Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy
Abstract
:1. Introduction
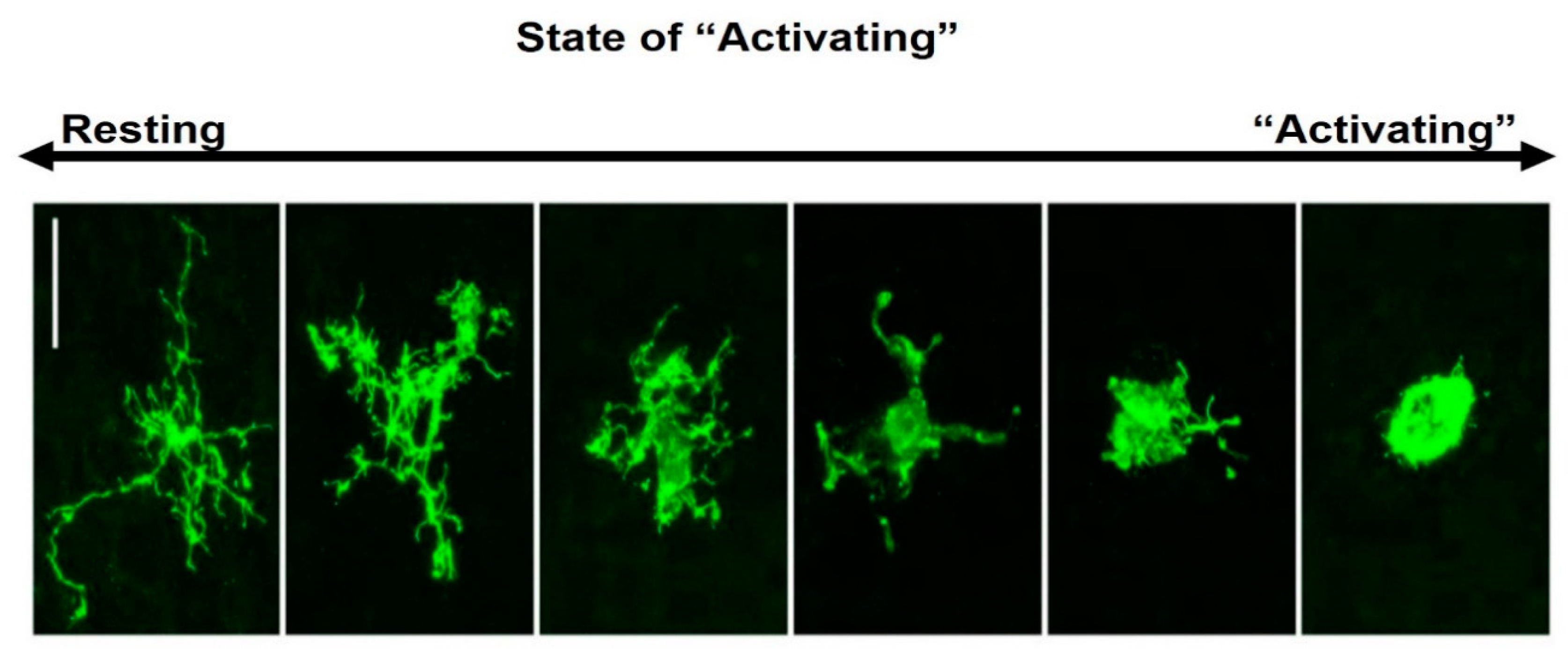
2. Microglial Morphology, “Activation” States and Functions in the Brain
3. Microglial Responses in Ischaemic Stroke
4. Pharmacological Modulation of Microglia Activation in Ischaemic Stroke
4.1. Minocycline
4.2. Metformin
4.3. Statins
4.4. Indomethacin
4.5. Noggin
4.6. PPAR-R
4.7. TNF-α Antagonists
4.8. Fingolimod
4.9. Colony Stimulating Factor Receptor Inhibitors
5. Cellular Therapies for Stroke That Target Microglia
6. Challenges for Stroke Therapies Targeting Microglia
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Musuka, T.D.; Wilton, S.B.; Traboulsi, M.; Hill, M.D. Diagnosis and management of acute ischemic stroke: Speed is critical. CMAJ 2015, 187, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.; Sharma, S.; Hassan, K. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010, 17, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Guruswamy, R.; ElAli, A. Complex Roles of Microglial Cells in Ischemic Stroke Pathobiology: New Insights and Future Directions. Int. J. Mol. Sci. 2017, 18, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Willing, A. Enhancing endogenous capacity to repair a stroke-damaged brain: An evolving field for stroke research. Prog. Neurobiol. 2018, 163, 5–26. [Google Scholar] [CrossRef]
- Woiciechowsky, C.; Asadullah, K.; Nestler, D.; Eberhardt, B.; Platzer, C.; Schöning, B.; Glöckner, F.; Lanksch, W.; Volk, H.; Döcke, W. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat. Med. 1998, 4, 808–813. [Google Scholar] [CrossRef]
- Offner, H.; Subramanian, S.; Parker, S.; Wang, C.; Afentoulis, M.; Lewis, A.; Vandenbark, A.; Hurn, P. Splenic Atrophy in Experimental Stroke Is Accompanied by Increased Regulatory T Cells and Circulating Macrophages. J. Immunol. 2006, 176, 6523–6531. [Google Scholar] [CrossRef] [Green Version]
- Winklewski, P.J.; Radkowski, M.; Demkow, U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J. Neuroinflamm. 2014, 11, 213. [Google Scholar] [CrossRef]
- Prass, K.; Meisel, C.; Höflich, C.; Braun, J.; Halle, E.; Wolf, T.; Ruscher, K.; Victorov, I.; Priller, J.; Dirnagl, U.; et al. Stroke-induced Immunodeficiency Promotes Spontaneous Bacterial Infections and Is Mediated by Sympathetic Activation Reversal by Poststroke T Helper Cell Type 1–like Immunostimulation. J. Exp. Med. 2003, 198, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Z.; Huang, X.; Zhao, T.; Qiao, M.; Zhao, X.; Zhao, M.; Xu, L.; Zhao, Y.; Wu, L.; Wu, K.; et al. Hypoxia augments LPS-induced inflammation and triggers high altitude cerebral edema in mice. Brain Behav. Immun. 2017, 64, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Kumar, S.; Selim, M.; Caplan, L. Medical complications after stroke. Lancet Neurol. 2010, 9, 105–118. [Google Scholar] [CrossRef]
- Balami, J.S.; Chen, R.L.; Grunwald, I.Q.; Buchan, A.M. Acute neurological complications in ischaemic stroke. Lancet Neurol. 2011, 10, 357–371. [Google Scholar] [CrossRef]
- Balami, J.S.; Chen, R.L.; Buchan, A.M. Stroke syndromes and clinical management. QJM 2013, 106, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Hu, Z.; Chen, R.; Zhang, D.; Xu, L.; Wang, J.; Wei, L. Socioeconomic deprivation and survival after stroke in China: A systematic literature review and a new population-based cohort study. BMJ Open 2015, 5, e005688. [Google Scholar] [CrossRef] [PubMed]
- Bovim, M.R.; Askim, T.; Lydersen, S.; Fjærtoft, H.; Indredavik, B. Complications in the first week after stroke: A 10-year comparison. BMC Neurol. 2016, 16, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, S.K.; Nguyen, M.H. Elderly Stroke Rehabilitation: Overcoming the Complications and Its Associated Challenges. Curr. Gerontol. Geriatr. Res. 2018, 2018, 9853837. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Bruce, A.; Boling, W.; Kindy, M.; Peschon, J.; Kraemer, P.; Carpenter, M.; Holtsberg, F.; Mattson, M. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat. Med. 1996, 2, 788–794. [Google Scholar] [CrossRef]
- Famakin, B. The Immune Response to Acute Focal Cerebral Ischemia and Associated Post-stroke Immunodepression: A Focused Review. Aging Dis. 2014, 5, 307–326. [Google Scholar]
- Zhang, S. Microglial activation after ischaemic stroke. Stroke Vasc. Neurol. 2019, 4, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Jayaraj, R.; Azimullah, S.; Beiram, R.; Jalal, F.; Rosenberg, G. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflam. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alliot, F.; Godin, I.; Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev. Brain Res. 1999, 117, 145–152. [Google Scholar] [CrossRef]
- Rayasam, A.; Hsu, M.; Kijak, J.; Kissel, L.; Hernandez, G.; Sandor, M.; Fabry, Z. Immune responses in stroke: How the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology 2018, 154, 363–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, A.; Encinas, J.; Deudero, J.; Chancey, J.; Enikolopov, G.; Overstreet-Wadiche, L.; Tsirka, S.; Maletic-Savatic, M. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilarte, T.; Loth, M.; Guariglia, S. TSPO Finds NOX2 in Microglia for Redox Homeostasis. Trends Pharmacol. Sci. 2016, 37, 334–343. [Google Scholar] [CrossRef]
- Trang, T.; Beggs, S.; Salter, M. Brain-derived neurotrophic factor from microglia: A molecular substrate for neuropathic pain. Neuron. Glia Biol. 2011, 7, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Honda, S.; Tohyama, Y.; Imai, Y.; Kohsaka, S.; Kurihara, T. Neurotrophin secretion from cultured microglia. J. Neurosci. Res. 2001, 65, 322–331. [Google Scholar] [CrossRef]
- Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013, 16, 543–551. [Google Scholar] [CrossRef]
- Schafer, D.; Lehrman, E.; Kautzman, A.; Koyama, R.; Mardinly, A.; Yamasaki, R.; Ransohoff, R.; Greenberg, M.; Barres, B.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, R.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, I.; Augestad, I.L.; Håberg, A.K.; Sandvig, A. Neuroplasticity in stroke recovery. The role of microglia in engaging and modifying synapses and networks. Eur. J. Neurosci. 2018, 47, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.; Perry, V.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Grabert, K.; Michoel, T.; Karavolos, M.; Clohisey, S.; Baillie, J.; Stevens, M.; Freeman, T.; Summers, K.; McColl, B. Microglial brain region−dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Sankoski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; Coenen, V.A.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Balami, J.; Esiri, M.; Chen, L.; Buchan, A. Ischemic stroke in the elderly: An overview of evidence. Nat. Rev. Neurol. 2010, 6, 256–265. [Google Scholar] [CrossRef]
- Chen, C.; Preston, J.; Zhou, S.; Fuller, H.; Morgan, D.; Chen, R. Proteomic analysis of age-related changes in ovine cerebrospinal fluid. Exp. Gerontol. 2018, 108, 181–188. [Google Scholar] [CrossRef]
- Holtman, I.; Skola, D.; Glass, C. Transcriptional control of microglia phenotypes in health and disease. J. Clin. Investig. 2017, 127, 3220–3229. [Google Scholar] [CrossRef] [Green Version]
- Dubbelaar, M.; Kracht, L.; Eggen, B.; Boddeke, E. The Kaleidoscope of Microglial Phenotypes. Front. Immunol. 2018, 9, 1753. [Google Scholar] [CrossRef]
- Arcuri, C.; Mecca, C.; Bianchi, R.; Giambanco, I.; Donato, R. The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS. Front. Mol. Neurosci. 2017, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Ritzel, R.; McCullough, L.D.; Liu, F. Microglia and ischemic stroke: A double-edged sword. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 73–90. [Google Scholar]
- Sominsky, L.; De Luca, S.; Spencer, S. Microglia: Key players in neurodevelopment and neuronal plasticity. Int. J. Biochem. Cell Biol. 2018, 94, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Han, X.; Li, Q.; Yang, Q.; Wang, J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 2017, 13, 420–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leo, J.; Sorkin, L.; Watkins, L. Immune and Glial Regulation of Pain. J. MSK Pain. 2007, 16, 297–311. [Google Scholar]
- Gulyás, B.; Tóth, M.; Schain, M.; Airaksinen, A.; Vas, A.; Kostulas, K.; Lindström, P.; Hillert, J.; Halldin, C. Evolution of microglial activation in ischaemic core and peri-infarct regions after stroke: A PET study with the TSPO molecular imaging biomarker [11C] vinpocetine. J. Neurol. Sci. 2012, 320, 110–117. [Google Scholar] [CrossRef]
- Monif, M.; Reid, C.; Powell, K.; Smart, M.; Williams, D. The P2X7 receptor drives microglial activation and proliferation: A trophic role for P2X7R pore. J. Neurosci. 2009, 29, 3781–3791. [Google Scholar] [CrossRef]
- Bianco, F.; Ceruti, S.; Colombo, A.; Fumagalli, M.; Ferrari, D.; Pizzirani, C.; Matteoli, M.; Di Virgilio, F.; Abbracchio, M.; Verderio, C. A role for P2X7 in microglial proliferation. J. Neurochem. 2006, 99, 745–758. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Giuliani, A.L.; Vultaggio-Poma, V.; Falzoni, S.; Sarti, A.C. Non-nucleotide Agonists Triggering P2X7 Receptor Activation and Pore Formation. Front. Pharmacol. 2018, 9, 39. [Google Scholar] [CrossRef]
- Anderson, C.; Bergher, J.; Swanson, R. ATP-induced ATP release from astrocytes. J. Neurochem. 2004, 88, 246–256. [Google Scholar] [CrossRef]
- Taylor, D.; Diemel, L.; Cuzner, M.; Pocock, J. Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromo- granin A, a peptide up-regulated in Alzheimer’s disease. J. Neurochem. 2002, 82, 1179–1191. [Google Scholar] [CrossRef]
- Dello Russo, C.; Lisi, L.; Tringali, G.; Navarra, P. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem. Pharmacol. 2009, 78, 1242–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardo, A.; Levi, G.; Minghetti, L. Role of the peroxisome pro- liferator-activated receptor- (PPAR-γ) and its natural ligand 15- 12, 14-prostaglandin J2 in the regulation of microglial functions. Eur. J. Neurosci. 2000, 12, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Patzer, A.; Gohlke, P.; Herdegen, T.; Culman, J. The intra-cerebral application of the PPARγ-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur. J. Neurosci. 2005, 22, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, T.; Higashi, Y.; Suh, S.; Escartin, C.; Nagasawa, K.; Swanson, R. Zinc triggers microglial activation. J. Neurosci. 2008, 28, 5827–5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mander, P.; Jekabsone, A.; Brown, G. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J. Immunol. 2006, 176, 1046–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefano, L.; Racchetti, G.; Bianco, F.; Passini, N.; Gupta, R.; Bordignon, P.; Meldolesi, J. The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J. Neurochem. 2009, 110, 284–294. [Google Scholar] [CrossRef]
- Benakis, C.; Garcia-Bonilla, L.; Iadecola, C.; Anrather, J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci. 2015, 8, 461. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia And Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism Against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef]
- Cherry, J.; Olschowka, J.; O’Banion, M. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- De Bilbao, F.; Arsenijevic, D.; Moll, T.; Garcia-Gabay, I.; Vallet, P.; Langhans, W.; Giannakopoulos, P. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J. Neurochem. 2009, 110, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhandapani, K.; Brann, D. Transforming Growth Factor-β: A Neuroprotective Factor in Cerebral Ischemia. Cell Biochem. Biophys. 2003, 39, 13–22. [Google Scholar] [CrossRef]
- Wilcock, D. A Changing Perspective on the Role of Neuroinflammation in Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 495243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambertsen, K.L.; Clausen, B.H.; Babcock, A.A.; Gregersen, R.; Fenger, C.; Nielsen, H.H.; Haugaard, L.S.; Wirenfeldt, M.; Nielsen, M.; Dagnaes-Hansen, F.; et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J. Neurosci. 2009, 29, 1319–1330. [Google Scholar] [CrossRef]
- Yang, G.-Y.; Gong, C.; Qin, Z.; Ye, W.; Mao, Y.; Bertz, A.L. Inhibition of TNFα attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport 1998, 9, 2131–2134. [Google Scholar] [CrossRef]
- Poh, L.; Kang, S.W.; Baik, S.H.; Ng, G.Y.Q.; She, D.T.; Balaganapathy, P.; Dheen, S.T.; Magnus, T.; Gelderblom, M.; Sobey, C.G.; et al. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav. Immun. 2019, 75, 34–47. [Google Scholar] [CrossRef]
- Denes, A.; Vidyasagar, R.; Feng, J.; Narvainen, J.; McColl, B.; Kauppinen, R.; Allan, S. Proliferating Resident Microglia after Focal Cerebral Ischaemia in Mice. J. Cereb. Blood Flow Metab. 2007, 27, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wang, Z.; Wei, X.; Han, H.; Meng, X.; Zhang, Y.; Shi, W.; Li, F.; Xin, T.; Pang, Q.; et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J. Cereb. Blood Flow Metab. 2014, 34, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, M.; Miura, M.; Toriyabe, M.; Koyama, M.; Hatakeyama, M.; Ishikawa, M.; Nakajima, T.; Onodera, O.; Takahashi, T.; Nishizawa, M.; et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.-Y.; Zhang, S.; Gao, Y.; Wang, Z.-Z.; Chen, N.-H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharmacol. 2015, 25, 377–382. [Google Scholar] [CrossRef]
- Chen, H.; Chopp, M.; Zhang, R.L.; Bodzin, G.; Chen, Q.; Rusche, J.R.; Todd III, R.F. Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann. Neurol. 1994, 35, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp. Neurol. 2018, 300, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yanuck, S. Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders. Front. Psych. 2019, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Del Zoppo, G.J.; Milner, R.; Mabuchi, T.; Hung, S.; Wang, X.; Berg, G.I.; Koziol, J.A. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 2007, 38, 646–651. [Google Scholar] [CrossRef]
- Greenberg, D.; Jin, K. Vascular endothelial growth factors (VEGFs) and stroke. Cell. Mol. Life Sci. 2013, 70, 1753–1761. [Google Scholar] [CrossRef]
- Kaur, C.; Rathnasamy, G.; Ling, E.A. Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J. Neuroimmune Pharmacol. 2013, 8, 66–78. [Google Scholar] [CrossRef]
- Narantuya, D.; Nagai, A.; Sheikh, A.; Masuda, J.; Kobayashi, S.; Yamaguchi, S.; Kim, S. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioural improvement. PLoS ONE 2010, 5, 11746. [Google Scholar] [CrossRef]
- Madinier, A.; Bertrand, N.; Mossiat, C.; Prigent-Tessier, A.; Beley, A.; Marie, C.; Garnier, P. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS ONE 2009, 4, e8101. [Google Scholar] [CrossRef] [Green Version]
- Rajan, W.; Wojtas, B.; Gielniewski, B.; Gieryng, A.; Zawadzka, M.; Kaminska, B. Dissecting functional phenotypes of microglia and macrophages in the rat brain after transient cerebral ischemia. Glia 2019, 67, 232–245. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.K.; Miller, S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElAli, A.; Rivest, S. Microglia Ontology and Signaling. Front. Cell Dev. Biol. 2016, 4, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, T.; Fujimura, M.; Noshita, N.; Kim, G.; Saito, A.; Hayashi, T.; Narasimhan, P.; Maier, C.; Chan, P. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRX 2004, 1, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Ling, E.; Kaur, C. Glutamate Receptors in Microglia. CNS Neurol. Disord. Drug Targets 2013, 12, 773–784. [Google Scholar] [CrossRef]
- Taylor, R.; Sansing, L. Microglial Responses after Ischemic Stroke and Intracerebral Hemorrhage. Clin. Dev. Immunol. 2013, 2013, 746068. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, R.; Zhu, X.; Smerin, D.; Zhong, Y.; Gu, L.; Fang, W.; Xiong, X. The Involvement and Therapy Target of Immune Cells after Ischemic Stroke. Front. Immunol. 2019, 10, 2167. [Google Scholar] [CrossRef] [Green Version]
- Perego, C.; Fumagalli, S.; De Simoni, M. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.; Chen, S.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [Green Version]
- Boche, D.; Perry, V.; Nicoll, J. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Jonathan, R.; Weinstein, I.; Koerner, P.; Möller, T. Microglia in ischemic brain injury. Future Neurol. 2010, 5, 227–246. [Google Scholar]
- Yang, Y.; Salayandia, V.; Thompson, J.; Yang, L.; Estrada, E.; Yang, Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood–brain barrier remodeling and alternative microglia/macrophage activation during recovery. J. Neuroinflamm. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagan, S.; Cronic, L.; Hess, D. Minocycline Development for Acute Ischemic Stroke. Transl. Stroke Res. 2011, 2, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veltkamp, R.; Gill, D. Clinical Trials of Immunomodulation in Ischemic Stroke. Neurotherapeutics 2016, 13, 791–800. [Google Scholar] [CrossRef]
- Machado, L.S.; Kozak, A.; Ergul, A.; Hess, D.C.; Borlonga, C.V.; Fagan, S.C. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an Antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Yew, W.P.; Diukic, N.D.; Javaseelan, J.S.P.; Walker, F.R.; Roos, K.A.A.; Chataway, T.K.; Muyderman, H.; Sims, N.R. Early treatment with minocycline following stroke in rats improves functional recovery and differentially modifies responses of peri-infarct microglia and astrocytes. J. Neuroinflamm. 2019, 16, 6. [Google Scholar] [CrossRef]
- Tsuji, M.; Wilson, M.A.; Lange, M.S.; Johnston, M.V. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp. Neurol. 2004, 189, 58–65. [Google Scholar] [CrossRef]
- Hanlon, L.A.; Huh, J.W.; Raghupathi, R. Minocycline Transiently Reduces Microglia/Macrophage Activation but Exacerbates Cognitive Deficits Following Repetitive Traumatic Brain Injury in the Neonatal Rat. J. Neuropath. Exp. Neurol. 2016, 75, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Kupsch, K.; Hertel, S.; Kreutzmann, P.; Wolf, G.; Wallesch, C.; Siemen, D.; Schönfeld, P. Impairment of mitochondrial function by minocycline. FEBS J. 2009, 276, 1729–1738. [Google Scholar] [CrossRef]
- Diguet, E.; Gross, C.; Tison, F.; Bezard, E. Rise and fall of minocycline in neuroprotection: Need to promote publication of negative results. Exp. Neurol. 2004, 189, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lampl, Y.; Boaz, M.; Gilad, R.; Lorberboym, M.; Dabby, R.; Rapoport, A.; Anca-Hershkowitz, M.; Sadeh, M. Minocycline Treatment In Acute Stroke: An Open-Label, Evaluator-Blinded Study. Neurology 2007, 69, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Padma Srivastava, M.V.; Bhasin, A.; Bhatia, R.; Garg, A.; Gaikwad, S.; Prasad, K.; Singh, M.B.; Tripathi, M. Efficacy of minocycline in acute ischemic stroke: A single-blinded, placebo-controlled trial. Neurol. India 2012, 60, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fagan, S.; Waller, J.; Nichols, F.; Edwards, D.; Pettigrew, L.; Clark, W.; Hall, C.; Switzer, J.; Ergul, A.; Hess, D. Minocycline to Improve Neurologic Outcome in Stroke (MINOS). Stroke 2010, 41, 2283–2287. [Google Scholar] [CrossRef] [Green Version]
- Kohler, E.; Prentice, D.A.; Bates, T.R.; Hankey, G.J.; Claxton, A.; van Heerden, J.; Blacker, D. Intravenous minocycline in acute stroke: A randomized, controlled pilot study and meta-analysis. Stroke 2013, 44, 2493–2499. [Google Scholar] [CrossRef]
- Switzer, J.; Hess, D.; Ergul, A.; Waller, J.; Machado, L.; Portik-Dobos, V.; Pettigrew, L.; Clark, W.; Fagan, S. Matrix Metalloproteinase-9 in an Exploratory Trial of Intravenous Minocycline for Acute Ischemic Stroke. Stroke 2011, 42, 2633–2635. [Google Scholar] [CrossRef] [Green Version]
- Neuroprotection with Minocycline Therapy for Acute Stroke Recovery Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT00930020 (accessed on 27 February 2020).
- Amiri-Nikpour, M.; Nazarbaghi, S.; Hamdi-Holasou, M.; Rezaei, Y. An Open-Label Evaluator-Blinded Clinical Study of Minocycline Neuroprotection in Ischemic Stroke: Gender-Dependent Effect. Acta Neurol. Scand. 2014, 131, 45–50. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, T.; Zhang, Q.; Cao, L.; Tan, M.; Wang, H.; Ding, Z.; Tan, L.; Yu, J. Chronic Metformin Preconditioning Provides Neuroprotection via Suppression of NF-κB-Mediated Inflammatory Pathway in Rats with Permanent Cerebral Ischemia. Mol. Neurobiol. 2014, 52, 375–385. [Google Scholar] [CrossRef]
- Deng, T.; Zheng, Y.R.; Hou, W.W.; Yuan, Y.; Shen, Z. Pre-stroke metformin treatment is neuroprotective involving AMPK reduction. Neurochem. Res. 2016, 41, 2719–2727. [Google Scholar] [CrossRef]
- Jia, J.; Cheng, J.; Ni, J.; Zhen, X. Neuropharmacological Actions of Metformin in Stroke. Curr. Neuropharmacol. 2015, 13, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014, 40. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMP-activated protein kinase (AMPK) signaling pathway coordinates cell growth, autophagy, & metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [PubMed]
- Gunn, A.; Singh, A.; Diao, A.; Chen, R.L. Pharmacological modulation of autophagy for neuroprotection in ischaemic stroke. J. Exp. Stroke Trans. Med. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Gu, J.; Ye, S.; Wang, S.; Sun, W.; Hu, Y. Metformin inhibits nuclear factor-κB activation and inflammatory cytokines expression induced by high glucose via adenosine monophosphate-activated protein kinase activation in rat glomerular mesangial cells in vitro. Chin. Med. J. 2014, 127, 1755–1760. [Google Scholar]
- Isoda, K.; Young, J.; Zirlik, A.; MacFarlane, L.; Tsuboi, N.; Gerdes, N.; Schönbeck, U.; Libby, P. Metformin Inhibits Proinflammatory Responses and Nuclear Factor-Κb In Human Vascular Wall Cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 611–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, V.; Amani, F.; Faraji-Almoti, A. Impact of Metformin on the Severity and Outcomes of Ischemic Stroke. Int. J. Basic Clin. Pharmacol. 2018, 7, 744. [Google Scholar] [CrossRef] [Green Version]
- Willey, J.; Elkind, M. 3-Hydroxy-3-methylglutaryl–Coenzyme a Reductase Inhibitors in the Treatment of Central Nervous System Diseases. Arch. Neurol. 2010, 67, 1062–1067. [Google Scholar] [CrossRef]
- Kim, S.; Kang, H.; Jhon, M.; Kim, J.W.; Lee, J.Y.; Walker, A.J.; Agustini, B.; Kim, J.M.; Berk, M. Statins and Inflammation: New Therapeutic Opportunities in Psychiatry. Front. Psychiatry 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A. High-Dose Atorvastatin after Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef]
- Ballantyne, C. Current and future aims of lipid-lowering therapy: Changing paradigms and lessons from the heart protection study on standards of efficacy and safety. Am. J. Cardiol. 2003, 92, 3–9. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Dong, L.; Wen, Y.; Cui, L. The Many Roles of Statins in Ischemic Stroke. Curr. Neuropharmacol. 2015, 12, 564–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellner, C.; Connolly, E. Neuroprotective Strategies for Intracerebral Hemorrhage: Trials and Translation. Stroke 2010, 41, S99–S102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kata, D.; Földesi, I.; Feher, L.; Hackler, L.; Puskas, L.; Gulya, K. Rosuvastatin enhances anti-inflammatory and inhibits pro-inflammatory functions in cultured microglial cells. Neuroscience 2016, 314, 47–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.; Song, D.; Nam, H.; Kim, E.; Kim, Y.; Lee, K.; Yoo, J.; Lee, B.; Yoon, B.; Kim, J. Effect and Safety of Rosuvastatin in Acute Ischemic Stroke. J. Stroke 2016, 18, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Montaner, J.; Bustamante, A.; García-Matas, S.; Martínez-Zabaleta, M.; Jiménez, C.; de la Torre, J.; Rubio, F.; Segura, T.; Masjuán, J.; Cánovas, D.; et al. Combination of Thrombolysis and Statins in Acute Stroke Is Safe. Stroke 2016, 47, 2870–2873. [Google Scholar] [CrossRef]
- Elkind, M.; Sacco, R.; MacArthur, R.; Peerschke, E.; Neils, G.; Andrews, H.; Stillman, J.; Corporan, T.; Leifer, D.; Liu, R.; et al. High-Dose Lovastatin for Acute Ischemic Stroke: Results of the Phase I Dose Escalation Neuroprotection with Statin Therapy for Acute Recovery Trial (NeuSTART). Cerebrovasc. Dis. 2009, 28, 266–275. [Google Scholar] [CrossRef]
- Reis, C.; Akyol, O.; Ho, W.M.; Araujo, C.; Huang, L.; Applegate, I.I.; Zhang, J.H. Phase I and Phase II Therapies for Acute Ischemic Stroke: An Update on Currently Studied Drugs in Clinical Research. Biomed. Res. Int. 2017, 2017, 4863079. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.; Cardoso, M.; Sampaio, A.; Barbosa, M.; Souza, C.; da Silva, M.; Ferreira, E.; Freire, M.; Lima, R.; Gomes-Leal, W. Indomethacin treatment reduces microglia activation and increases numbers of neuroblasts in the subventricular zone and ischaemic striatum after focal ischaemia. J. Biosci. 2016, 41, 381–394. [Google Scholar] [CrossRef]
- Sandu, R.; Uzoni, A.; Coman, C.; Popa-Wagner, A. Cerebral ischemia in the aged. Limited anti-inflammatory efficacy of the indomethacin treatment. Rom. J. Morphol. Embryol. 2015, 56, 1111–1117. [Google Scholar]
- Bok, S.; Wang, T.; Lee, C.; Jeon, S.; Kim, Y.; Kim, J.; Hong, B.; Yoon, C.; Kim, S.; Lee, S.; et al. In vivo imaging of activated microglia in a mouse model of focal cerebral ischemia by two-photon microscopy. Biomed. Opt. Express 2015, 6, 3303. [Google Scholar] [CrossRef] [Green Version]
- Hoehn, B.; Palmer, T.; Steinberg, G. Neurogenesis in Rats After Focal Cerebral Ischemia is Enhanced by Indomethacin. Stroke 2005, 36, 2718–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vemuganti, R. Therapeutic Potential of PPAR-γ activation In Stroke. PPAR Res. 2008, 2008, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Lim, S.; Jeong, S.; Kang, J.; Park, E. Noggin Improves Ischemic Brain Tissue Repair and Promotes Alternative Activation of Microglia in Mice. Brain Behav. Immun. 2014, 40, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Sharma, S.; Gupta, P.; Saini, A.; Kaushal, C. The Peroxisome Proliferator-Activated Receptor: A Family of Nuclear Receptors Role in Various Diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, Y.; Guo, Y.; Wu, S.; Chen, W.; Liu, N.; Wang, Y.; Geng, D. 1, 25-D3 Protects From Cerebral Ischemia by Maintaining BBB Permeability via PPAR-γ Activation. Front. Cell. Neurosci. 2018, 17, 480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L. Peroxisome Proliferator-Activated Receptor Gamma Agonists for Preventing Recurrent Stroke and Other Vascular Events in Patients with Stroke or Transient Ischaemic Attack. Cochrane Database Syst. Rev. 2019, 10, CD010693. [Google Scholar]
- Tobinick, E.; Rodriguez-Romanacce, H.; Levine, A.; Ignatowski, T.; Spengler, R. Immediate Neurological Recovery Following Perispinal Etanercept Years After Brain Injury. Clin. Drug Investig. 2014, 34, 361–366. [Google Scholar] [CrossRef]
- Wu, M.; Huang, C.; Chio, C.; Tsai, K.; Chang, C.; Lin, N.; Lin, M. Inhibition of Peripheral TNF-α and Downregulation of Microglial Activation by Alpha-Lipoic Acid and Etanercept Protect Rat Brain Against Ischemic Stroke. Mol. Neurobiol. 2015, 53, 4961–4971. [Google Scholar] [CrossRef]
- Banno, M.; Mizuno, T.; Kato, H.; Zhang, G.; Kawanokuchi, J.; Wang, J.; Kuno, R.; Jin, S.; Takeuchi, H.; Suzumura, A. The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology 2005, 48, 283–290. [Google Scholar] [CrossRef]
- Mizuma, A.; Yenari, M. Anti-Inflammatory Targets for the Treatment of Reperfusion Injury in Stroke. Front. Neurol. 2017, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Isahaya, K.; Yamada, K.; Yamatoku, M.; Sakurai, K.; Takaishi, S.; Kato, B.; Hirayama, T.; Hasegawa, Y. Effects of Edaravone, a Free Radical Scavenger, on Serum Levels of Inflammatory Biomarkers in Acute Brain Infarction. J. Stroke Cerebrovasc. Dis. 2012, 21, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Boutin, H.; Murray, K.; Pradillo, J.; Maroy, R.; Smigova, A.; Gerhard, A.; Jones, P.; Trigg, W. 18F-GE-180: A novel TSPO radiotracer compared to 11C-R-PK11195 in a preclinical model of stroke. Eur. J. Nuclear Med. Mol. Imaging 2014, 42, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 2013, 256, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Erblich, B.; Zhu, L.; Etgen, A.M.; Dobrenis, K.; Pollard, J.W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE 2011, 6, e26317. [Google Scholar] [CrossRef] [Green Version]
- Stanley, E.R.; Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 2014, 6, a021857. [Google Scholar] [CrossRef] [Green Version]
- Wieghofer, P.; Knobeloch, K.P.; Prinz, M. Genetic targeting of microglia. Glia 2015, 63, 1–22. [Google Scholar] [CrossRef]
- Elmore, M.R.; Lee, R.J.; West, B.L.; Green, K.N. Characterizing newly repopulated microglia in the adult mouse: Impacts on animal behavior, cell morphology, and neuroinflammation. PLoS ONE 2015, 10, e0122912. [Google Scholar] [CrossRef]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef] [Green Version]
- Dagher, N.N.; Najafi, A.R.; Kayala, K.M.; Elmore, M.R.; White, T.E.; Medeiros, R.; West, B.L.; Green, K.N. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J. Neuroinflamm. 2015, 12, 139. [Google Scholar] [CrossRef] [Green Version]
- Olmos-Alonso, A.; Schetters, S.T.; Sri, S.; Askew, K.; Mancuso, R.; Vargas-Caballero, M.; Holscher, C.; Perry, V.H.; Gomez-Nicola, D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 2016, 139, 891–907. [Google Scholar] [CrossRef] [Green Version]
- Sosna, J.; Philipp, S.; Albay, R., III; Reyes-Ruiz, J.M.; Baglietto-Vargas, D.; Laferla, F.M.; Glabe, C.G. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Szalay, G.; Martinecz, B.; Lenart, N.; Környei, Z.; Orsolits, B.; Judák, L.; Császár, E.; Fekete, R.; West, B.L.; Katona, G.; et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016, 7, 11499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.N.; Shi, S.X.; Li, Z.; Li, M.; Wood, K.; Gonzales, R.J.; Liu, Q. Depletion of microglia exacerbates postischemic inflammation and brain injury. J. Cereb. Blood Flow Metab. 2017, 37, 2224–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otxoa-de-Amezaga, A.; Miró-Mur, F.; Pedragosa, J.; Gallizioli, M.; Justicia, C.; Gaja-Capdevila, N.; Ruíz-Jaen, F.; Salas-Perdomo, A.; Bosch, A.; Calvo, M.; et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019, 137, 321–341. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, M.; Lo, E.; Iadecola, C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Soriano, S.; Coxon, A.; Wang, Y.; Frosch, M.; Lipton, S.; Hickey, P.; Mayadas, T. Mice Deficient in Mac-1 (CD11b/CD18) Are Less Susceptible to Cerebral Ischemia/Reperfusion Injury. Stroke 1999, 30, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Ge, R.; Tornero, D.; Hirota, M.; Monni, E.; Laterza, C.; Lindvall, O.; Kokaia, Z. Choroid plexus-cerebrospinal fluid route for monocyte-derived macrophages after stroke. J. Neuroinflamm. 2017, 14, 153. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef]
- Benakis, C.; Llovera, G.; Liesz, A. The meningeal and choroidal infiltration routes for leukocytes in stroke. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418783708. [Google Scholar] [CrossRef]
- Imai, F.; Suzuki, H.; Oda, J.; Ninomiya, T.; Ono, K.; Sano, H.; Sawada, M. Neuroprotective Effect of Exogenous Microglia in Global Brain Ischemia. J. Cereb. Blood Flow Metab. 2007, 27, 488–500. [Google Scholar] [CrossRef] [Green Version]
- Ekdahl, C.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Satani, N.; Savitz, S. Is Immunomodulation a Principal Mechanism Underlying How Cell-Based Therapies Enhance Stroke Recovery? Neurotherapeutics 2016, 13, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernykh, E.; Shevela, E.; Starostina, N.; Morozov, S.; Davydova, M.; Menyaeva, E.; Ostanin, A. Safety and Therapeutic Potential of M2 Macrophages in Stroke Treatment. Cell Transplant. 2016, 25, 1461–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.; Nair, V.; Sarkar, R.; Gorthi, S.; et al. Intravenous Autologous Bone Marrow Mononuclear Stem Cell Therapy for Ischemic Stroke. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Migliati, E.; Parsha, K.; Schaar, K.; Xi, X.; Aronowski, J.; Savitz, S. Intra-Arterial Delivery Is Not Superior to Intravenous Delivery of Autologous Bone Marrow Mononuclear Cells in Acute Ischemic Stroke. Stroke 2013, 44, 3463–3472. [Google Scholar] [CrossRef] [Green Version]
- Ransohoff, R. A Polarizing Question: Do M1 And M2 Microglia Exist? Nat. Neurosci. 2016, 19, 987. [Google Scholar] [CrossRef]
- Hellwig, S.; Heinrich, A.; Biber, K. The brain’s best friend: Microglial neurotoxicity revisited. Front. Cell. Neurosci. 2013, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, R.; Burm, S.M.; Bajramovic, J.J. An Overview of in vitro Methods to Study Microglia. Front. Cell Neurosci. 2018, 12, 242. [Google Scholar] [CrossRef]
- Dirnagl, U. Modeling immunity and inflammation in stroke: Can mice be trusted? Stroke 2014, 45, e177–e178. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Yang, J. What do experimental models teach us about comorbidities in stroke? Stroke 2018, 49, 501–507. [Google Scholar] [CrossRef]
- Da Fonseca, A.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.; Freitas, C.; Lima, F. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xing, H.; Wan, L.; Jiang, X.; Wang, C.; Wu, Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed. Pharmacother. 2018, 105, 518–525. [Google Scholar] [CrossRef] [PubMed]
| Biological Process | Mediators | Effects Potentially Beneficial Post-Ischaemia | Effects Potentially Deleterious Post-Ischaemia |
| Pro-inflammatory | TNF-α | Possibly neuroprotective; exacerbated infarct in TNF-α-/- KO mice [64] | Exacerbated infarct volume, oedema [58,65] |
| Inflammasome | In ischaemic conditions, NLRC4 inflammasome complex induces pyroptotic microglial death, exacerbating inflammatory damage [66] NLRC4-/- KO mice have lesser neurological deficits post tMCAo [67] NLRP3-/- KO mice have lesser BBB breakdown, infarct size, oedema, neurological deficits post tMCAo [68] | ||
| IL-1β, IL-6, IL-12, IL-23, IFN-γ | Prolonged/heightened inflammation. Bystander tissue damage [66] | ||
| Anti-inflammatory | TGF-β, IL-4, IL-10, IL-13 | Reduced inflammatory damage. Pro-regeneration. Upregulated Bcl-2, Bcl-x1. Enhances dendritic spine formation and synaptogenesis in cultured neurons and provides negative feedback in the production of inflammatory cytokines. Inhibit the activity of caspase-3, upregulate the level of GSH and NGF [69] | |
| Chemotaxis | CD11b, CD16, CD32, CCL2/MCP1 | Facilitate microglial migration to injury sites [70] | CCL2-/- KO mice show decreased injury post-ischaemia [59]; anti-CD11b antibody reduced infarct size in rat tMCAo [71] |
| Phagocytosis | CD11c (ITGAX) | Clears damaged cells, neurotoxic molecules and molecules inhibitory to repair. Early infiltrating macrophages are CD11c−, but CD11c+ macrophages may outnumber CD11c+ microglia by 3 d post-stroke [63]. Dendritic cells are CD11c+ [72] | Microglia may phagocytose damaged neurons, which could otherwise have recovered [73,74] |
| ROS, RNS | iNOS, NO− | Disrupts BBB, facilitating infiltration of peripheral immune cells and toxic molecules from serum. Oxidises PS on the surface of neurons and promotes neuron loss through phagocytosis. Exacerbates glutamate excitotoxicity. Damaging to oligodendroglia. | |
| Anti-oxidants | GSH, HO-1 | Inhibit oxidation of PS and promote neuron repair by reducing the phagocytic capacity of microglia [59] | |
| ECM-degrading enzymes | MMP-3, MMP-9 | Degrade extracellular matrix proteins, reduce the integrity of BBB; MMP-9 upregulated by signals from serum [75] | |
| Angiogenesis | VEGF, BDNF, progranulin, MMP-9 | Facilitate axonal outgrowth and angiogenesis. MMP-9 degrades chondroitin sulphate proteoglycan, a component of the EC matrix that is reported to inhibit axonal growth [59] | Microglia express VEGF receptor; VEGF is associated with increased BBB permeability [76] |
| Neurotoxic molecules | Glutamate | Neurotoxic; microglia upregulation of GluR2-4 AMPA receptors is associated with axon and oligodendrocyte damage [77] | |
| Neuroprotective molecules | bFGF | Promotes mitosis of oligodendrocyte precursor cells [78] | |
| IGF-1, GDNF, thrombospondins, erythropoietin | Promote plasticity [79]; thrombospondins may be more highly expressed in macrophages [80] | ||
| BDNF | ~30% OX42+ cells expressed BDNF post-ischaemia; associated with plasticity (neuronal regeneration) [79] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawlinson, C.; Jenkins, S.; Thei, L.; Dallas, M.L.; Chen, R. Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy. Brain Sci. 2020, 10, 159. https://doi.org/10.3390/brainsci10030159
Rawlinson C, Jenkins S, Thei L, Dallas ML, Chen R. Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy. Brain Sciences. 2020; 10(3):159. https://doi.org/10.3390/brainsci10030159
Chicago/Turabian StyleRawlinson, Charlotte, Stuart Jenkins, Laura Thei, Mark L. Dallas, and Ruoli Chen. 2020. "Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy" Brain Sciences 10, no. 3: 159. https://doi.org/10.3390/brainsci10030159
APA StyleRawlinson, C., Jenkins, S., Thei, L., Dallas, M. L., & Chen, R. (2020). Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy. Brain Sciences, 10(3), 159. https://doi.org/10.3390/brainsci10030159







