Association between Behavioral Ambidexterity and Brain Health
Abstract
1. Introduction
2. Materials and methods
2.1. Subjects
2.2. Psychological Scales
2.3. MRI Data Acquisition
2.4. MRI Data Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- March, J.G. Exploration and exploitation in organizational learning. Organ. Sci. 1991, 2, 71–87. [Google Scholar] [CrossRef]
- Raisch, S.; Birkinshaw, J. Organizational ambidexterity: Antecedents, outcomes, and moderators. J. Manag. 2008, 34, 375–409. [Google Scholar] [CrossRef]
- Sheremata, W.A. Centrifugal and centripetal forces in radical new product development under time pressure. Acad. Manag. Rev. 2008, 25, 389–408. [Google Scholar] [CrossRef]
- Good, D.; Michel, E.J. Individual ambidexterity: Exploring and exploiting in dynamic contexts. J. Psychol. 2013, 147, 435–453. [Google Scholar] [CrossRef]
- Rosing, K.; Zacher, H. Individual ambidexterity: The duality of exploration and exploitation and its relationship with innovative performance. Eur. J. Work Organ. Psychol. 2017, 26, 694–709. [Google Scholar] [CrossRef]
- O’Reilly, C.A., III; Tushman, M.L. Organizational ambidexterity: Past, present, and future. Acad. Manag. Perspect. 2013, 27, 324–338. [Google Scholar] [CrossRef]
- Baror, S.; Bar, M. Associative activation and its relation to exploration and exploitation in the brain. Psychol. Sci. 2016, 27, 776–789. [Google Scholar] [CrossRef]
- Blanchard, T.C.; Gershman, S.J. Pure correlates of exploration and exploitation in the human brain. Cogn. Affect. Behav. Neurosci. 2018, 18, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Daw, N.D.; O’doherty, J.P.; Dayan, P.; Seymour, B.; Dolan, R.J. Cortical substrates for exploratory decisions in humans. Nature 2006, 441, 876. [Google Scholar] [CrossRef] [PubMed]
- Laureiro-Martínez, D.; Brusoni, S.; Canessa, N.; Zollo, M. Understanding the exploration–exploitation dilemma: An fMRI study of attention control and decision-making performance. Strateg. Manag. J. 2015, 36, 319–338. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y. MRI-based Brain Healthcare Quotients: A bridge between neural and behavioral analyses for keeping the brain healthy. PLoS ONE 2017, 12, e0187137. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, K.; Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y.; Watanabe, Y. Association of fatigue and stress with gray matter volume. Front. Behav. Neurosci. 2018, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Kashdan, T.B.; Gallagher, M.W.; Silvia, P.J.; Winterstein, B.P.; Breen, W.E.; Terhar, D.; Steger, M.F. The curiosity and exploration inventory-II: Development, factor structure, and psychometrics. J. Res. Pers. 2009, 43, 987–998. [Google Scholar] [CrossRef]
- Duckworth, A.L.; Quinn, P.D. Development and validation of the Short Grit Scale (GRIT–S). J. Pers. Assess. 2009, 91, 166–174. [Google Scholar] [CrossRef]
- Chak, A. Teachers’ and parents’ conceptions of children’s curiosity and exploration. Int. J. Early Years Educ. 2007, 15, 141–159. [Google Scholar] [CrossRef]
- Dale, G.; Sampers, D.; Loo, S.; Green, C.S. Individual differences in exploration and persistence: Grit and beliefs about ability and reward. PLoS ONE 2018, 13, e0203131. [Google Scholar] [CrossRef]
- Ryan, R.M.; Deci, E.L. Intrinsic and extrinsic motivations: Classic definitions and new directions. Contemp. Educ. Psychol. 2000, 25, 54–67. [Google Scholar] [CrossRef]
- Mussel, P. Introducing the construct curiosity for predicting job performance. J. Organ. Behav. 2013, 34, 453–472. [Google Scholar] [CrossRef]
- Celik, P.; Storme, M.; Davila, A.; Myszkowski, N. Work-related curiosity positively predicts worker innovation. J. Manag. Dev. 2016, 35, 1184–1194. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Eskreis-Winkler, L.; Duckworth, A.L.; Shulman, E.P.; Beal, S. The grit effect: Predicting retention in the military, the workplace, school and marriage. Front. Psychol. 2014, 5, 36. [Google Scholar] [CrossRef]
- Robertson-Kraft, C.; Duckworth, A.L. True grit: Trait-level perseverance and passion for long-term goals predicts effectiveness and retention among novice teachers. Teach. Coll. Rec. 2014, 116, 3. [Google Scholar]
- Duckworth, A.L.; Kirby, T.A.; Tsukayama, E.; Berstein, H.; Ericsson, K.A. Deliberate practice spells success: Why grittier competitors triumph at the National Spelling Bee. Soc. Psychol. Personal. Sci. 2011, 2, 174–181. [Google Scholar] [CrossRef]
- Strayhorn, T.L. What role does grit play in the academic success of black male collegians at predominantly white institutions? J. Afr. Am. Stud. 2014, 18, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Dai, J.; Li, J.; Wang, X.; Chen, T.; Yang, X.; He, M.; Gong, Q. Neuroanatomical correlates of grit: Growth mindset mediates the association between gray matter structure and trait grit in late adolescence. Hum. Brain Mapp. 2018, 39, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Liljeholm, M.; O’Doherty, J.P. Contributions of the striatum to learning, motivation, and performance: An associative account. Trends Cogn. Sci. 2012, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Shohamy, D. Learning and motivation in the human striatum. Curr. Opin. Neurobiol. 2011, 21, 408–414. [Google Scholar] [CrossRef]
- Sherer, M.; Maddux, J.E.; Mercandante, B.; Prentice-Dunn, S.; Jacobs, B.; Rogers, R.W. The self-efficacy scale: Construction and validation. Psychol. Rep. 1982, 51, 663–671. [Google Scholar] [CrossRef]
- Rosenberg, M. Society and the Adolescent Self-Image; Princeton University Press: Princeton, NJ, USA, 1965. [Google Scholar]
- Scheier, M.F.; Carver, C.S.; Bridges, M.W. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J. Pers. Soc. Psychol. 1994, 67, 1063–1078. [Google Scholar] [CrossRef]
- Davis, J.C.; Nagamatsu, L.S.; Hsu, C.L.; Beattie, B.L.; Liu-Ambrose, T. Self-efficacy is independently associated with brain volume in older women. Age Ageing 2012, 41, 495–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Honicke, T.; Broadbent, J. The influence of academic self-efficacy on academic performance: A systematic review. Educ. Res. Rev. 2016, 17, 63–84. [Google Scholar] [CrossRef]
- Beattie, S.; Woodman, T.; Fakehy, M.; Dempsey, C. The role of performance feedback on the self-efficacy–performance relationship. Sport Exerc. Perform. Psychol. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Çetin, F.; Aşkun, D. The effect of occupational self-efficacy on work performance through intrinsic work motivation. Manag. Res. Rev. 2018, 41, 186–201. [Google Scholar] [CrossRef]
- Guntzviller, L.M.; King, A.J.; Jensen, J.D.; Davis, L.A. Self-efficacy, health literacy, and nutrition and exercise behaviors in a low-income, Hispanic population. J. Immigr. Minor. Health 2017, 19, 489–493. [Google Scholar] [CrossRef]
- Reilly, E.; Dhingra, K.; Boduszek, D. Teachers’ self-efficacy beliefs, self-esteem, and job stress as determinants of job satisfaction. Int. J. Educ. Manag. 2014, 28, 365–378. [Google Scholar] [CrossRef]
- Gustems-Carnicer, J.; Calderón, C.; Santacana, M.F. Psychometric properties of the Life Orientation Test (LOT-R) and its relationship with psychological well-being and academic progress in college students. Rev. Latinoam. Psicol. 2017, 49, 19–27. [Google Scholar] [CrossRef]
- Sadeghi, A.; Yousefi, A.; Khedmati, Z. The Role of Life Orientation and Cognitive Regulation on Decreasing Job Stress. Health 2018, 10, 268–281. [Google Scholar] [CrossRef]
- Korn, C.W.; Sharot, T.; Walter, H.; Heekeren, H.R.; Dolan, R.J. Depression is related to an absence of optimistically biased belief updating about future life events. Psychol. Med. 2014, 44, 579–592. [Google Scholar] [CrossRef]
- Duckworth, A.L.; Peterson, C.; Matthews, M.D.; Kelly, D.R. Grit: Perseverance and passion for long-term goals. J. Pers. Soc. Psychol. 2007, 92, 1087–1101. [Google Scholar] [CrossRef]
- DeVellis, R.F. Scale Development: Theory and Applications; Sage: Los Angeles, CA, USA, 2012. [Google Scholar]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Wang, H.; Li, J. How trait curiosity influences psychological well-being and emotional exhaustion: The mediating role of personal initiative. Pers. Individ. Differ. 2015, 75, 135–140. [Google Scholar] [CrossRef]
- Kang, M.J.; Hsu, M.; Krajbich, I.M.; Loewenstein, G.; McClure, S.M.; Wang, J.T.Y.; Camerer, C.F. The wick in the candle of learning: Epistemic curiosity activates reward circuitry and enhances memory. Psychol. Sci. 2009, 20, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Nojavanasghari, B.; Baltrušaitis, T.; Hughes, C.E.; Morency, L.P. The future belongs to the curious: Towards automatic understanding and recognition of curiosity in children. Workshop Child Comput. Interact. 2016, 2016, 16–22. [Google Scholar]
- Freeman, H.D.; Brosnan, S.F.; Hopper, L.M.; Lambeth, S.P.; Schapiro, S.J.; Gosling, S.D. Developing a comprehensive and comparative questionnaire for measuring personality in chimpanzees using a simultaneous top-down/bottom-up design. Am. J. Primatol. 2013, 75, 1042–1053. [Google Scholar] [CrossRef]
- Singh, K.; Jha, S.D. Positive and negative affect, and grit as predictors of happiness and life satisfaction. J. Indian Acad. Appl. Psychol. 2008, 34, 40–45. [Google Scholar]
- Sheridan, Z.; Boman, P.; Mergler, A.; Furlong, M.J. Examining well-being, anxiety, and self-deception in university students. Cogent Psychol. 2015, 2, 993850. [Google Scholar] [CrossRef]
- Datu, J.A.D.; King, R.B.; Valdez, J.P.M.; Eala, M.S.M. Grit is associated with lower depression via meaning in life among Filipino high school students. Youth Soc. 2018, 51, 865–876. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Nichols, T.E.; Laird, A.R.; Hoffstaedter, F.; Amunts, K.; Fox, P.T.; Bzdok, D.; Eickhoff, C.R. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 2016, 137, 70–85. [Google Scholar] [CrossRef]
- Seo, Y.W.; Chae, S.W.; Lee, K.C. The impact of absorptive capacity, exploration, and exploitation on individual creativity: Moderating effect of subjective well-being. Comput. Hum. Behav. 2015, 42, 68–82. [Google Scholar] [CrossRef]
- Hills, T.T.; Todd, P.M.; Lazer, D.; Redish, A.D.; Couzin, I.D. Cognitive Search Research Group. Exploration versus exploitation in space, mind, and society. Trends Cogn. Sci. 2015, 19, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.H.; Lee, K.C.; Lee, D.S. Network structure, organizational learning culture, and employee creativity in system integration companies: The mediating effects of exploitation and exploration. Comput. Hum. Behav. 2015, 42, 167–175. [Google Scholar] [CrossRef]
- Kokubun, K.; Yamakawa, Y. Association between food patterns and gray matter volume. Front. Hum. Neurosci. 2019, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Anandarajan, M.; Dai, Q. Experiencing flow with instant messaging and its facilitating role on creative behaviors. Comput. Hum. Behav. 2010, 26, 1009–1018. [Google Scholar] [CrossRef]
- Črepinšek, M.; Liu, S.H.; Mernik, M. Exploration and exploitation in evolutionary algorithms: A survey. ACM Comput. Surv. 2013, 45, 35. [Google Scholar] [CrossRef]
- Na-Nan, K.; Sanamthong, E. Self-efficacy and employee job performance. Int. J. Qual. Reliab. Manag. 2019, 37, 1–17. [Google Scholar] [CrossRef]
- McCann, T.V.; Clark, E.; Lu, S. The self-efficacy model of medication adherence in chronic mental illness. J. Clin. Nurs. 2008, 17, 329–340. [Google Scholar] [CrossRef]
- Kershaw, T.; Ellis, K.R.; Yoon, H.; Schafenacker, A.; Katapodi, M.; Northouse, L. The interdependence of advanced cancer patients’ and their family caregivers’ mental health, physical health, and self-efficacy over time. Ann. Behav. Med. 2015, 49, 901–911. [Google Scholar] [CrossRef]
- Matsudaira, I.; Yokota, S.; Hashimoto, T.; Takeuchi, H.; Asano, K.; Asano, M.; Sassa, Y.; Taki, Y.; Kawashima, R. Parental praise correlates with posterior insular cortex gray matter volume in children and adolescents. PLoS ONE 2016, 11, e0154220. [Google Scholar] [CrossRef]
- Sugiura, A.; Aoki, R.; Murayama, K.; Yomogida, Y.; Haji, T.; Saito, A.; Hasegawa, T.; Matsumoto, K. Regional gray matter volume in the posterior precuneus is associated with general self-efficacy. Neuroreport 2016, 27, 1350–1353. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, F.; Huang, L.; Liu, J. Neural correlates of biased responses: The negative method effect in the Rosenberg Self-Esteem Scale is associated with right amygdala volume. J. Personal. 2016, 84, 623–632. [Google Scholar] [CrossRef] [PubMed]

| Scale | Number of Items Comprising the Scale | Cronbach’s α | Original Name | Response Scale | Sample Item | Source |
|---|---|---|---|---|---|---|
| Curiosity | 10 | 0.898 | Trait Curiosity and Exploration Inventory II | 5 points from 1 (very slightly or not at all) to 5 (extremely) | I actively seek as much information as I can in new situations. | Kashdan et al. [14] |
| Grit | 8 | 0.777 | Short Grit Scale | 5 points from 1 (not like me at all) to 5 (very much like me) | Setbacks don’t discourage me. | Duckworth and Quinn [15] |
| Self-efficacy | 23 | 0.906 | General Self-Efficacy Scale | 5 points from 1 (strongly disagree) to 5 (strongly agree) | When I make plans, I am certain I can make them work. | Sherer et al. [29] |
| Self-esteem | 10 | 0.873 | Rosenberg Self-Esteem Scale | 4 points from 1 (strongly disagree) to 4 (strongly agree) | On the whole, I am satisfied with myself. | Rosenberg et al. [30] |
| Optimism | 6 | 0.688 | Life Orientation Test | 5 points from 1 (strongly disagree) to 5 (strongly agree) | In uncertain times, I usually expect the best. | Scheier et al. [31] |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| GM-BHQ | 98.003 | 8.868 | 103.660 | 7.613 | 4.827 | *** |
| Curiosity | 2.610 | 0.791 | 2.487 | 0.740 | 1.135 | |
| Grit | 3.267 | 0.660 | 3.273 | 0.540 | 0.072 | |
| Self-efficacy | 3.314 | 0.622 | 3.360 | 0.501 | 0.567 | |
| Self-esteem | 2.889 | 0.563 | 2.900 | 0.525 | 0.139 | |
| Optimism | 3.175 | 0.601 | 3.270 | 0.548 | 1.166 | |
| Age | 44.864 | 13.462 | 43.866 | 10.775 | 0.577 | |
| n | % | n | % | χ2 | ||
| Kyoto | 57 | 55.3 | 58 | 59.8 | 9.844 | ** |
| Tokyo | 23 | 22.3 | 32 | 33.0 | ||
| Kobe | 23 | 22.3 | 7 | 7.2 | ||
| Kyoto | Tokyo | Kobe | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F (2, 197) | p | |
| GM-BHQ | 101.199 | 9.936 | 99.213 | 6.047 | 101.828 | 7.806 | 1.235 | |
| Curiosity | 2.528 | 0.779 | 2.484 | 0.709 | 2.757 | 0.811 | 1.348 | |
| Grit | 3.266 | 0.640 | 3.227 | 0.525 | 3.363 | 0.603 | 0.490 | |
| Self-efficacy | 3.356 | 0.554 | 3.282 | 0.624 | 3.362 | 0.505 | 0.349 | |
| Self-esteem | 2.929 | 0.530 | 2.740 | 0.568 | 3.047 | 0.497 | 3.726 | * |
| Optimism | 3.248 | 0.607 | 3.091 | 0.566 | 3.356 | 0.426 | 2.375 | |
| Age | 43.348 | 14.180 | 48.273 | 6.066 | 41.200 | 11.006 | 4.364 | * |
| Variable | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | GM-BHQ | 100.747 | 8.735 | 0.188 ** | 0.154 * | 0.155 * | 0.002 | 0.052 | |
| 2 | Curiosity | 2.550 | 0.767 | 0.105 | 0.328 *** | 0.555 *** | 0.333 *** | 0.277 *** | |
| 3 | Grit | 3.270 | 0.604 | -0.060 | 0.314 *** | 0.662 *** | 0.469 *** | 0.215 ** | |
| 4 | Self-efficacy | 3.337 | 0.566 | -0.062 | 0.529 *** | 0.676 *** | 0.674 *** | 0.459 *** | |
| 5 | Self-esteem | 2.895 | 0.543 | -0.140 * | 0.319 *** | 0.490 *** | 0.687 *** | 0.569 *** | |
| 6 | Optimism | 3.221 | 0.576 | 0.047 | 0.268 *** | 0.213 ** | 0.453 *** | 0.560 *** | |
| 7 | Age | 44.380 | 12.213 | -0.763 *** | -0.028 | 0.197 ** | 0.214 *** | 0.192 ** | 0.009 |
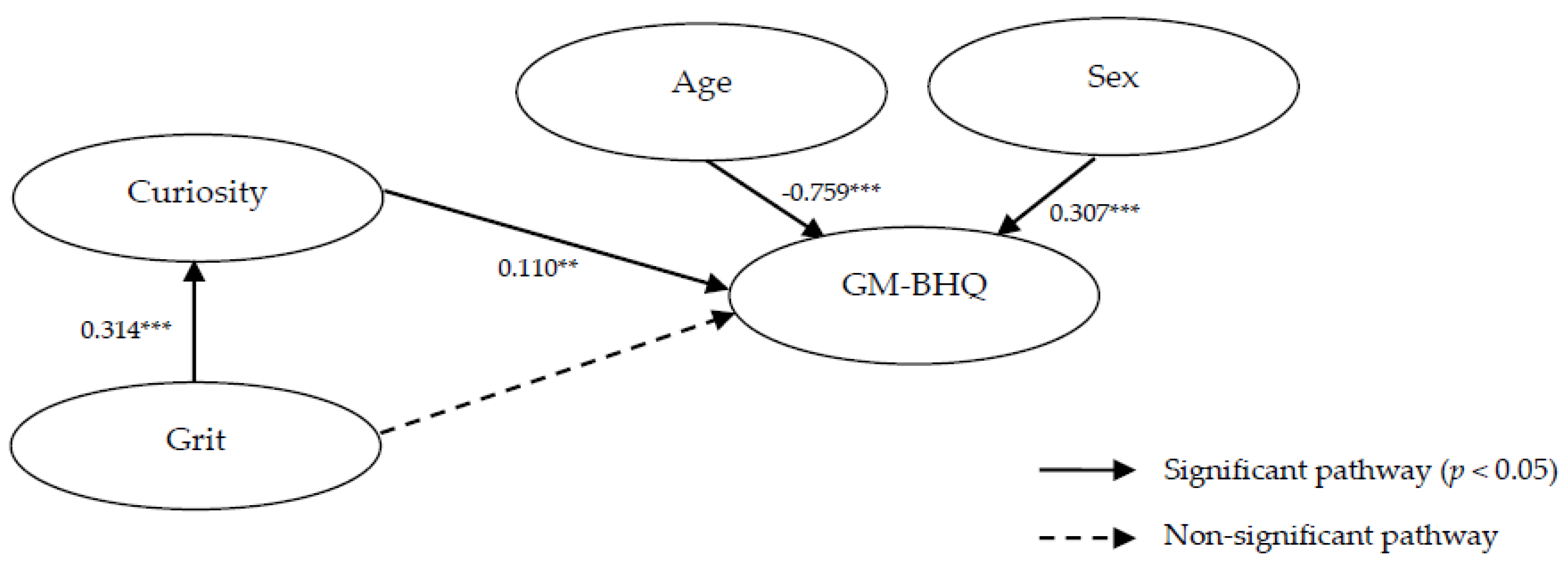
| Effect (standardized) | |||||
|---|---|---|---|---|---|
| Path | Direct | Indirect | Total | ||
| Curiosity | → | GM-BHQ | 0.110 | 0.110 | |
| Grit | → | Curiosity | 0.314 | 0.314 | |
| Age | → | GM-BHQ | −0.759 | −0.759 | |
| Sex | → | GM-BHQ | 0.307 | 0.307 | |
| Grit | → | GM-BHQ | 0.035 | 0.035 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokubun, K.; Yamakawa, Y.; Hiraki, K. Association between Behavioral Ambidexterity and Brain Health. Brain Sci. 2020, 10, 137. https://doi.org/10.3390/brainsci10030137
Kokubun K, Yamakawa Y, Hiraki K. Association between Behavioral Ambidexterity and Brain Health. Brain Sciences. 2020; 10(3):137. https://doi.org/10.3390/brainsci10030137
Chicago/Turabian StyleKokubun, Keisuke, Yoshinori Yamakawa, and Kazuo Hiraki. 2020. "Association between Behavioral Ambidexterity and Brain Health" Brain Sciences 10, no. 3: 137. https://doi.org/10.3390/brainsci10030137
APA StyleKokubun, K., Yamakawa, Y., & Hiraki, K. (2020). Association between Behavioral Ambidexterity and Brain Health. Brain Sciences, 10(3), 137. https://doi.org/10.3390/brainsci10030137





