Traumatic Brain Injury Patients Mortality and Serum Total Antioxidant Capacity
Abstract
1. Introduction
2. Methods
2.1. Design and Subjects
2.2. Serum Samples Collection
2.3. Determinations of Serum TAC Levels
2.4. Determination of Serum Malondialdehyde Levels
2.5. Statistical Methods
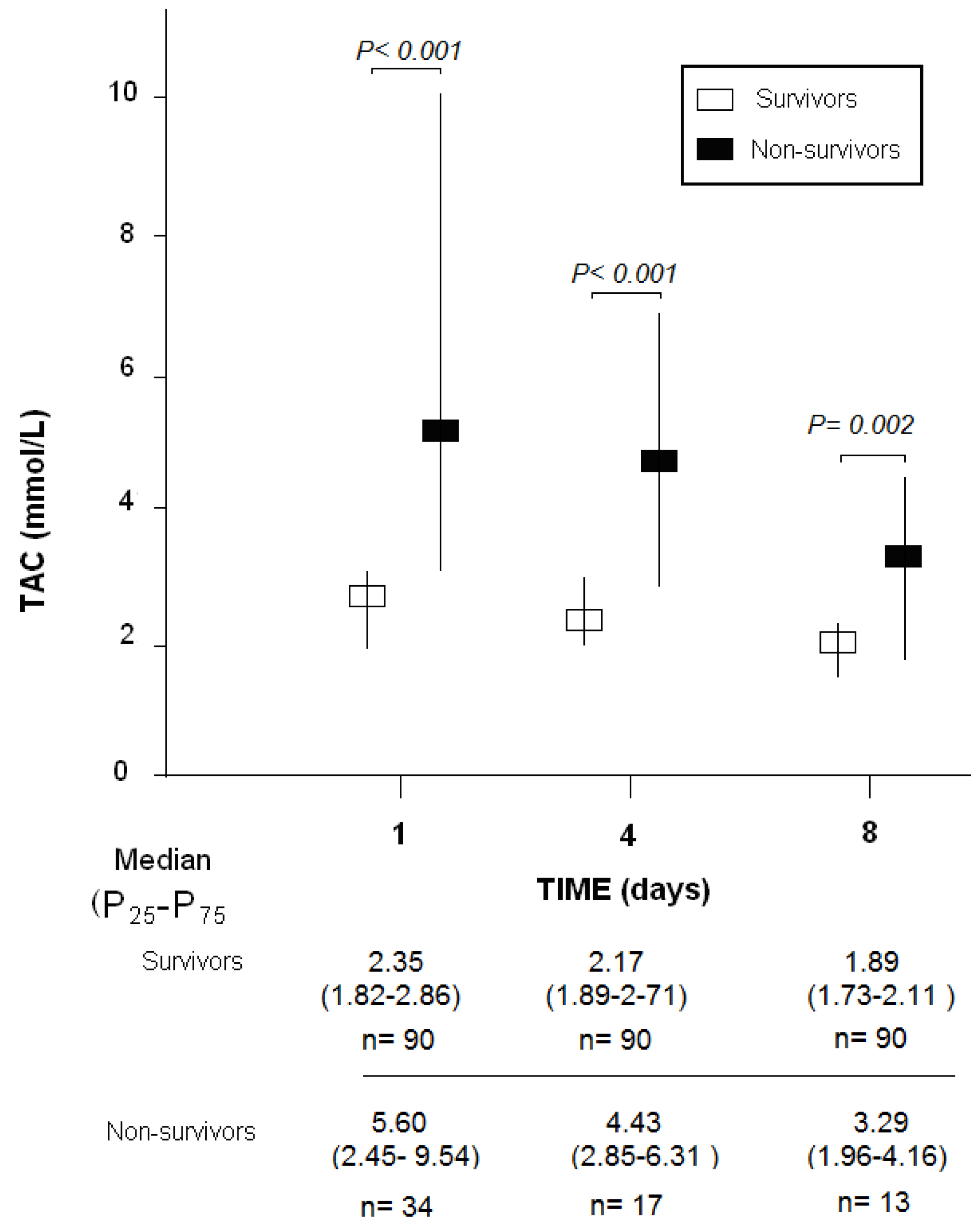
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 2007, 24, S1–S106. [Google Scholar]
- Ikeda, Y.; Long, D.M. The molecular basis of brain injury and brain edema: The role of oxygen free radicals. Neurosurgery 1990, 27, 1–11. [Google Scholar] [CrossRef]
- McCall, J.M.; Braughler, J.M.; Hall, E.D. Lipid peroxidation and the role of oxygen radicals in CNS injury. Acta Anaesthesiol. Belg. 1987, 38, 373–379. [Google Scholar]
- Warner, D.S.; Sheng, H.; Batinić-Haberle, I. Oxidants, antioxidants and the ischemic brain. J. Exp. Biol. 2004, 207, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D. Lipid antioxidants in acute central nervous system injury. Ann. Emerg. Med. 1993, 22, 1022–1027. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J.; Vilches-Arenas, Á.; Gordillo-Escobar, E.; Ruiz de Azúa-López, Z.; Murillo-Cabezas, F. Prognostic value of total antioxidant capacity to predict functional outcome in traumatic brain injury patients. Clin. Chem. Lab. Med. 2017, 55, e265–e267. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, H.S.; Erel, O.; Karakayali, O.; Neselioglu, S.; Tanriverdi, F.; Coskun, F.; Kahraman, A.F. Oxidative stress in isolated blunt traumatic brain injury. Sci. Res. Essays 2010, 5, 2832–2836. [Google Scholar]
- Lorente, L.; Martín, M.M.; Almeida, T.; Abreu-González, P.; Ramos, L.; Argueso, M.; Riaño-Ruiz, M.; Solé-Violán, J.; Jiménez, A. Total antioxidant capacity is associated with mortality of patients with severe traumatic brain injury. BMC Neurol. 2015, 15, 115. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Long, W.B., Jr. The Injury Severity Score: A Method for Describing Patients with Multiple Injuries and Evaluating Emergency Care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessement of coma and impaired conciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Van Berkum Clark, M.; Eisenberg, H.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 1992, 9 (Suppl. 1), S287–S292. [Google Scholar]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Ramos, L.; Cáceres, J.J.; Argueso, M.; Solé-Violán, J.; Jiménez, A.; García-Marín, V. Maintained high sustained serum malondialdehyde levels after severe brain trauma injury in non-survivor patients. BMC Res. Notes 2019, 12, 789. [Google Scholar] [CrossRef]
- Kikugawa, K.; Kojima, T.; Yamaki, S.; Kosugi, H. Interpretation of the thiobarbituric acid reactivity of rat liver and brain homogenates in the presence of ferric ion and ethylediaminotetraacetic acid. Anal. Biochem. 1992, 202, 249–255. [Google Scholar] [CrossRef]
- Messenge, C.; Margail, I.; Verrechia, C.; Allix, M. Protective effect of melatonin in a model of traumatic brain injury in mice. J. Pineal Res. 1998, 25, 41–46. [Google Scholar] [CrossRef]
- Horakova, L.; Onrejickova, O.; Barchrrata, K.; Vajdova, M. Preventive effect of several antioxidants after oxidative stress on rat brain homogenates. Gen. Physiol. Biophys. 2000, 19, 195–205. [Google Scholar]
- Kerman, M.; Cirak, B.; Ozguner, M.F.; Dagtekin, A.; Sutcu, R.; Altuntas, I.; Delibas, N. Does melatonin protect or treat brain damage from traumatic oxidative stress? Exp. Brain Res. 2005, 163, 406–410. [Google Scholar] [CrossRef]
- Ozsüer, H.; Görgülü, A.; Kiriş, T.; Cobanoğlu, S. The effects of memantine on lipid peroxidation following closed-head trauma in rats. Neurosurg. Rev. 2005, 28, 143–147. [Google Scholar] [CrossRef]
- Saniova, B.; Drobny, M.; Lehotsky, J.; Sulaj, M.; Schudichova, J. Biochemical and clinical improvement of cytotoxic state by amantadine sulphate. Cell Mol. Neurobiol. 2006, 26, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Vaishnav, R.A.; Mustafa, A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics 2010, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Ikram, M.; Ullah, N.; Alam, S.I.; Park, H.Y.; Badshah, H.; Choe, K.; Kim, M.O. Neurological Enhancement Effects of Melatonin against Brain Injury-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration via AMPK/CREB Signaling. Cells 2019, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, C.; Zhang, K.; Lan, X.; Chen, X.; Zang, W.; Wang, Z.; Guan, F.; Zhu, C.; Yang, X.; et al. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic. Biol. Med. 2019, 131, 345–355. [Google Scholar] [CrossRef] [PubMed]
| Non-Surviving (n = 34) | Surviving (n = 90) | p-Value | |
|---|---|---|---|
| GCS-m (p 25–75) | 4 (3–7) | 7 (5–8) | <0.001 |
| Gender female-n (%) | 13 (38.2) | 15 (16.7) | 0.02 |
| Age (years)-m (p 25–75) | 65 (55–75) | 46 (28–62) | <0.001 |
| ISS-m (p 25–75) | 25 (25–25) | 25 (25–34) | 0.28 |
| Marshall computer tomography-n (%) | 0.01 | ||
| Diffuse injury I | 0 | 0 | |
| Diffuse injury II | 5 (14.7) | 25 (27.8) | |
| Diffuse injury III | 6 (17.6) | 15 (16.7) | |
| Diffuse injury IV | 9 (26.5) | 13 (14.4) | |
| Evacuated mass lesion V | 6 (17.6) | 32 (35.6) | |
| Non-evacuated mass lesion VI | 8 (23.5) | 5 (5.6) | |
| PaO2 (mmHg)-m (p 25–75) | 142 (97–195) | 148 (110–242) | 0.45 |
| PaO2/FIO2 ratio-m (p 25–75) | 294 (167–395) | 336 (246–400) | 0.11 |
| Platelets-m*103/mm3 (p 25–75) | 172 (125–232) | 182 (135–238) | 0.49 |
| aPTT (seconds)-m (p 25–75) | 29 (25–37) | 28 (25–31) | 0.25 |
| Fibrinogen (mg/dl)-m (p 25–75) | 348 (300–475) | 371 (286–471) | 0.70 |
| INR-m (p 25–75) | 1.12 (1.03–1.48) | 1.11 (1.00–1.24) | 0.19 |
| Leukocytes-m*103/mm3 (p 25–75) | 14.9 (9.7–21.6) | 13.9 (10.1–19.0) | 0.47 |
| Hemoglobin (g/dL)-m (p 25–75) | 11.9 (10.0–13.7) | 11.2 (10.0–13.0) | 0.73 |
| Bilirubin (mg/dl)-m (p 25–75) | 0.70 (0.53–1.05) | 0.60 (0.40–0.80) | 0.06 |
| Glycemia (g/dL)-m (p 25–75) | 160 (125–191) | 139 (121–167) | 0.11 |
| Sodium (mEq/L)- m (p 25–75) | 141 (136–147) | 140 (138–143) | 0.41 |
| Creatinine (mg/dl)-m (p 25–75) | 0.80 (0.70–1.10) | 0.80 (0.70–1.00) | 0.50 |
| Lactic acid (mmol/L)-m (p 25–75) | 2.30 (1.25–4.58) | 1.75 (1.10–2.50) | 0.08 |
| ICP (mmHg)-m (p 25–75) | 25 (11–30) | 15 (14–20) | 0.36 |
| CPP (mmHg)-m (p 25–75) | 61 (52–70) | 68 (57–70) | 0.60 |
| APACHE-II score-m (p 25–75) | 25 (23–28) | 18 (14–22) | <0.001 |
| TAC (mmol/mL)-m (p 25–75) | 5.60 (2.45–9.54) | 2.35 (1.82–2.86) | <0.001 |
| Day 1 | Day 4 | Day 8 | |
|---|---|---|---|
| Cut-off of TAC (pg/mL) | >4.32 | >2.67 | >2.79 |
| Specificity (95% CI) | 93% (86–98%) | 76% (65–84%) | 93% (86–97%) |
| Sensitivity (95% CI) | 59% (41–75%) | 82% (57–96%) | 69% (38–90%) |
| Negative predicted value (95% CI) | 86% (80–90%) | 96% (89–98%) | 95% (90–98%) |
| Positive predicted value (95% CI) | 77% (59–88%) | 39% (29–49%) | 60% (39–77%) |
| Negative likelihood ratio (95% CI) | 0.4 (0.3–0.7) | 0.2 (0.1–0.7) | 0.3 (0.1–0.7) |
| Positive likelihood ratio (95% CI) | 8.8 (3.9–20.1) | 3.4 (2.2–5.2) | 10.4 (4.4–24.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; González-Rivero, A.F.; Abreu-González, P.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; et al. Traumatic Brain Injury Patients Mortality and Serum Total Antioxidant Capacity. Brain Sci. 2020, 10, 110. https://doi.org/10.3390/brainsci10020110
Lorente L, Martín MM, Pérez-Cejas A, González-Rivero AF, Abreu-González P, Ramos L, Argueso M, Solé-Violán J, Cáceres JJ, Jiménez A, et al. Traumatic Brain Injury Patients Mortality and Serum Total Antioxidant Capacity. Brain Sciences. 2020; 10(2):110. https://doi.org/10.3390/brainsci10020110
Chicago/Turabian StyleLorente, Leonardo, María M. Martín, Antonia Pérez-Cejas, Agustín F. González-Rivero, Pedro Abreu-González, Luis Ramos, Mónica Argueso, Jordi Solé-Violán, Juan J. Cáceres, Alejandro Jiménez, and et al. 2020. "Traumatic Brain Injury Patients Mortality and Serum Total Antioxidant Capacity" Brain Sciences 10, no. 2: 110. https://doi.org/10.3390/brainsci10020110
APA StyleLorente, L., Martín, M. M., Pérez-Cejas, A., González-Rivero, A. F., Abreu-González, P., Ramos, L., Argueso, M., Solé-Violán, J., Cáceres, J. J., Jiménez, A., & García-Marín, V. (2020). Traumatic Brain Injury Patients Mortality and Serum Total Antioxidant Capacity. Brain Sciences, 10(2), 110. https://doi.org/10.3390/brainsci10020110





