in Brain Diseases. J. Caffeine Adenosine Res. 2019, 9, 73–88. [Google Scholar] [CrossRef]
- Hohoff, C.; Garibotto, V.; Elmenhorst, D.; Baffa, A.; Kroll, T.; Hoffmann, A.; Schwarte, K.; Zhang, W.; Arolt, V.; Deckert, J.; et al. Association of adenosine receptor gene polymorphisms and in vivo adenosine A1 receptor binding in the human brain. Neuropsychopharmacology 2014, 39, 2989–2999. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Ardais, A.P.; Bastos, C.R.; Gazal, M.; Jansen, K.; de Mattos Souza, L.; da Silva, R.A.; Kaster, M.P.; Lara, D.R.; Ghisleni, G. Impact of genetic variations in ADORA2A gene on depression and symptoms: A cross-sectional population-based study. Purinergic Signal. 2019, 15, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gass, N.; Ollila, H.M.; Utge, S.; Partonen, T.; Kronholm, E.; Pirkola, S.; Suhonen, J.; Silander, K.; Porkka-Heiskanen, T.; Paunio, T. Contribution of adenosine related genes to the risk of depression with disturbed sleep. J. Affect. Disord. 2010, 126, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Vereczkei, A.; Abdul-Rahman, O.; Halmai, Z.; Nagy, G.; Szekely, A.; Somogyi, A.; Faludi, G.; Nemoda, Z. Association of purinergic receptor P2RX7 gene polymorphisms with depression symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Halmai, Z.; Dome, P.; Vereczkei, A.; Abdul-Rahman, O.; Szekely, A.; Gonda, X.; Faludi, G.; Sasvari-Szekely, M.; Nemoda, Z. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J. Affect. Disord. 2013, 150, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Lucae, S.; Salyakina, D.; Barden, N.; Harvey, M.; Gagné, B.; Labbé, M.; Binder, E.B.; Uhr, M.; Paez-Pereda, M.; Sillaber, I.; et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 2006, 15, 2438–2445. [Google Scholar] [CrossRef]
- Soronen, P.; Mantere, O.; Melartin, T.; Suominen, K.; Vuorilehto, M.; Rytsälä, H.; Arvilommi, P.; Holma, I.; Holma, M.; Jylhä, P.; et al. P2RX7 gene is associated consistently with mood disorders and predicts clinical outcome in three clinical cohorts. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156B, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Czamara, D.; Müller-Myhsok, B.; Lucae, S. The P2RX7 polymorphism rs2230912 is associated with depression: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Agren, H.; Niklasson, F.; Hällgren, R. Brain purinergic activity linked with depressive symptomatology: Hypoxanthine and xanthine in CSF of patients with major depressive disorders. Psychiatry Res. 1983, 9, 179–189. [Google Scholar] [CrossRef]
- Niklasson, F.; Agren, H.; Hällgren, R. Purine and monoamine metabolites in cerebrospinal fluid: Parallel purinergic and monoaminergic activation in depressive illness? J. Neurol. Neurosur. Psychiatry 1983, 46, 255–260. [Google Scholar] [CrossRef]
- Elgün, S.; Keskinege, A.; Kumbasar, H. Dipeptidyl peptidase IV and adenosine deaminase activity. Decrease in depression. Psychoneuroendocrinology 1999, 24, 823–832. [Google Scholar] [CrossRef]
- Herken, H.; Gurel, A.; Selek, S.; Armutcu, F.; Ozen, M.E.; Bulut, M.; Kap, O.; Yumru, M.; Savas, H.A.; Akyol, O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: Impact of antidepressant treatment. Arch. Med. Res. 2007, 38, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.M.; Camara, S.; Tatschner, T.; Frangou, S.; Sheldrick, A.J.; Riederer, P.; Grünblatt, E. Increased xanthine oxidase in the thalamus and putamen in depression. World J. Biol. Psychiatry 2010, 11, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.M.; Neis, V.B.; Rieger, D.K.; Lopes, M.W.; Heinrich, I.A.; Costa, A.P.; Rodrigues, A.L.S.; Kaster, M.P.; Leal, R.B. Signaling pathways underlying the antidepressant-like effect of inosine in mice. Purinergic Signal. 2017, 13, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Kaster, M.P.; Budni, J.; Gazal, M.; Cunha, M.P.; Santos, A.R.; Rodrigues, A.L. The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A 2A receptors. Purinergic Signal. 2013, 9, 481–486. [Google Scholar] [CrossRef]
- Muto, J.; Lee, H.; Lee, H.; Uwaya, A.; Park, J.; Nakajima, S.; Nagata, K.; Ohno, M.; Ohsawa, I.; Mikami, T. Oral administration of inosine produces antidepressant-like effects in mice. Sci. Rep. 2014, 4, 4199. [Google Scholar] [CrossRef]
- Yuan, S.; Jiang, X.; Zhou, X.; Zhang, Y.; Teng, T.; Xie, P. Inosine alleviates depression-like behavior and increases the activity of the ERK-CREB signaling in adolescent male rats. Neuroreport 2018, 29, 1223–1229. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Bowman, G.L.; Shannon, J.; Frei, B.; Kaye, J.A.; Quinn, J.F. Uric acid as a CNS antioxidant. J. Alzheimers Dis. 2010, 19, 1331–1336. [Google Scholar] [CrossRef]
- Bartoli, F.; Clerici, M.; Crocamo, C.; Carrà, G. The antioxidant uric acid and depression: Clinical evidence and biological hypotheses. Acta Psychiatr. Scand. 2018, 137, 79. [Google Scholar] [CrossRef]
- Bartoli, F.; Trotta, G.; Crocamo, C.; Malerba, M.R.; Clerici, M.; Carrà, G. Antioxidant uric acid in treated and untreated subjects with major depressive disorder: A meta-analysis and meta-regression. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Snieder, H.; Penninx, B.W.J.H. Uric acid in major depressive and anxiety disorders. J. Affect. Disord. 2018, 225, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, M.K.; Kobylecki, C.J.; Afzal, S.; Nordestgaard, B.G. Association between the antioxidant uric acid and depression and antidepressant medication use in 96 989 individuals. Acta Psychiatr. Scand. 2017, 136, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Crocamo, C.; Mazza, M.G.; Clerici, M.; Carrà, G. Uric acid levels in subjects with bipolar disorder: A comparative meta-analysis. J. Psychiatr. Res. 2016, 81, 133–139. [Google Scholar] [CrossRef]
- Bartoli, F.; Crocamo, C.; Gennaro, G.M.; Castagna, G.; Trotta, G.; Clerici, M.; Carrà, G. Exploring the association between bipolar disorder and uric acid: A mediation analysis. J. Psychosom. Res. 2016, 84, 56–59. [Google Scholar] [CrossRef]
- Bartoli, F.; Crocamo, C.; Dakanalis, A.; Brosio, E.; Miotto, A.; Capuzzi, E.; Clerici, M.; Carrà, G. Purinergic system dysfunctions in subjects with bipolar disorder: A comparative cross-sectional study. Compr. Psychiatry 2017, 73, 1–6. [Google Scholar] [CrossRef]
- Bartoli, F.; Crocamo, C.; Clerici, M.; Carrà, G. Allopurinol as add-on treatment for mania symptoms in bipolar disorder: Systematic review and meta-analysis of randomised controlled trials. Br. J. Psychiatry 2017, 210, 10–15. [Google Scholar] [CrossRef]
- Dos Santos Oliveira, P.M.; Santos, V.; Coroa, M.; Ribeiro, J.; Madeira, N. Serum uric acid as a predictor of bipolarity in individuals with a major depressive episode. Bipolar Disord. 2019, 21, 235–243. [Google Scholar] [CrossRef]
- Goodman, A.M.; Wheelock, M.D.; Harnett, N.G.; Mrug, S.; Granger, D.A.; Knight, D.C. The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience 2016, 339, 396–401. [Google Scholar] [CrossRef]
- Sohn, H.; Kwon, M.S.; Lee, S.W.; Oh, J.; Kim, M.K.; Lee, S.H.; Lee, K.S.; Kim, B. Effects of Uric Acid on the Alterations of White Matter Connectivity in Patients with Major Depression. Psychiatry Investig. 2018, 15, 593–601. [Google Scholar] [CrossRef]
- Ali-Sisto, T.; Tolmunen, T.; Toffol, E.; Viinamäki, H.; Mäntyselkä, P.; Valkonen-Korhonen, M.; Honkalampi, K.; Ruusunen, A.; Velagapudi, V.; Lehto, S.M. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology 2016, 70, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, L.; Lan, X.; Cohen, D.; Zhang, Y.; Ravindran, A.V.; Yuan, S.; Zheng, P.; Coghill, D.; Yang, L.; et al. Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents. Mol. Psychiatry 2019, 24, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Blardi, P.; de Lalla, A.; Urso, R.; Auteri, A.; Dell’Erba, A.; Bossini, L.; Castrogiovanni, P. Activity of citalopram on adenosine and serotonin circulating levels in depressed patients. J. Clin. Psychopharmacol. 2005, 25, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Ahmed, A.T.; Arnold, M.; Liu, D.; Luo, C.; Zhu, H.; Mahmoudiandehkordi, S.; Neavin, D.; Louie, G.; Dunlop, B.W.; et al. Metabolomic signature of exposure and response to citalopram/escitalopram in depressed outpatients. Transl. Psychiatry 2019, 9, 173. [Google Scholar] [CrossRef] [PubMed]
| Purinergic Metabolite/Enzyme | Variation |
|---|---|
| Adenosine | Increase after antidepressant treatment [99,100] |
| Inosine | Decrease in adults [97], children and adolescents [98] with depression |
| Hypoxanthine | Decrease in children and adolescents with depression [98] |
| Xanthine | Increase in adults with depression [97] |
| Uric acid | Decrease in depression, increase after antidepressant treatment [87] |
| Adenosine Deaminase | Decrease [78] or increase [79] in depression and after antidepressant treatment [79] |
| Xanthine Oxidase | Increase in depression and decrease after antidepressant treatment [79] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoli, F.; Burnstock, G.; Crocamo, C.; Carrà, G. Purinergic Signaling and Related Biomarkers in Depression. Brain Sci. 2020, 10, 160. https://doi.org/10.3390/brainsci10030160
Bartoli F, Burnstock G, Crocamo C, Carrà G. Purinergic Signaling and Related Biomarkers in Depression. Brain Sciences. 2020; 10(3):160. https://doi.org/10.3390/brainsci10030160
Chicago/Turabian StyleBartoli, Francesco, Geoffrey Burnstock, Cristina Crocamo, and Giuseppe Carrà. 2020. "Purinergic Signaling and Related Biomarkers in Depression" Brain Sciences 10, no. 3: 160. https://doi.org/10.3390/brainsci10030160
APA StyleBartoli, F., Burnstock, G., Crocamo, C., & Carrà, G. (2020). Purinergic Signaling and Related Biomarkers in Depression. Brain Sciences, 10(3), 160. https://doi.org/10.3390/brainsci10030160







