Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
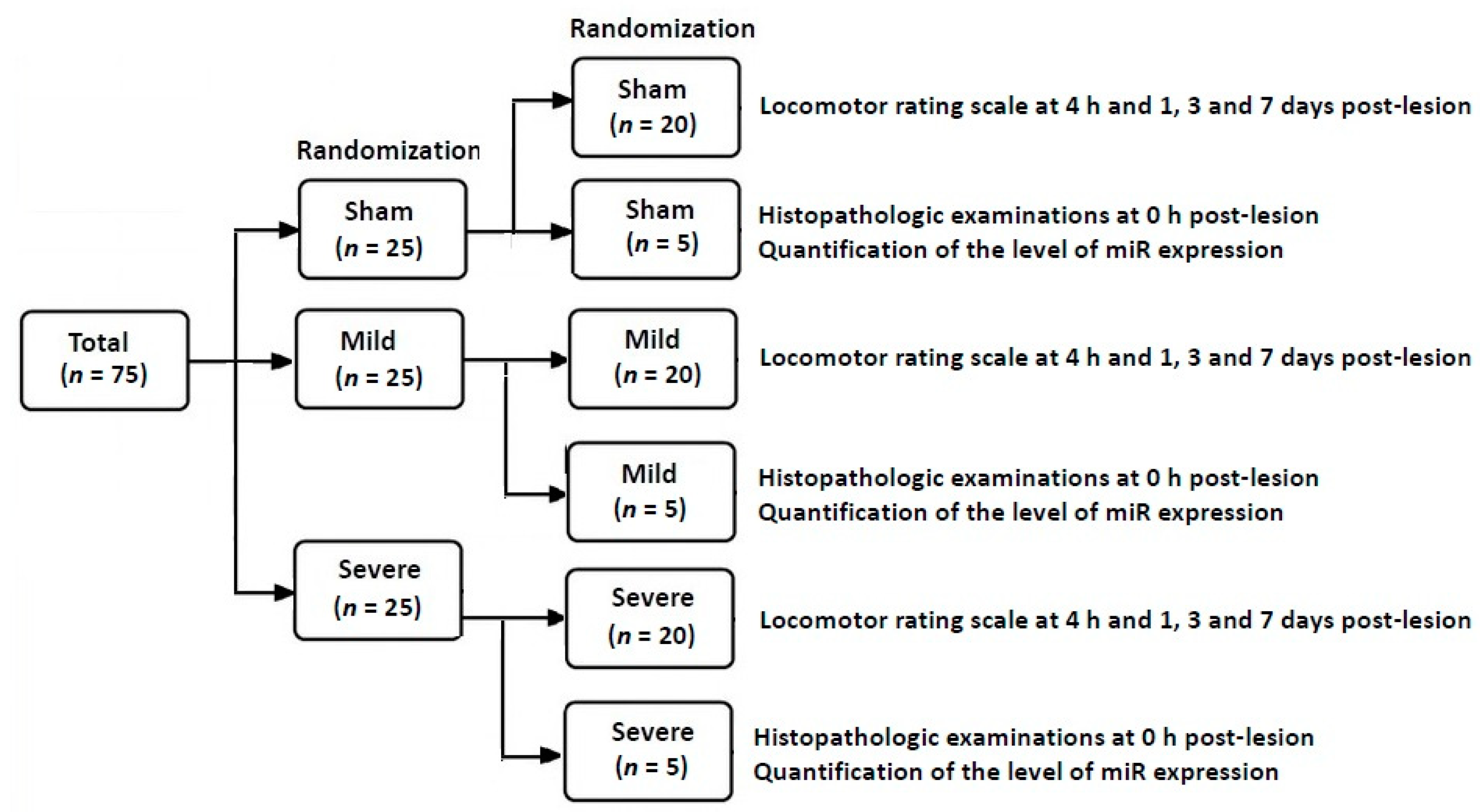
2.1. Experimental Design and Setting
2.2. Experimental Procedures
2.2.1. Establishment of an SD Model of TSCI
- (1)
- The sham group (n = 25): The SD rats undergoing insertion of an uninflated balloon catheter
- (2)
- The mild group (n = 25): The SD rats undergoing insertion of a 20-μL balloon catheter
- (3)
- The severe group (n = 25): The SD rats undergoing insertion of a 50-μL balloon catheter inflated at a volume of 50 μL.
2.2.2. Validation of an SD Model of TSCI
2.2.3. Isolation of miR Samples and the RT-PCR
2.3. Data Analysis
3. Results
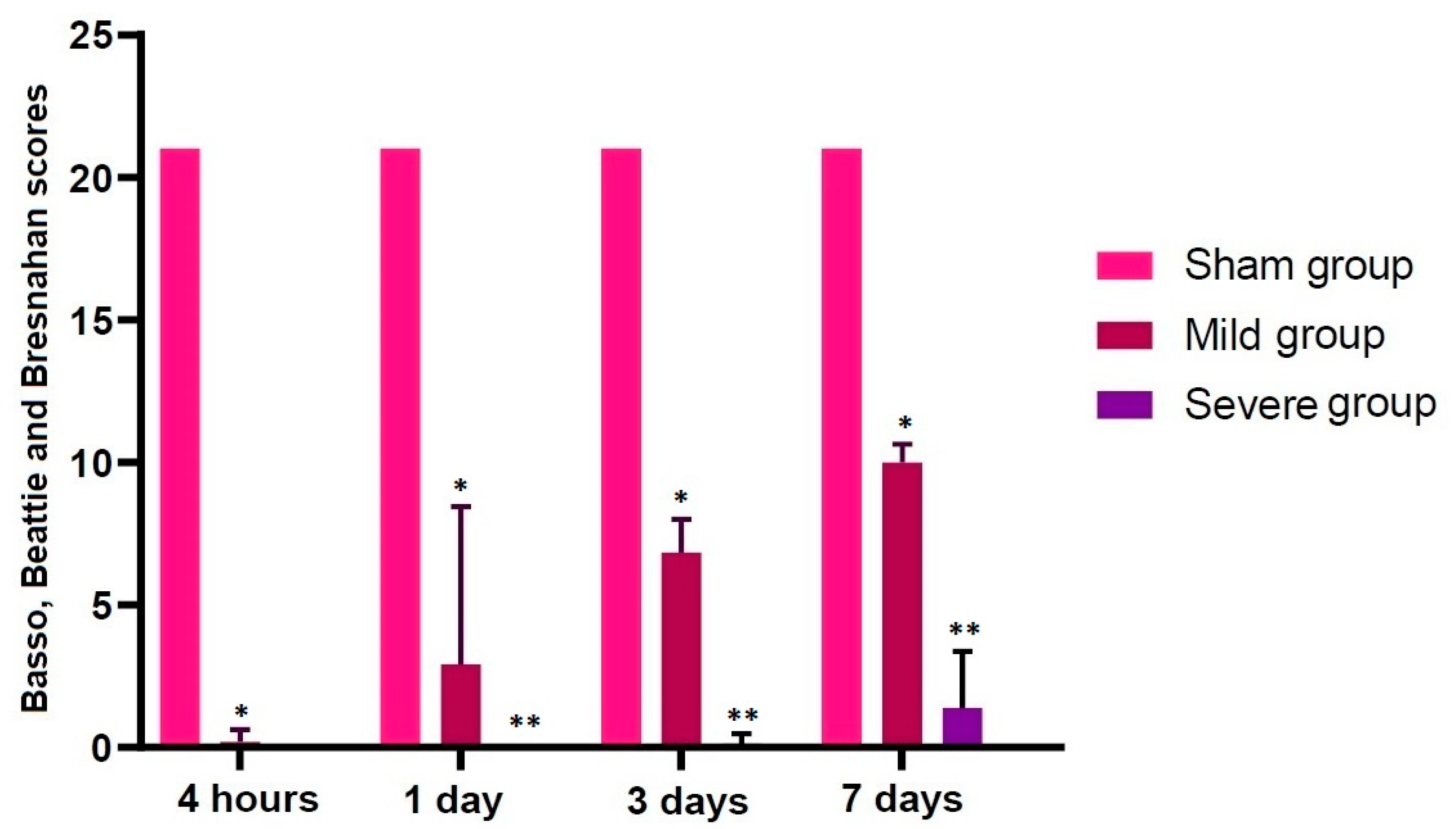
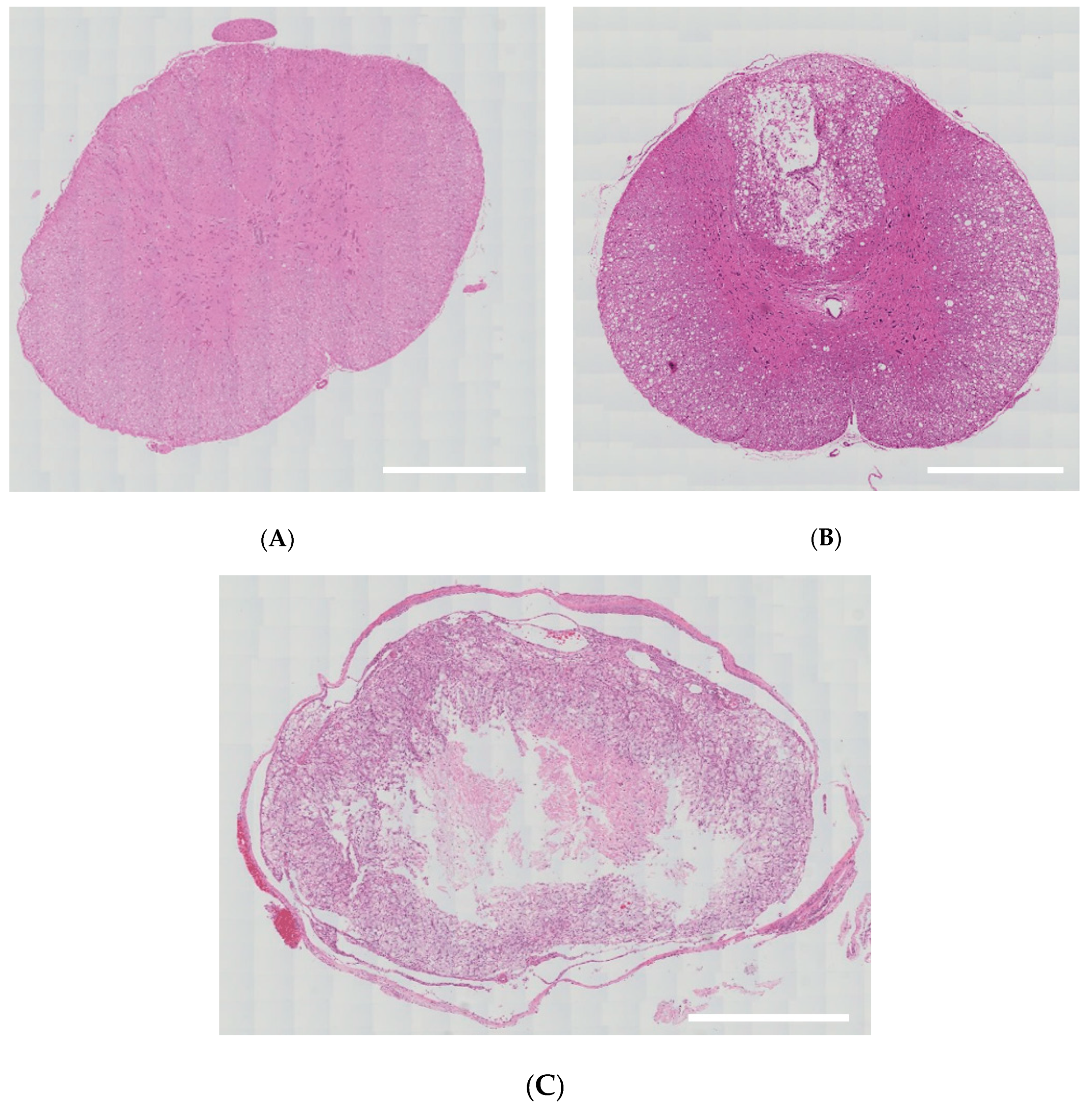
3.1. Validation of an SD Model of TSCI
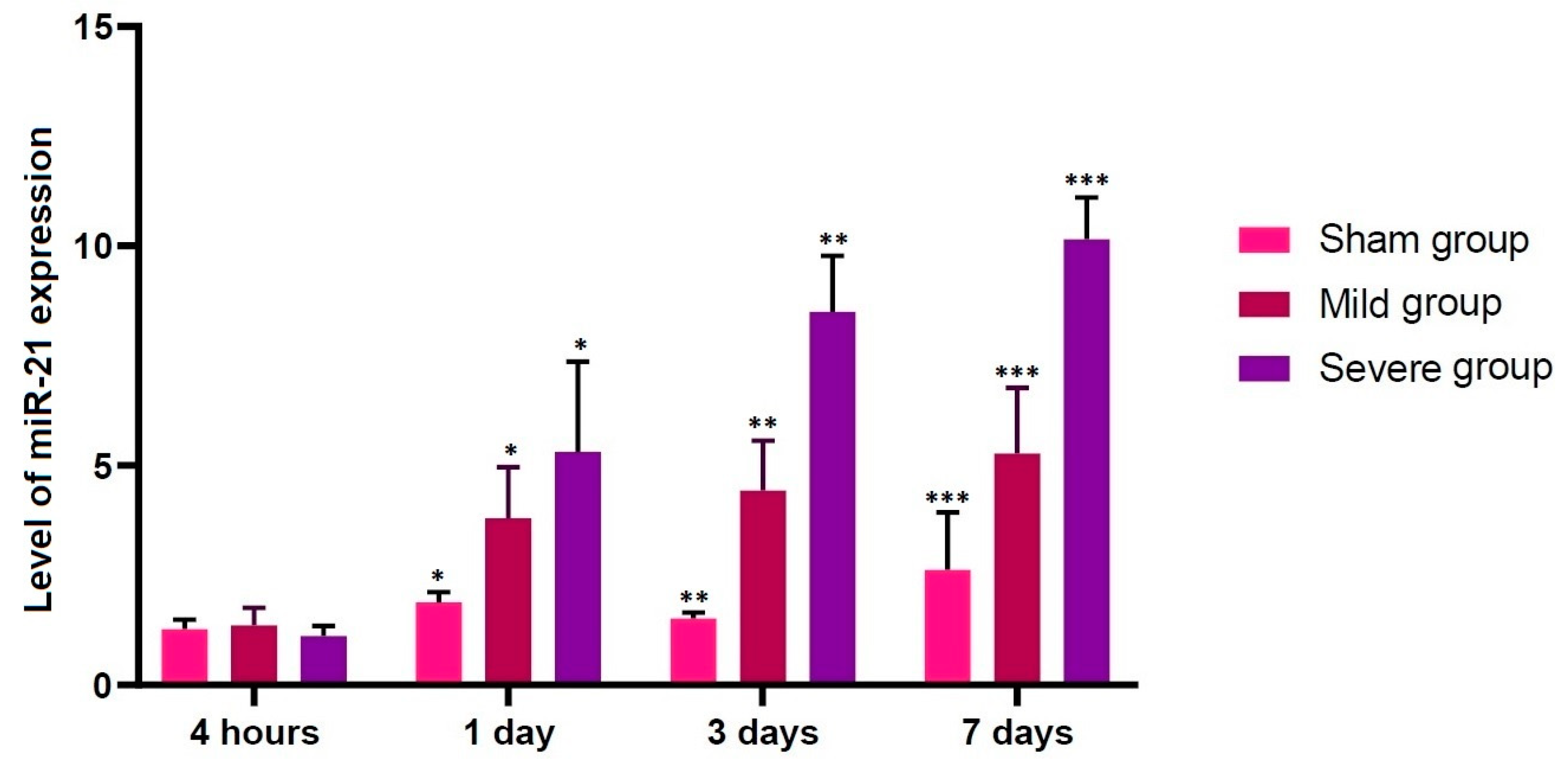
3.2. Alterations in the Level of miR-21 Expression according to the Time Course
3.3. Alterations in the Level of miR-223 Expression according to the Time Course
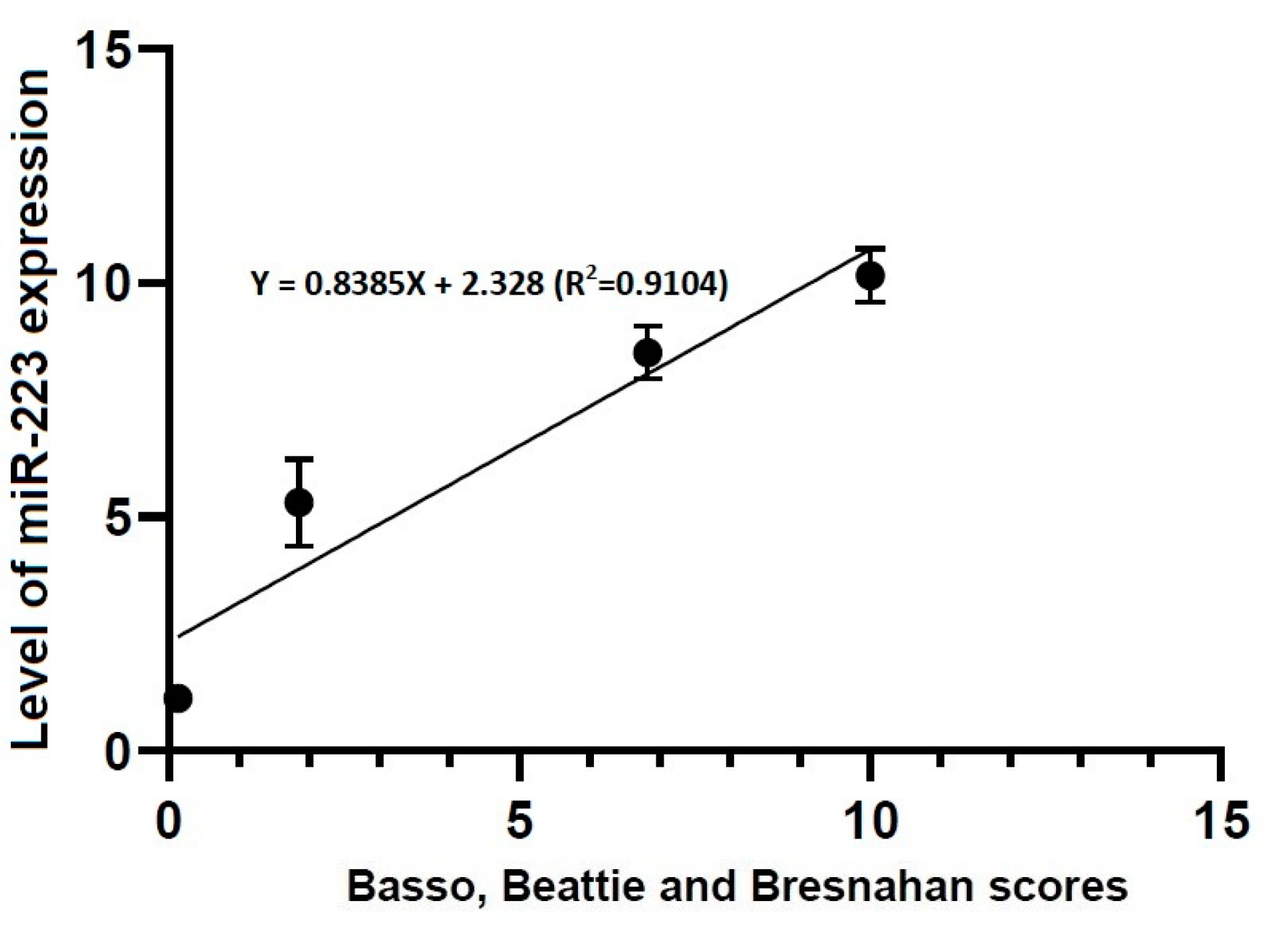
3.4. Correlations between Locomotor Rating Scale Scores and the Level of miR-21 or -223 Expression
4. Discussion
- (1)
- No differences in the level of miR-21 expression were found at the first time point studied (4 h post-lesion) between the three experimental groups, whereas such differences were significant at all the other time points (p < 0.05).
- (2)
- There were significant alterations in the level of miR-223 expression at all time points studied through all the experimental groups (p < 0.05).
- (3)
- Locomotor rating scale scores had a linear relationship with the level of miR-21 expression (R2 = 0.4363, Y = 1.661X + 3.096) and that of miR-223 one (R2 = 0.9104, Y = 0.8385X + 2.328).
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cripps, R.A.; Lee, B.B.; Wing, P.; Weerts, E.; Mackay, J.; Brown, D. A global map for traumatic spinal cord injury epidemiology: Towards a living data repository for injury prevention. Spinal Cord 2011, 49, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- García-Alté, A.; Pérez, K.; Novoa, A.; Suelves, J.M.; Bernabeu, M.; Vidal, J.; Arrufat, V.; Santamariña-Rubio, E.; Ferrando, J.; Cogollos, M.; et al. Spinal cord injury and traumatic brain injury: A cost-of-illness study. Neuroepidemiology 2012, 39, 103–108. [Google Scholar]
- Branco, F.; Cardenas, D.D.; Svircev, J.N. Spinal cord injury: A comprehensive review. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 651–679. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar]
- Center NSCIS. Spinal cord injury facts and figures at a glance. J. Spinal Cord Med. 2014, 37, 117–118. [Google Scholar] [CrossRef]
- Han, Z.A.; Lee, B.S.; Kim, W.; Lee, S.J.; Im, H.J.; Kim, C.; Song, K.; Ko, H.Y.; Bang, M.S.; Park, C.I. People with spinal cord injury in Korea. Am. J. Phys. Med. Rehabil. 2017, 96, S83–S85. [Google Scholar] [CrossRef]
- Bracken, M.B. Steroids for acute spinal cord injury. Cochrane Database Syst. Rev. 2012, 1, CD001046. [Google Scholar] [CrossRef] [PubMed]
- Budh, C.N.; Osteråker, A.L. Life satisfaction in individuals with a spinal cord injury and pain. Clin. Rehabil. 2007, 21, 89–96. [Google Scholar] [CrossRef]
- Ramer, L.M.; Ramer, M.S.; Steeves, J.D. Setting the stage for functional repair of spinal cord injuries: A cast of thousands. Spinal Cord 2005, 43, 134–161. [Google Scholar] [CrossRef]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Brechtel, K.; Mueller, C.A.; Failli, V.; Kaps, H.P.; Tuli, S.K.; Schluesener, H.J. Experimental strategies to promote spinal cord regeneration—An integrative perspective. Prog. Neurobiol. 2006, 78, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Wang, Y.; Akyol, O.; Ho, W.M.; Ii, R.A.; Stier, G.; Martin, R.; Zhang, J.H. What’s new in traumatic brain injury: Update on tracking, monitoring and treatment. Int. J. Mol. Sci. 2015, 16, 11903–11965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pearse, D.D. Therapeutic hypothermia in spinal cord injury: The status of its use and open questions. Int. J. Mol. Sci. 2015, 16, 16848–16879. [Google Scholar] [CrossRef] [PubMed]
- Liao, L. Evaluation and management of neurogenic bladder: What is new in China? Int. J. Mol. Sci. 2015, 16, 18580–18600. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinov, D.; Shapiro, L.A. Overview of traumatic brain injury: An immunological context. Brain Sci. 2017, 7, 11. [Google Scholar] [CrossRef]
- Gutiérrez, Á.; Sepúlveda-Muñoz, D.; Gil-Agudo, Á.; de los Reyes Guzmán, A. Serious game platform with haptic feedback and EMG monitoring for upper limb rehabilitation and smoothness quantification on spinal cord injury patients. Appl. Sci. 2020, 10, 963. [Google Scholar] [CrossRef]
- Otzel, D.M.; Lee, J.; Ye, F.; Borst, S.E.; Yarrow, J.F. Activity-based physical rehabilitation with adjuvant testosterone to promote neuromuscular recovery after spinal cord injury. Int. J. Mol. Sci. 2018, 19, 1701. [Google Scholar] [CrossRef]
- Pereira, I.M.; Marote, A.; Salgado, A.J.; Silva, N.A. Filling the gap: Neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals 2019, 12, 65. [Google Scholar] [CrossRef]
- Hu, J.Z.; Huang, J.H.; Zeng, L.; Wang, G.; Cao, M.; Lu, H.B. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J. Neurotrauma 2013, 30, 1349–1360. [Google Scholar] [CrossRef]
- Izumi, B.; Nakasa, T.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Yamamoto, R.; Nakamae, T.; Ohta, R.; Fujioka, Y.; Yamasaki, K.; et al. MicroRNA-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neurosci. Lett. 2011, 492, 114–118. [Google Scholar] [CrossRef]
- Vanický, I.; Urdzíková, L.; Saganová, K.; Cízková, D.; Gálik, J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J. Neurotrauma 2001, 18, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Lee, J.H.; Chung, D.J.; Yang, W.J.; Lee, A.J.; Choi, C.B.; Chang, H.S.; Kim, D.H.; Chung, H.J.; Suh, H.J.; et al. Improved rat spinal cord injury model using spinal cord compression by percutaneous method. J. Vet. Sci. 2013, 14, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, C.B.; Chung, D.J.; Kang, E.H.; Chang, H.S.; Hwang, S.H.; Han, H.; Choe, B.Y.; Sur, J.H.; Lee, S.Y.; et al. Development of an improved canine model of percutaneous spinal cord compression injury by balloon catheter. J. Neurosci. Methods 2008, 167, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chung, W.H.; Kang, E.H.; Chung, D.J.; Choi, C.B.; Chang, H.S.; Lee, J.H.; Hwang, S.H.; Han, H.; Choe, B.Y.; et al. Schwann cell-like remyelination following transplantation of human umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs with acute spinal cord injury. J. Neurol. Sci. 2011, 300, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Chambers, C.; Shuai, B. Profiling microRNA expression in Arabidopsis pollen using microRNA array and real-time PCR. BMC Plant Biol. 2009, 9, 87. [Google Scholar] [CrossRef]
- Nakanishi, K.; Nakasa, T.; Tanaka, N.; Ishikawa, M.; Yamada, K.; Yamasaki, K.; Kamei, N.; Izumi, B.; Adachi, N.; Miyaki, S.; et al. Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal Cord 2010, 48, 192–196. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, H.; Liao, Z.; Wang, Y.; Hu, X.; Chen, X.; Xu, L.; Hu, Z. MiR-203 enhances let-7 biogenesis by targeting LIN28B to suppress tumor growth in lung cancer. Sci. Rep. 2017, 7, 42680. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Fan, J.M.; Zhang, Z.M.; Ouyang, J.L.; Ni, T.T.; Wu, H.X.; Li, H. MicroRNA-204 targets signal transducer and activator of transcription 5 expression and inhibits proliferation of B-cell lymphoma cells. Mol. Med. Rep. 2015, 11, 4567–4572. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.C.; Ferreira, F.M.; Nakaya, H.I.; Baron, M.A.; Vilar-Pereira, G.; Pereira, I.R.; Silva, A.M.; Real, J.M.; De, B.T.; Chevillard, C.; et al. MicroRNA transcriptome profiling in heart of trypanosoma cruzi-infected mice: Parasitological and cardiological outcomes. PLoS Negl. Trop. Dis. 2015, 9, e0003828. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.; McDaniel, K.; Han, Y.; Liu, X.; Kennedy, L.; Yang, F.; McCarra, J.; Zhou, T.; Glaser, S.; Venter, J.; et al. Regulation of the extrinsic apoptotic pathway by microRNA-21 in alcoholic liver injury. J. Biol. Chem. 2014, 289, 27526–27539. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N.J. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- Nieto-Diaz, M.; Esteban, F.J.; Reigada, D.; Muñoz-Galdeano, T.; Yunta, M.; Caballero-López, M.; Navarro-Ruiz, R.; Del Águila, A.; Maza, R.M. MicroRNA dysregulation in spinal cord injury: Causes, consequences and therapeutics. Front. Cell. Neurosci. 2014, 8, 53. [Google Scholar] [CrossRef]
- Dong, J.; Lu, M.; He, X.; Xu, J.; Qin, J.; Cheng, Z.; Liang, B.; Wang, D.; Li, H. Identifying the role of microRNAs in spinal cord injury. Neurol. Sci. 2014, 35, 1663–1671. [Google Scholar] [CrossRef]
- Fineberg, S.K.; Kosik, K.S.; Davidson, B.L. MicroRNAs potentiate neural development. Neuron 2009, 64, 303–309. [Google Scholar] [CrossRef]
- Genovese, T.; Esposito, E.; Mazzon, E.; Di, P.R.; Caminiti, R.; Bramanti, P.; Cappelani, A.; Cuzzocrea, S. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J. Neurochem. 2009, 108, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, A.G.; Faden, A.I. Mechanisms of neural cell death: Implications for development of neuroprotective treatment strategies. NeuroRx 2004, 1, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Stoica, B.A.; Fricke, S.; Di, G.S.; Faden, A.I. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain 2007, 130, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.R.; Uchida, K.; Nakajima, H.; Watanabe, S.; Nakamura, M.; Johnson, W.E.; Baba, H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J. Neuroinflamm. 2012, 9, 40. [Google Scholar] [CrossRef]
- Lüningschrör, P.; Hauser, S.; Kaltschmidt, B.; Kaltschmidt, C. MicroRNAs in pluripotency, reprogramming and cell fate induction. Biochim. Biophys. Acta 2013, 1833, 1894–1903. [Google Scholar] [CrossRef]
- Chan, J.A.; Krichevsky, A.M.; Kosik, K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005, 65, 6029–6033. [Google Scholar] [CrossRef]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef]
- Sahni, V.; Mukhopadhyay, A.; Tysseling, V.; Hebert, A.; Birch, D.; Mcguire, T.L.; Stupp, S.I.; Kessler, J.A. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J. Neurosci. 2010, 30, 1839–1855. [Google Scholar] [CrossRef]
- Zhou, S.; Ding, F.; Gu, X. Non-coding RNAs as emerging regulators of neural injury responses and regeneration. Neurosci. Bull. 2016, 32, 253–264. [Google Scholar] [CrossRef]
- Liu, J. Control of protein synthesis and mRNA degradation by microRNAs. Curr. Opin. Cell Biol. 2008, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chao, K.; Ng, S.C.; Bai, A.H.; Yu, Q.; Yu, J.; Li, M.; Cui, Y.; Chen, M.; Hu, J.F.; et al. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhou, H.; Lu, L.; Li, X.; Fu, Z.; Liu, J.; Kang, Y.; Wei, Z.; Pan, B.; Liu, L.; et al. The roles of microRNAs in spinal cord injury. Int. J. Neurosci. 2017, 127, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Washington, P.M.; Knoblach, S.M.; Hoffman, E.; Faden, A.I. Delayed inflammatory mRNA and protein expression after spinal cord injury. J. Neuroinflamm. 2011, 8, 130. [Google Scholar] [CrossRef]
- Strickland, E.R.; Hook, M.A.; Balaraman, S.; Huie, J.R.; Grau, J.W.; Miranda, R.C. MicroRNA dysregulation following spinal cord contusion: Implications for neural plasticity and repair. Neuroscience 2011, 186, 146–160. [Google Scholar] [CrossRef]
- Urbanek, M.O.; Nawrocka, A.U.; Krzyzosiak, W.J. Small RNA detection by in situ hybridization methods. Int. J. Mol. Sci. 2015, 16, 13259–13286. [Google Scholar] [CrossRef]
- Baril, P.; Ezzine, S.; Pichon, C. Monitoring the spatiotemporal activities of miRNAs in small animal models using molecular imaging modalities. Int. J. Mol. Sci. 2015, 16, 4947–4972. [Google Scholar] [CrossRef]
- Miya Shaik, M.; Tamargo, I.A.; Abubakar, M.B.; Kamal, M.A.; Greig, N.H.; Gan, S.H. The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes 2018, 9, 174. [Google Scholar] [CrossRef]
- Marí-Alexandre, J.; Sánchez-Izquierdo, D.; Gilabert-Estellés, J.; Barceló-Molina, M.; Braza-Boïls, A.; Sandoval, J. MiRNAs regulation and its role as biomarkers in endometriosis. Int. J. Mol. Sci. 2016, 17, 93. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Wienholds, E.; de Bruijn, E.; Kauppinen, S.; Plasterk, R.H. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat. Methods 2006, 3, 27–29. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Frisén, J. Stem cells for spinal cord repair. Cell Stem Cell 2008, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord Med. 2017, 40, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Gospodarev, V.; Reis, H.; Wilkinson, M.; Gaio, J.; Araujo, C.; Chen, S.; Zhang, J.H. Traumatic brain injury and stem cell: Pathophysiology and update on recent treatment modalities. Stem Cells Int. 2017, 2017, 6392592. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Duroux-Richard, I.; Firat, H.; Schordan, E.; Apparailly, F. MicroRNAs: Key regulators to understand osteoclast differentiation? Front. Immunol. 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.C.P.D.; Carneiro, F.D.; Almeida, K.C.; Fernandes-Santos, C. Role of miRNAs on the pathophysiology of cardiovascular diseases. Arq. Bras. Cardiol. 2018, 111, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Banik, N.L.; Kurowska, E.; Hogan, E.L. Myelin recovery in multiple sclerosis: The challenge of remyelination. Brain Sci. 2013, 3, 1282–1324. [Google Scholar] [CrossRef]
- Stallings, R.L. MicroRNA involvement in the pathogenesis of neuroblastoma: Potential for microRNA mediated therapeutics. Curr. Pharm. Des. 2009, 15, 456–462. [Google Scholar] [CrossRef]
- Pal, M.K.; Jaiswar, S.P.; Dwivedi, V.N.; Tripathi, A.K.; Dwivedi, A.; Sankhwar, P. MicroRNA: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol. Med. 2015, 12, 328–341. [Google Scholar]
- Jurkovicova, D.; Smolkova, B.; Magyerkova, M.; Sestakova, Z.; Kajabova, V.H.; Kulcsar, L.; Zmetakova, I.; Kalinkova, L.; Krivulcik, T.; Karaba, M.; et al. Down-regulation of traditional oncomiRs in plasma of breast cancer patients. Oncotarget 2017, 8, 77369–77384. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Carotenuto, A.; Patel, A.A.; Kalani, M.Y.; Yagmurlu, K.; Lemole, G.M.J.; Preul, M.C.; Theodore, N. The role of microRNA markers in the diagnosis, treatment, and outcome prediction of spinal cord injury. Front. Surg. 2016, 3, 56. [Google Scholar] [CrossRef]
- Kreth, S.; Hübner, M.; Hinske, L.C. MicroRNAs as clinical biomarkers and therapeutic tools in perioperative medicine. Anesth. Analg. 2018, 126, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Carè, A.; Bellini, T. “Bridging the gap” everything that could have been avoided if we had applied gender medicine, pharmacogenetics and personalized medicine in the gender-omics and sex-omics era. Int. J. Mol. Sci. 2019, 21, 296. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [PubMed]
- Taneja, A.; Della, P.O.; Danhof, M. Challenges in translational drug research in neuropathic and inflammatory pain: The prerequisites for a new paradigm. Eur. J. Clin. Pharmacol. 2017, 73, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Yunta, M.; Nieto-Díaz, M.; Esteban, F.J.; Caballero-López, M.; Navarro-Ruíz, R.; Reigada, D.; Pita-Thomas, D.W.; del, Á.A.; Muñoz-Galdeano, T.; Maza, R.M. MicroRNA dysregulation in the spinal cord following traumatic injury. PLoS ONE 2012, 7, e34534. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, O.G.; Pan, L.; Sahni, V.; McGuire, T.L.; Gruner, K.; Tourtellotte, W.G.; Kessler, J.A. MicroRNA-21 regulates astrocytic response following spinal cord injury. J. Neurosci. 2012, 32, 17935–17947. [Google Scholar] [CrossRef] [PubMed]


| Group | Time Points | |||
|---|---|---|---|---|
| 4 H | 1 Day | 3 Days | 7 Days | |
| Sham | 21.00000 | 21.00000 | 21.00000 | 21.00000 |
| Mild | 0.12500 * | 1.85714 * | 6.83333 * | 10.00000 * |
| Severe | 0.00000 | 0.00000 ** | 0.12500 ** | 1.37500 ** |
| Group | Time Points | |||
|---|---|---|---|---|
| 4 H | 1 Day | 3 Days | 7 Days | |
| Sham | 1.27396 ± 0.09624 | 1.88336 ± 0.10513 * | 1.51919 ± 0.05863 ** | 2.62678 ± 0.58438 *** |
| Mild | 1.36184 ± 0.17934 | 3.80291 ± 0.52056 * | 4.43207 ± 0.50885 ** | 5.27714 ± 0.66994 *** |
| Severe | 1.11534 ± 0.10416 | 5.30909 ± 0.92265 * | 8.50091 ± 0.56952 ** | 10.1606 ± 0.42516 *** |
| Group | Time Points | |||
|---|---|---|---|---|
| 4 h | 1 Day | 3 Days | 7 Days | |
| Sham | 3.068116 ± 1.348989 * | 5.35168 ± 0.95415 ** | 1.40717 ± 0.17022 *** | 1.37411 ± 0.30366 **** |
| Mild | 8.465987 ± 1.812612 * | 11.6884 ± 1.89249 ** | 3.47943 ± 0.33655 *** | 2.67426 ± 0.59617 **** |
| Severe | 12.8211 ± 0.921337 * | 26.8762 ± 3.33326 ** | 11.0146 ± 0.9859 *** | 6.48124 ± 0.44328 **** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-J.; Chung, W.-H.; Do, S.-H.; Lee, J.-H.; Kim, H.-y. Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury. Brain Sci. 2020, 10, 141. https://doi.org/10.3390/brainsci10030141
Chung H-J, Chung W-H, Do S-H, Lee J-H, Kim H-y. Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury. Brain Sciences. 2020; 10(3):141. https://doi.org/10.3390/brainsci10030141
Chicago/Turabian StyleChung, Hyo-Jin, Wook-Hun Chung, Sun-Hee Do, Jae-Hoon Lee, and Hwi-yool Kim. 2020. "Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury" Brain Sciences 10, no. 3: 141. https://doi.org/10.3390/brainsci10030141
APA StyleChung, H.-J., Chung, W.-H., Do, S.-H., Lee, J.-H., & Kim, H.-y. (2020). Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury. Brain Sciences, 10(3), 141. https://doi.org/10.3390/brainsci10030141






