Exercise Training Results in Lower Amyloid Plaque Load and Greater Cognitive Function in an Intensity Dependent Manner in the Tg2576 Mouse Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
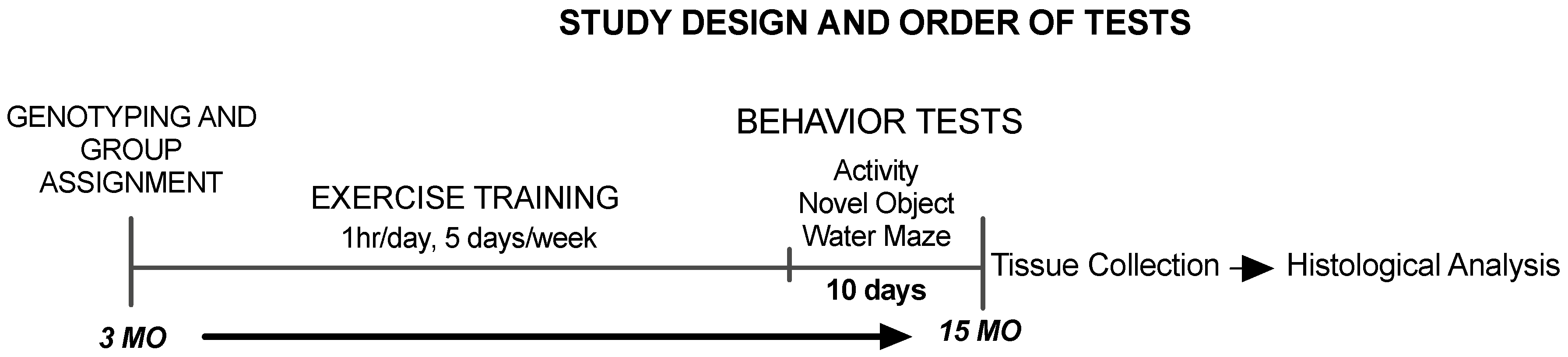
2.1. Animals and Experimental Design
2.2. Tissue Preparation
2.3. Soleus Muscle Citrate Synthase Analysis
2.4. Brain Tissue Sectioning and Amyloid Plaque Staining
2.5. Open Field Test
2.6. Novel Object Recognition Test
2.7. Morris Water Maze Test
2.8. Statistical Analysis
3. Results
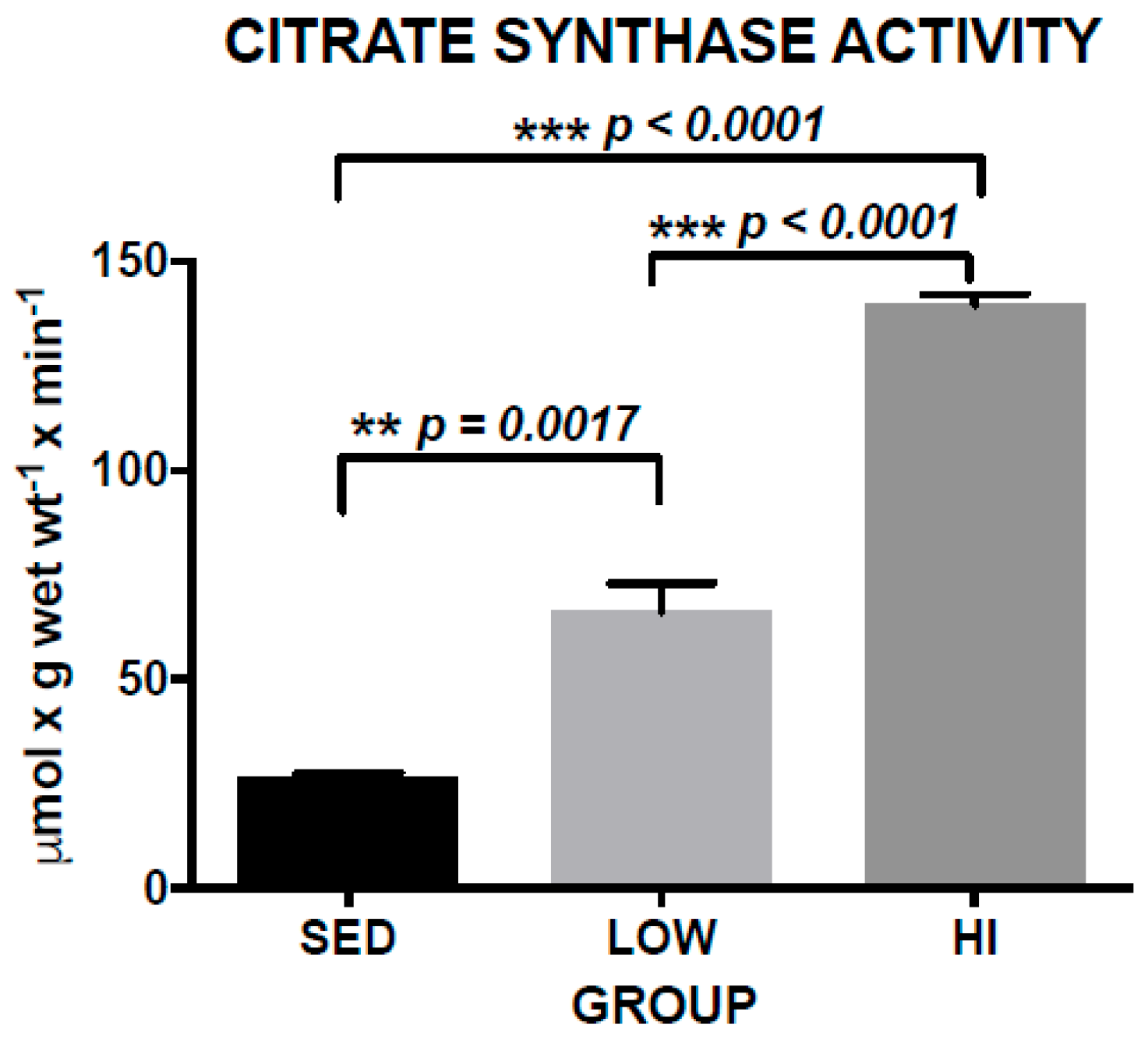
3.1. Increased Skeletal Muscle Citrate Synthase Activity (CS) is a Marker of Exercise Training Effects
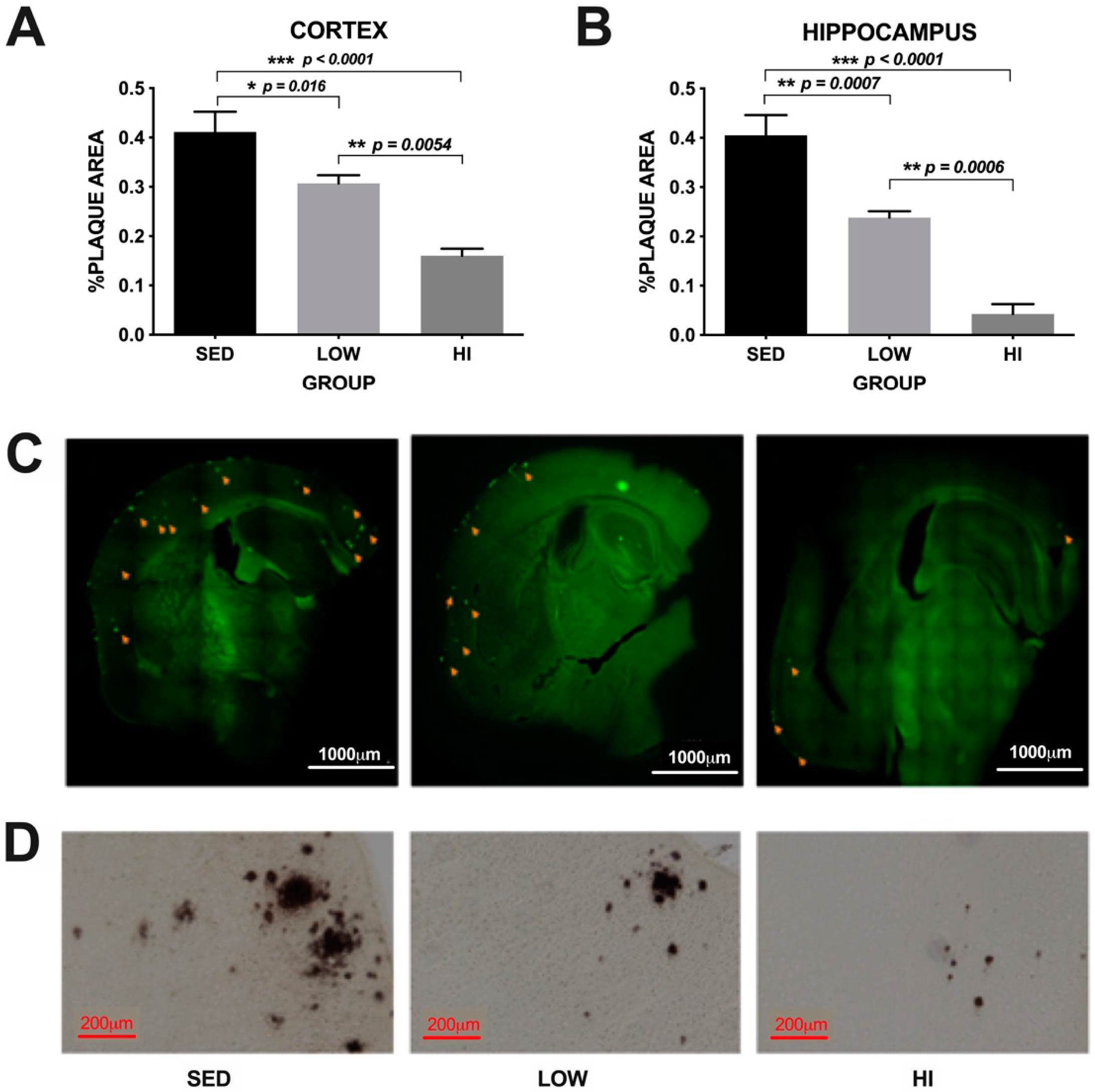
3.2. Exercise Training Reduces Amyloid Plaque Deposition in an Intensity-Dependent Manner in Tg2576 Mice
3.3. Exercise Training Does not Affect Locomotor Activity in Open Field Test
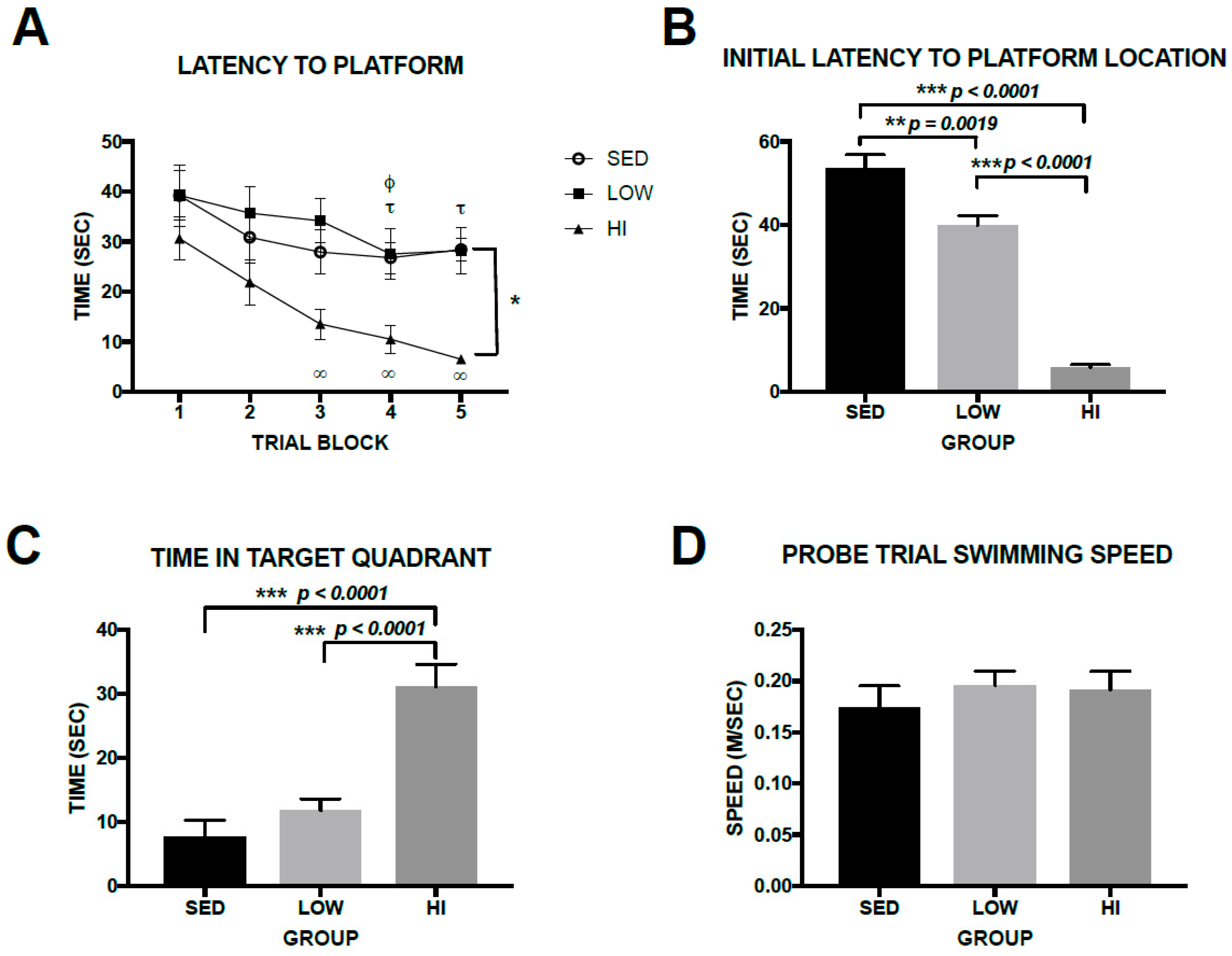
3.4. High-Intensity Exercise Training Had Robust Effects on Spatial Learning and Memory in Tg2576 Mice, Whereas Effects Associated with Low-Intensity Exercise Training Were Subtle
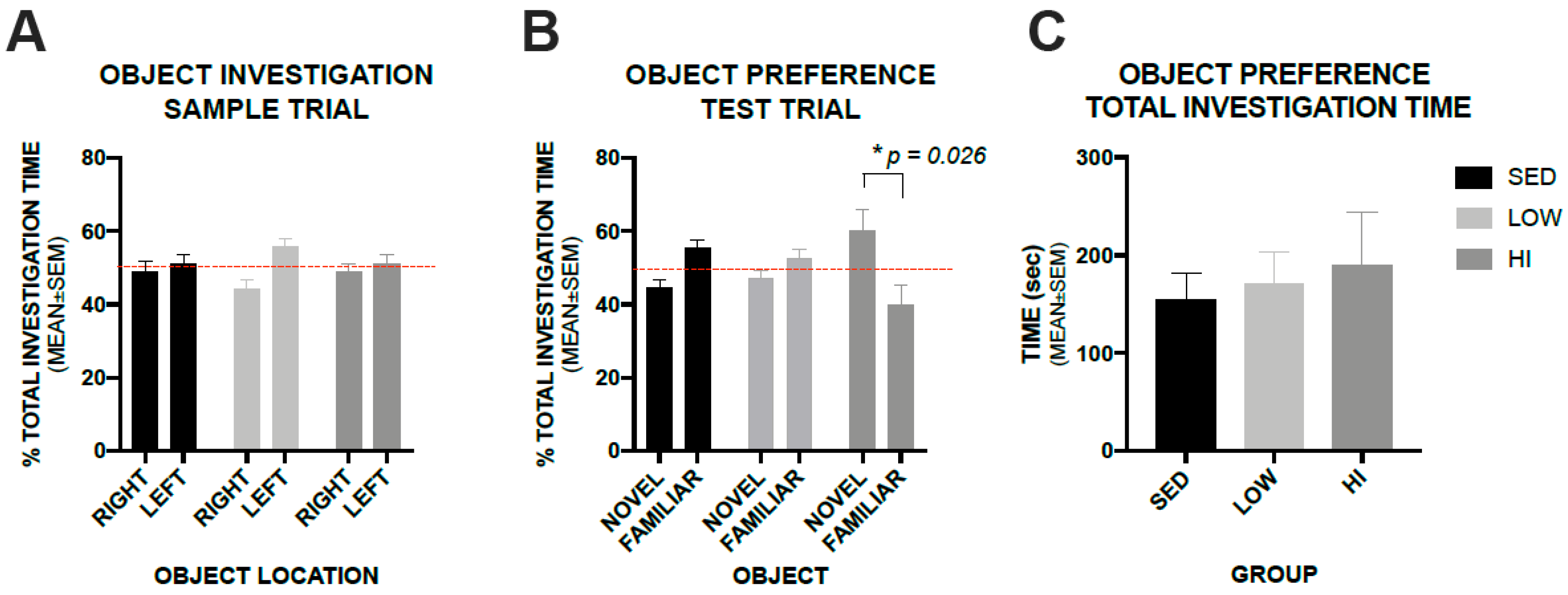
3.5. High-Intensity Exercise Training Positively Impacts Recognition Memory in Tg2576 Mice as Assessed by a Novel Object Recognition Test, whereas, Low-Intensity Exercise Training Does not
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ab | Beta amyloid fragment of the amyloid precursor protein |
| AD | Alzheimer’s disease |
| CS | Citrate synthase |
| DAB | 3,3’-diaminobenzidine |
| ET | Exercise training |
| HI | High-intensity exercise group |
| ISF | Interstitial fluid |
| LOW | Low-intensity exercise group |
| MWM | Morris Water Maze |
| NOR | Novel Object Recognition |
| SED | Sedentary group |
References
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Yuede, C.M.; Timson, B.F.; Hettinger, J.C.; Yuede, K.M.; Edwards, H.M.; Lawson, J.E.; Zimmerman, S.D.; Cirrito, J.R. Interactions between stress and physical activity on Alzheimer’s disease pathology. Neurobiol. Stress. 2018, 8, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Blackwell, T.; Stone, K.L.; Goldman, S.E.; Hillier, T.; Yaffe, K. Cognition in older women: The importance of daytime movement. J. Am. Geriatr. Soc. 2008, 56, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Geda, Y.E.; Roberts, R.O.; Knopman, D.S.; Christianson, T.J.; Pankrataz, V.S.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Petersen, R.C.; Rocca, W.A. Physical exercise, aging, and mild cognitive impairment: A population-based study. Arch. Neurol. 2010, 67, 80–86. [Google Scholar] [CrossRef]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer’s disease. JAMA 2008, 300, 1027–1037. [Google Scholar] [CrossRef]
- Rovio, S.; Karehholt, I.; Helkala, E.I.; Viitanen, M.; Winblad, B.; Tuomilehto, J.; Soininen, H.; Nissinen, A.; Kivielto, M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005, 4, 705–711. [Google Scholar] [CrossRef]
- Taaffee, D.R.; Irie, F.; Masaki, K.H.; Abbott, R.D.; Petrovitch, H.; Ross, G.W.; White, L.R. Physical activity, physical function, and incident dementia in elderly men: The Honolulu-Asia aging study. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 529–535. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Leem, Y.H.; Cho, I.H.; Yang, C.H.; Chae, K.R.; Hwang, D.Y.; Cho, J.Y. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw transgenic model. Int. J. Mol. Med. 2008, 22, 529–539. [Google Scholar]
- Um, H.S.; Kang, E.B.; Koo, J.H.; Kim, H.T.; Lee, J.; Kim, E.J.; Yang, C.H.; An, G.Y.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci. Res. 2011, 69, 161–173. [Google Scholar] [CrossRef]
- Cho, J.Y.; Um, H.S.; Kang, E.B.; Cho, I.H.; Kim, C.H.; Cho, J.S.; Hwang, D.Y. The combination of exercise training and α-lipoic acid treatment has therapeutic effects on the pathogenic phenotypes of Alzheimer’s disease in NSE/APPsw-transgenic mice. Int. J. Mol. Med. 2010, 25, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.B.; Kwon, I.S.; Koo, J.H.; Kim, J.; Kim, C.H.; Lee, J.; Yang, C.H.; Lee, Y.I.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death and inflammation during Aβ-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis 2013, 11, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.I.; Zhao, G.; Zhang, H.; Shi, L.D. Long term treadmill exercise inhibits the progression of Alzheimer’s disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice. Behav. Brain Res. 2013, 256, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.C.; Huang, H.J.; Liang, K.C.; Hsieh-Li, H.M. Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise. Brain Res. 2011, 1403, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuede, C.M.; Zimmerman, S.D.; Dong, H.; Kling, M.J.; Bero, A.W.; Holtzman, D.M.; Timson, B.F.; Csernansky, J.G. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol. Dis. 2009, 35, 426–432. [Google Scholar] [CrossRef]
- Gimenez-Llort, L.; Garcia, Y.; Buccieri, K.; Revilla, S.; Sunol, C.; Cristofol, R.; Sanfeliu, C. Gender-Specific Neuroimmunoendocrine Response to Treadmill Exercise in 3xTg-AD Mice. Int. J. Alzheimer’s Dis. 2010. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Li, S.C.; Sun, Y.X.; Zhang, X.S.; Dong, Z.Z.; Zhong, P.; Sun, X.R. Long-term treadmill exercise improves spatial memory of male APPswe/PS1dE9 mice by regulation of BDNF expression and microglia activation. Biol. Sport. 2015, 32, 295–300. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, H.L.; Zhang, H.; Tong, X.J. Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice. Neuroscience 2015, 298, 357–366. [Google Scholar] [CrossRef]
- Cho, J.Y.; Shin, M.K.; Kim, D.; Lee, I.; Kim, S.; Kang, H. Treadmill running reverses cognitive declines due to Alzheimer’s disease. Med. Sci. Sports Exerc. 2015, 47, 1814–1824. [Google Scholar] [CrossRef]
- Liu, H.I.; Zhao, G.; Cai, K.; Zhao, H.H.; Shi, L.D. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav. Brain Res. 2011, 218, 308–314. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine (ACSM) Position Stand: Quantity and quality of exercise for developine and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults-guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; May, P.C.; O’Dell, M.A.; Taylor, J.W.; Parsadanian, M.; Cramer, J.W.; Audia, J.E.; Nissen, J.S.; Bales, K.R.; Paul, S.M.; et al. In vivo assessment of brain intersitital fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 2003, 23, 8844–8853. [Google Scholar] [CrossRef]
- Bero, A.W.; Yan, P.; Roh, J.H.; Cirrito, J.R.; Stewart, F.R.; Raichle, M.E.; Lee, J.-M.; Holtzman, D.M. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011, 14, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Bero, A.W.; Cirrito, J.R.; Xiao, Q.; Hu, X.; Wang, Y.; Gonzales, E.; Holtzman, D.M.; Lee, J.-M. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci. 2009, 29, 10706–10714. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.M.; Girens, R.N.; Larson, S.K.; Jones, M.R.; Restivo, J.L.; Holtzman, D.M.; Cirrito, J.R.; Yuede, C.M.; Zimmerman, S.D.; Timson, B.F. A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 85, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Selko, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Morris, J.N.; Chave, S.P.; Adam, C.; Sirey, C.; Epstein, L.; Sheehan, D.J. Vigorous exercise in leisure time and the incidence of coronary heart disease. Lancet 1973, 1, 333–339. [Google Scholar] [CrossRef]
- Williams, P.D. Relationship of heart disease risk factors to exercise quantity and intensity. Arch. Intern. Med. 1998, 158, 237–245. [Google Scholar] [CrossRef]
- Blair, S.N.; Jackson, A.S. Physical fitness and activity as separate heart disease risk factors: A meta analysis. Med. Sci. Sports Exerc. 2001, 33, 762–764. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reductiion in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Kirk, S.; Sharp, C.F.; Elbaum, N.; Endres, D.B.; Simons, S.M.; Mohler, J.G.; Rude, R.K. Effect of long-distance running on bone mass in women. J. Bone Miner. Res. 1989, 4, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.A.; Lane, N.E.; Bjorkengren, A.; Bloch, D.A.; Fries, J.F. Impact of running on lumbar bone density: A 5-year longitudinal study. J. Rheumatol. 1992, 19, 1759–1763. [Google Scholar] [PubMed]
- Dunn, A.L.; Dishman, R.K. Exercise and the neurobiology of depression. Exerc. Sport Sci. Rev. 1991, 19, 41–98. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Birnbaum, A.S.; Kubik, M.Y.; Dishman, R.K. Naturally occurring changes in physical activity are inversely related to depressive symptoms during early adolescence. Psychosom. Med. 2004, 66, 336–342. [Google Scholar] [PubMed]
- Friedenreich, C.M.; Cust, A.E. Physical activity and breast cancer risk: Impact of timing, type and dose of activity and population subgroup effects. Br. J. Sports Med. 2008, 42, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.R.; Heesch, K.C.; Brown, W.J. A systematic review of the association between physical activity and colorectal cancer risk. Scand. J. Med. Sci. Sports 2009, 19, 764–781. [Google Scholar] [CrossRef]
- Yanagita, S.; Amemiya, S.; Suzuki, S.; Kita, I. Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats. Life Sci. 2007, 80, 356–363. [Google Scholar] [CrossRef]
- Dong, H.; Goico, B.; Martin, M.; Csernansky, C.A.; Bertchume, A.; Csernansky, J.G. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience 2004, 127, 601–609. [Google Scholar] [CrossRef]
- Dong, H.; Yuede, C.M.; Yoo, H.-S.; Martin, M.V.; Deal, C.; Mace, A.G.; Csernansky, J.G. Corticosterone and related receptor expression are associated with increased β-amyloid plaques in isolated Tg2576 mice. Neuroscience 2008, 155, 154–163. [Google Scholar] [CrossRef]
- Dong, H.; Csernansky, J.G. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J. Alzheimer’s Dis. 2009, 18, 459–469. [Google Scholar] [CrossRef]
- Kang, J.-E.; Cirrito, J.R.; Dong, H.; Csernansky, J.G.; Holtzman, D.M. Acute stress increases interstitial fluid amyloid-β via corticotropin-releasing factor and neuronal activity. Proc. Natl. Acad. Sci. USA 2007, 104, 10673–10678. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary exercise decreases amyloid load in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2005, 25, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mesa, Y.; Gimenez-Llort, L.; Lopez, L.C.; Venegas, C.; Cristofol, R.; Escames, G.; Acuna-Castroviejo, D.; Sanfeliu, C. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol. Aging. 2012, 33, e13–e1124. [Google Scholar] [CrossRef] [PubMed]
- Garacia-Mesa, Y.; Colie, S.; Corpas, R.; Cristofol, R.; Comellas, F.; Nebreda, A.R.; Gimenez-Llort, L.; Sanfeliu, C. Oxidative stress is a central target for physical exercise neuroprotection against pathological brain aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Aranquiz, F.; Varela-Nallar, L.; Inestrosa, N.C. Voluntary Running Attenuates Memory Loss, Decreases Neuropathological Changes and Induces Neurogenesis in a Mouse Model of Alzheimer’s Disease. Brain Pathol. 2016, 26, 62–74. [Google Scholar] [CrossRef]
- Maliszewska-Cyna, E.; Xhima, K.; Aubert, A. A comparative study evaluating the impact of physical exercise on disease progression in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 53, 243–257. [Google Scholar] [CrossRef]
- Cracchiolo, J.R.; Mori, T.; Nazian, S.J.; Tan, J.; Potter, H.; Arendash, G.W. Enhanced cognitive activity-over and above social or physical activity-is required to protect Alzheimer’s mice against cognitive impairment, reduce Aβ deposition, and increase synaptic immunoreactivity. Neurobiol. Learn Mem. 2007, 88, 277–294. [Google Scholar] [CrossRef]
- Richter, H.; Ambree, O.; Lewejohann, L.; Herring, A.; Keyvani, K.; Paulus, W.; Palme, R.; Touma, C.; Schabitz, W.R.; Sachser, N. Wheel-running in a transgenic mouse model of Alzheimer’s disease: Protection of symptom? Behav. Brain Res. 2008, 190, 74–84. [Google Scholar] [CrossRef]
- Wolf, S.A.; Kronenberg, G.; Lehmann, K.; Blankenship, A.; Overall, R.; Staufenbiel, M.; Kempermann, G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol. Psychiatry 2006, 60, 1314–1323. [Google Scholar] [CrossRef]
- Parachikova, A.; Nichol, K.E.; Cotman, C.W. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol. Dis. 2008, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mesa, Y.; Lopez-Ramos, J.C.; Gimenez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; LeFerla, F.M.; Cristofol, R.; Delgado-Garcia, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimer’s Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, R.; Zimmerman, S.D.; Yuede, K.M.; Cirrito, J.R.; Tai, L.M.; Timson, B.F.; Yuede, C.M. Exercise Training Results in Lower Amyloid Plaque Load and Greater Cognitive Function in an Intensity Dependent Manner in the Tg2576 Mouse Model of Alzheimer’s Disease. Brain Sci. 2020, 10, 88. https://doi.org/10.3390/brainsci10020088
Thomas R, Zimmerman SD, Yuede KM, Cirrito JR, Tai LM, Timson BF, Yuede CM. Exercise Training Results in Lower Amyloid Plaque Load and Greater Cognitive Function in an Intensity Dependent Manner in the Tg2576 Mouse Model of Alzheimer’s Disease. Brain Sciences. 2020; 10(2):88. https://doi.org/10.3390/brainsci10020088
Chicago/Turabian StyleThomas, Riya, Scott D. Zimmerman, Kayla M. Yuede, John R. Cirrito, Leon M. Tai, Benjamin F. Timson, and Carla M. Yuede. 2020. "Exercise Training Results in Lower Amyloid Plaque Load and Greater Cognitive Function in an Intensity Dependent Manner in the Tg2576 Mouse Model of Alzheimer’s Disease" Brain Sciences 10, no. 2: 88. https://doi.org/10.3390/brainsci10020088
APA StyleThomas, R., Zimmerman, S. D., Yuede, K. M., Cirrito, J. R., Tai, L. M., Timson, B. F., & Yuede, C. M. (2020). Exercise Training Results in Lower Amyloid Plaque Load and Greater Cognitive Function in an Intensity Dependent Manner in the Tg2576 Mouse Model of Alzheimer’s Disease. Brain Sciences, 10(2), 88. https://doi.org/10.3390/brainsci10020088



