Treatment-Resistant Depression in a Real-World Setting: First Interim Analysis of Characteristics, Healthcare Resource Use, and Utility Values of the FondaMental Cohort
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chevance, A.; Gaillard, R. La dépression, du mal-être à la maladie. Bull. Epidémiol Hebd 2018, 32, 636–637. [Google Scholar]
- IsHak, W.W.; Mirocha, J.; James, D.; Tobia, G.; Vilhauer, J.; Fakhry, H.; Pi, S.; Hanson, E.; Nashawati, R.; Peselow, E.D.; et al. Quality of life in major depressive disorder before/after multiple steps of treatment and one-year follow-up. Acta Psychiatr. Scand. 2015, 131, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Nierenberg, A.A.; Husain, M.M.; Trivedi, M.H.; Fava, M.; Warden, D.; Wisniewski, S.R.; Miyahara, S.; Rush, A.J. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: A STAR*D report. Psychol. Med. 2010, 40, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Balestri, M.; Calati, R.; Souery, D.; Kautzky, A.; Kasper, S.; Montgomery, S.; Zohar, J.; Mendlewicz, J.; Serretti, A. Socio-demographic and clinical predictors of treatment resistant depression: A prospective European multicenter study. J. Affect. Disord. 2016, 189, 224–232. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Reps, J.; Fife, D.; Blacketer, C.; Stang, P.; Ryan, P. Finding treatment-resistant depression in real-world data: How a data-driven approach compares with expert-based heuristics. Depress. Anxiety 2018, 35, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Fagiolini, A.; Kupfer, D.J. Is treatment-resistant depression a unique subtype of depression? Biol. Psychiatry 2003, 53, 640–648. [Google Scholar] [CrossRef]
- Trevino, K.; McClintock, S.M.; McDonald Fischer, N.; Vora, A.; Husain, M.M. Defining treatment-resistant depression: A comprehensive review of the literature. Ann. Clin. Psychiatry 2014, 26, 222–232. [Google Scholar]
- Bennabi, D.; Yrondi, A.; Charpeaud, T.; Genty, J.-B.; Destouches, S.; Lancrenon, S.; Allaili, N.; Bellivier, F.; Bougerol, T.; Camus, V.; et al. Clinical guidelines for the management of depression with specific comorbid psychiatric conditions French recommendations from experts (the French Association for Biological Psychiatry and Neuropsychopharmacology and the fondation FondaMental). BMC Psychiatry 2019, 19, 50. [Google Scholar] [CrossRef] [Green Version]
- Charpeaud, T.; Genty, J.-B.; Destouches, S.; Yrondi, A.; Lancrenon, S.; Alaïli, N.; Bellivier, F.; Bennabi, D.; Bougerol, T.; Camus, V.; et al. French Society for Biological Psychiatry and Neuropsychopharmacology and Fondation FondaMental task force: Formal Consensus for the management of treatment-resistant depression. L’Encephale 2017, 43, S1–S24. [Google Scholar] [CrossRef]
- Kautzky, A.; Dold, M.; Bartova, L.; Spies, M.; Kranz, G.S.; Souery, D.; Montgomery, S.; Mendlewicz, J.; Zohar, J.; Fabbri, C.; et al. Clinical factors predicting treatment resistant depression: Affirmative results from the European multicenter study. Acta Psychiatr. Scand. 2019, 139, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ruhé, H.G.; van Rooijen, G.; Spijker, J.; Peeters, F.P.M.L.; Schene, A.H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 2012, 137, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Holtzmann, J.; Richieri, R.; Saba, G.; Allaïli, N.; Bation, R.; Moliere, F.; Nieto, I.; Bellivier, F.; Bennabi, D.; Bubrovszky, M.; et al. How to define treatment-resistant depression? Presse Médicale Paris Fr. 1983 2016, 45, 354–359. [Google Scholar] [CrossRef]
- Bennabi, D.; Aouizerate, B.; El-Hage, W.; Doumy, O.; Moliere, F.; Courtet, P.; Nieto, I.; Bellivier, F.; Bubrovsky, M.; Vaiva, G.; et al. Risk factors for treatment resistance in unipolar depression: A systematic review. J. Affect. Disord. 2015, 171, 137–141. [Google Scholar] [CrossRef]
- Amos, T.B.; Tandon, N.; Lefebvre, P.; Pilon, D.; Kamstra, R.L.; Pivneva, I.; Greenberg, P.E. Direct and Indirect Cost Burden and Change of Employment Status in Treatment-Resistant Depression: A Matched-Cohort Study Using a US Commercial Claims Database. J. Clin. Psychiatry 2018, 79. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, J.I.; Birnbaum, H.G.; Kidolezi, Y.; Subramanian, G.; Khan, S.A.; Stensland, M.D. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr. Med. Res. Opin. 2010, 26, 2475–2484. [Google Scholar] [CrossRef]
- OECD and European Union. Health at a Glance: Europe 2018: State of Health in the EU Cycle; OECD Publishing: Brussels, Belgium, 2018. [Google Scholar]
- Kubitz, N.; Mehra, M.; Potluri, R.C.; Garg, N.; Cossrow, N. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial claims database. PLoS ONE 2013, 8, e76882. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.J.; Grima, E.; Tan, M.; Rotzinger, S.; Lin, P.; Mcintyre, R.S.; Kennedy, S.H. Treatment-resistant depression in primary care across Canada. Can. J. Psychiatry Rev. Can. Psychiatr. 2014, 59, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Aaronson, S.T.; Sears, P.; Ruvuna, F.; Bunker, M.; Conway, C.R.; Dougherty, D.D.; Reimherr, F.W.; Schwartz, T.L.; Zajecka, J.M. A 5-Year Observational Study of Patients with Treatment-Resistant Depression Treated with Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am. J. Psychiatry 2017, 174, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Dunner, D.L.; Rush, A.J.; Russell, J.M.; Burke, M.; Woodard, S.; Wingard, P.; Allen, J. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J. Clin. Psychiatry 2006, 67, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Yrondi, A.; Bennabi, D.; Haffen, E.; Garnier, M.; Bellivier, F.; Bourgerol, T.; Camus, V.; D’Amato, T.; Doumy, O.; Haesebaert, F.; et al. Significant Need for a French Network of Expert Centers Enabling a Better Characterization and Management of Treatment-Resistant Depression (Fondation FondaMental). Front. Psychiatry 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, C.C. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. JAMA 1994, 272, 828–829. [Google Scholar] [CrossRef]
- Thase, M.E.; Rush, A.J. When at first you don’t succeed: Sequential strategies for antidepressant nonresponders. J. Clin. Psychiatry 1997, 58 (Suppl. 13), 23–29. [Google Scholar] [PubMed]
- Snaith, R.P.; Harrop, F.M.; Newby, D.A.; Teale, C. Grade scores of the Montgomery-Asberg Depression and the Clinical Anxiety Scales. Br. J. Psychiatry J. Ment. Sci. 1986, 148, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Freemantle, N.; Geddes, J.R.; Bhagwagar, Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: Systematic review and meta-analysis. Arch. Gen. Psychiatry 2006, 63, 1217–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posner, K.; Brown, G.K.; Stanley, B.; Brent, D.A.; Yershova, K.V.; Oquendo, M.A.; Currier, G.W.; Melvin, G.A.; Greenhill, L.; Shen, S.; et al. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 2011, 168, 1266–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Reenen, M.; Janssen, B. EQ-5D-5L User Guide. 2015. Available online: https://apersu.ca/wp-content/uploads/2020/10/EQ-5D-5L_User-Guide.pdf (accessed on 4 December 2020).
- Charlton, M.; Everson, G.T.; Flamm, S.L.; Kumar, P.; Landis, C.; Brown, R.S.; Fried, M.W.; Terrault, N.A.; O’Leary, J.G.; Vargas, H.E.; et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease. Gastroenterology 2015, 149, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Van Hout, B.; Janssen, M.F.; Feng, Y.-S.; Kohlmann, T.; Busschbach, J.; Golicki, D.; Lloyd, A.; Scalone, L.; Kind, P.; Pickard, A.S. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health J. Int. Soc. Pharm. Outcomes Res. 2012, 15, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, J.; de Pouvourville, G. Valuing EQ-5D using time trade-off in France. Eur. J. Health Econ. Hepac Health Econ. Prev. Care 2013, 14, 57–66. [Google Scholar] [CrossRef]
- Fedgchin, M.; Trivedi, M.; Daly, E.J.; Melkote, R.; Lane, R.; Lim, P.; Vitagliano, D.; Blier, P.; Fava, M.; Liebowitz, M.; et al. Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined with a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1). Int. J. Neuropsychopharmacol. 2019, 22, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined with a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry 2019, 176, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Nierenberg, A.A.; DeCecco, L.M. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: A focus on treatment-resistant depression. J. Clin. Psychiatry 2001, 62 (Suppl. 16), 5–9. [Google Scholar] [PubMed]
- Zimmerman, M.; Posternak, M.A.; Chelminski, I. Derivation of a definition of remission on the Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. J. Psychiatr. Res. 2004, 38, 577–582. [Google Scholar] [CrossRef]
- Haro, J.M.; Lamy, F.-X.; Jönsson, B.; Knapp, M.; Brignone, M.; Caillou, H.; Chalem, Y.; Hammer-Helmich, L.; Rive, B.; Saragoussi, D. Characteristics of patients with depression initiating or switching antidepressant treatment: Baseline analyses of the PERFORM cohort study. BMC Psychiatry 2018, 18, 80. [Google Scholar] [CrossRef]
- Dantchev, N.; Tcherny-Lessenot, S.; Picard, H.; Baraille, L.; Quail, D. Results of the French cohort of the European observational study FINDER: Quality of life of patients treated with antidepressants. L’Encephale 2013, 39, 101–108. [Google Scholar] [CrossRef]
- Cossais, S.; Schwarzinger, M.; Pol, S.; Fontaine, H.; Larrey, D.; Pageaux, G.-P.; Canva, V.; Mathurin, P.; Yazdanpanah, Y.; Deuffic-Burban, S. Quality of life in patients with chronic hepatitis C infection: Severe comorbidities and disease perception matter more than liver-disease stage. PLoS ONE 2019, 14, e0215596. [Google Scholar] [CrossRef]
- Wood, R.; Taylor-Stokes, G.; Smith, F.; Chaib, C. The humanistic burden of advanced non-small cell lung cancer (NSCLC) in Europe: A real-world survey linking patient clinical factors to patient and caregiver burden. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2019, 28, 1849–1861. [Google Scholar] [CrossRef]
- Ara, R.; Brazier, J.E. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health J. Int. Soc. Pharm. Outcomes Res. 2011, 14, 539–545. [Google Scholar] [CrossRef] [Green Version]
| Health State | At Each Visit | For Each Interval between Two Consecutive Visits |
|---|---|---|
| Major depressive episode (MDE) | MADRS score ≥28 at baseline, or ≥22 at a follow-up visit | (1) MDE at one visit and no ≥50% reduction in baseline MADRS score at the following visit OR (2) Any health state at a visit and at least one of the following: - MADRS score ≥22 at following visit - Hospitalisation for depression between visits - Suicide attempt between visits - Antidepressant treatment change between visits - Use of ECT or rTMS after ending antidepressant treatment use ≥28 days between visits |
| Response | ≥50% reduction in baseline MADRS score | Response at one visit and none of the following: - MADRS score ≥22 at following visit - Hospitalisation for depression between visits - Suicide attempt between visits - Antidepressant treatment change between visits - Use of ECT or rTMS after ending antidepressant treatment use ≥28 days between visits |
| Remission | MADRS score ≤10 | Remission at one visit and none of the following: - MADRS score ≥22 at following visit - Hospitalisation for depression between visits - Suicide attempt between visits - Antidepressant treatment change between visits - Use of ECT or rTMS after ending antidepressant treatment use of ≥28 days between visits |
| Recovery | MADRS score ≤10 for two consecutive visits, and none of the following between the two visits: - Hospitalisation for depression - Suicide attempt - Antidepressant treatment change - Use of ECT or rTMS after ending antidepressant treatment use ≥28 days | Recovery at one visit and none of the following: - MADRS score ≥22 at following visit - Hospitalisation for depression between visits - Suicide attempt between visits - Antidepressant treatment change between visits - Use of ECT or rTMS after ending antidepressant treatment use ≥28 days between visits |
| Demographic Characteristics | n Missing | |
|---|---|---|
| Number of patients | 252 | |
| Age in years, mean ± SD (median) | 53.1 ± 13.0 (53.5) | 0 |
| Sex, n (%) | ||
| Female | 159 (63.1%) | 0 |
| Male | 93 (36.9%) | |
| Education, n (%) | 0 | |
| Some secondary education | 95 (37.7%) | |
| Associate degree | 50 (19.8%) | |
| At least some college | 107 (42.5%) | |
| Employment status, n (%) | 23 | |
| Employed | 80 (34.9%) | |
| Unemployed | 44 (19.2%) | |
| Retired | 61 (26.6%) | |
| Student | 4 (1.7%) | |
| Pension | 15 (6.6%) | |
| Stay-at-home | 9 (3.9%) | |
| Other | 16 (7.0%) | |
| Marital status, n (%) | 23 | |
| Single | 46 (20.1%) | |
| Married/Civil Union | 142 (62.0%) | |
| Separated | 14 (6.1%) | |
| Divorced | 24 (10.5%) | |
| Widowed | 3 (1.3%) | |
| Household size, n (%) | 38 | |
| 1 | 66 (30.8%) | |
| 2 | 84 (39.3%) | |
| 3 | 28 (13.1%) | |
| >3 | 36 (16.8%) | |
| Clinical Characteristics | ||
| MADRS, mean ± SD (median) | 29.0 ± 6.9 (29.0) | 8 |
| Disease severity according to MADRS, n (%) | 8 | |
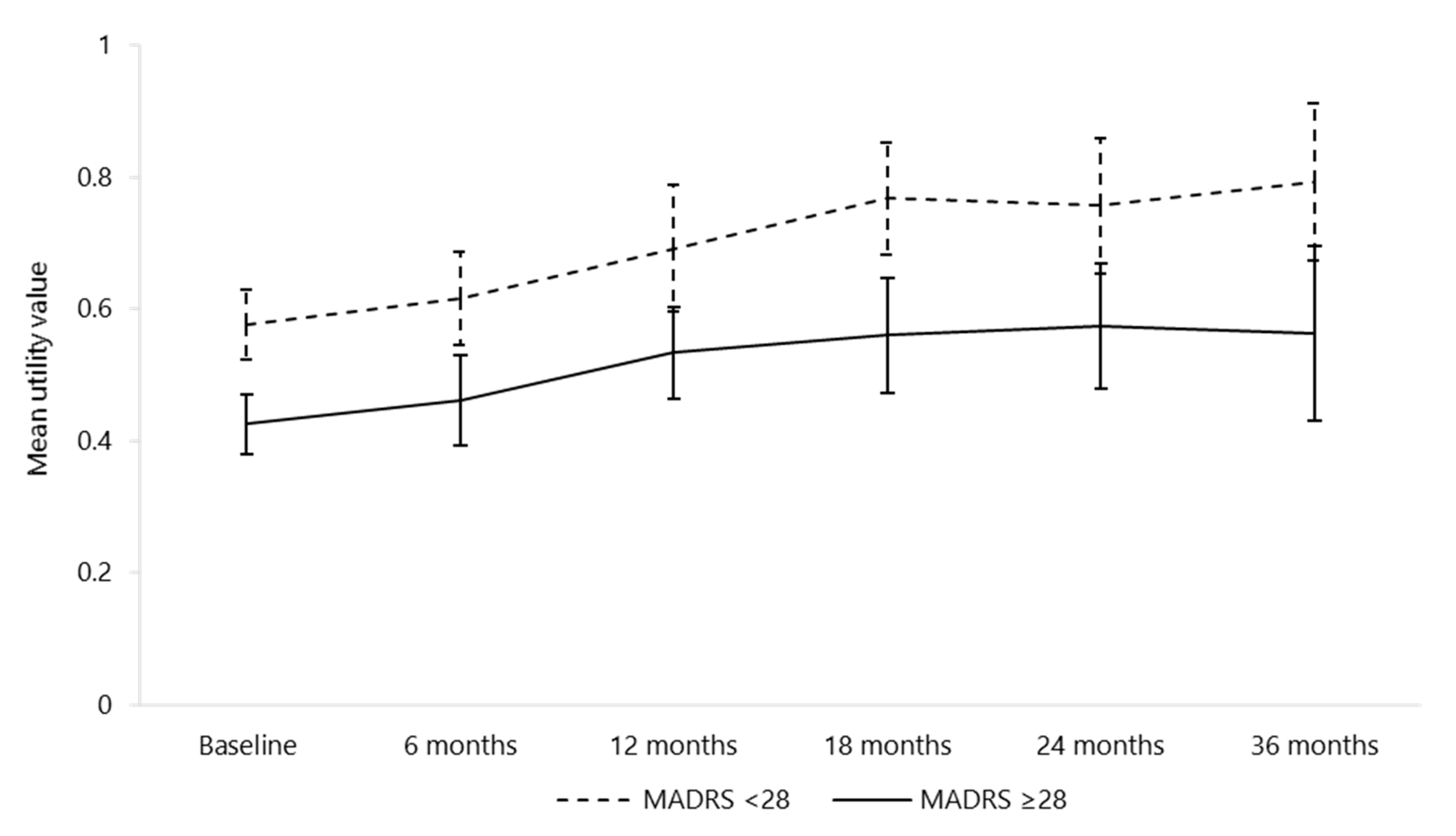
| MADRS <28 | 101 (41.4%) | |
| MADRS ≥28 | 143 (58.6%) | |
| Lifetime suicide severity, n (%) | 0 | |
| Wish to be dead | 168 (66.7%) | |
| Non-specific active suicidal thoughts | 130 (51.6%) | |
| Thoughts about the way in which to commit suicide | 103 (40.9%) | |
| Suicidal thoughts with intention to follow-through | 59 (23.4%) | |
| Construction of detailed scenario on how to commit suicide | 44 (17.5%) | |
| Attempted suicide | 65 (25.8%) | |
| Number of suicide attempts, mean ± SD (median) | ||
| Entire cohort | 0.5 ± 1.2 (0.0) | |
| Patients that attempted suicide at least once | 1.9 ± 1.7 (1.0) |
| Treatment Class/Treatment | n (%) | n Missing |
|---|---|---|
| Antidepressants | ||
| Treated at baseline or during follow-up | 185 (83.0%) | 29 * |
| Treated at baseline only (among treated patients) | 97 (64.7%) | 35 |
| First treatment recorded in the cohort | ||
| Serotonin–norepinephrine reuptake inhibitors (SNRIs) | 54 (29.2%) | |
| Venlafaxine | 44 (23.8%) | |
| Duloxetine | 10 (5.4%) | |
| Tricyclic antidepressants (TCA) | 49 (26.5%) | |
| Clomipramine | 40 (21.6%) | |
| Amitriptyline | 6 (3.2%) | |
| Maprotiline | 2 (1.1%) | |
| Imipramine | 1 (0.5%) | |
| Selective serotonin reuptake inhibitors (SSRIs) | 45 (24.3%) | |
| Fluoxetine | 20 (10.8%) | |
| Sertraline | 12 (6.5%) | |
| Paroxetine | 10 (5.4%) | |
| Escitalopram | 3 (1.6%) | |
| Alpha-2 antagonists | 24 (13.0%) | |
| Mirtazapine | 18 (9.7%) | |
| Mianserine | 6 (3.2%) | |
| Reversible inhibitor of monoamine oxidase A (RIMA) | 6 (3.2%) | |
| Moclobemide | 6 (3.2%) | |
| Non-selective, irreversible monoamine oxidase inhibitor (MAOI) | 3 (1.6%) | |
| Iproniazid | 3 (1.6%) | |
| Other | 4 (2.2%) | |
| Agomelatine | 4 (2.2%) | |
| Electroconvulsive therapy (ECT)/repetitive transcranial magnetic stimulation (rTMS) | ||
| Treated at baseline | ||
| Overall | 19 (9.2%)/8 (3.8%) | 46/44 |
| Patients with no antidepressant at baseline (n = 91) | 4 (4.7%)/2 (2.4%) | 6/7 |
| Treated at baseline or during follow-up | ||
| Overall | 58 (23.0%)/18 (7.1%) | 0 |
| Patients with no antidepressant at baseline or follow-up (n = 38) | 10 (26.3%)/3 (7.9%) | 0 |
| Healthcare Resource Use | MDE (n = 278) | Response (n = 22) | Remission (n = 38) | Recovery (n = 19) |
|---|---|---|---|---|
| Hospitalisations (all) | ||||
| Number of hospitalisations, mean (SD) | 1.1 (3.2) | 0.1 (0.2) | 0 (0.0) | 0 (0.0) |
| Patients with at least one hospitalisation, n (%) | 106 (38.1) | 1 (4.6) | 0 (0.0) | 0 (0.0) |
| Hospitalisations for depression | ||||
| Number of hospitalisations, mean (SD) | 0.9 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Patients with at least one hospitalisation, n (%) | 89 (32.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hospitalisations outside depression | ||||
| Number of hospitalisations, mean (SD) | 0.2 (0.6) | 0.1 (0.2) | 0 (0.0) | 0 (0.0) |
| Patients with at least one hospitalisation, n (%) | 32 (11.5) | 1 (4.6) | 0 (0.0) | 0 (0.0) |
| At least one ER visit, n (%) | 20 (7.2) | 1 (4.6) | 2 (5.3) | 2 (10.5) |
| At least one ambulance ride, n (%) | 9 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
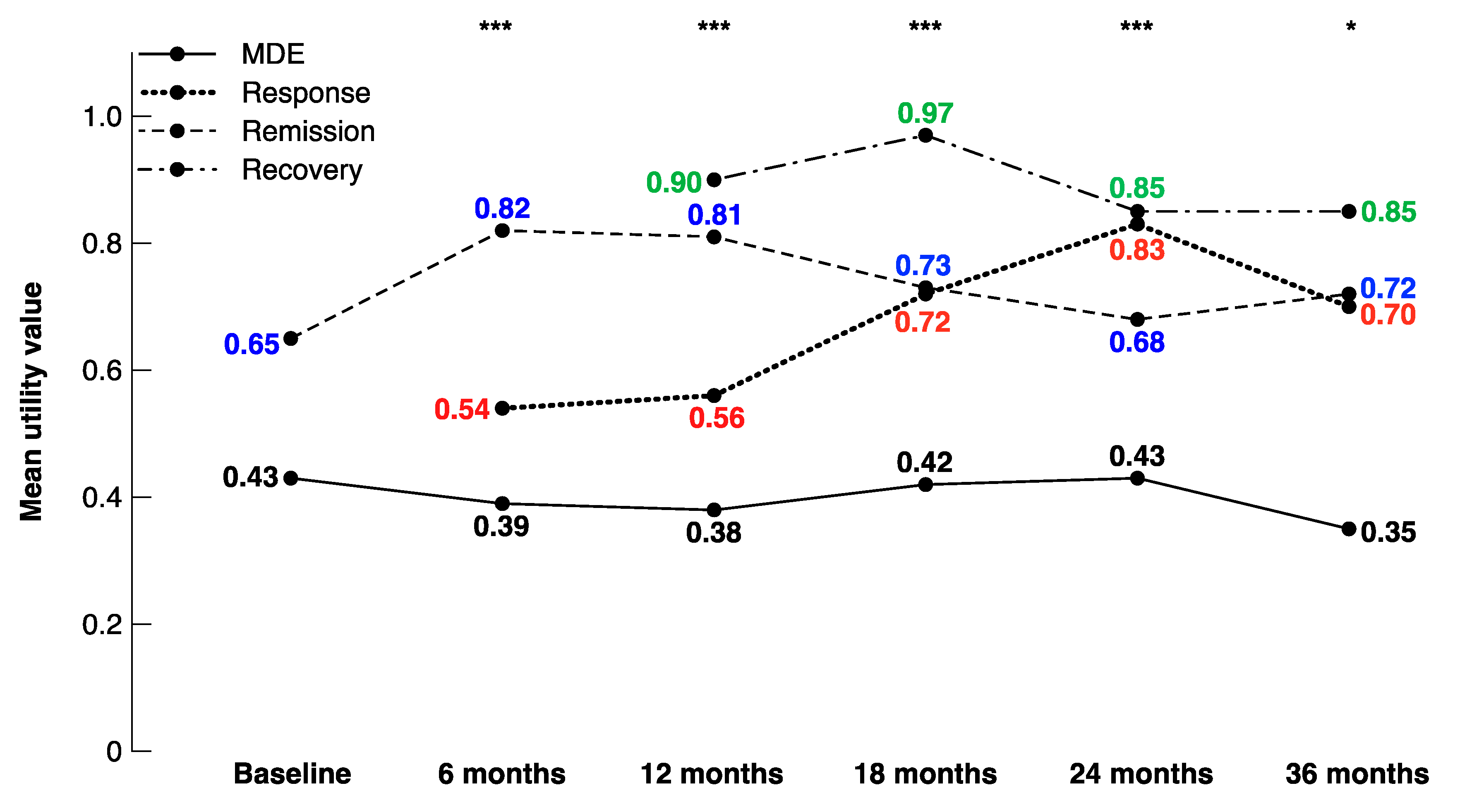
| Utility values | MDE (n = 341) | Response (n = 49) | Remission (n = 91) | Recovery (n = 40) |
| Mean (SD) | 0.41 (0.29) | 0.63 (0.24) | 0.80 (0.21) | 0.90 (0.15) |
| MDE | Response | Remission | Recovery | |
|---|---|---|---|---|
| Baseline | 143 | 0 | 9 | 0 |
| 6 months | 86 | 7 | 32 | 0 |
| 12 months | 49 | 14 | 28 | 9 |
| 18 months | 30 | 11 | 18 | 10 |
| 24 months | 25 | 7 | 17 | 18 |
| 36 months | 9 | 7 | 7 | 10 |
| 48 months | 2 | 3 | 4 | 2 |
| No ECT (n = 203) | ECT (n = 49) | p-Value | |
|---|---|---|---|
| Patient characteristics at baseline | |||
| Age in years, mean ± SD (median) | 52.7 ± 13.2 (53.1) | 54.9 ± 12.4 (55.7) | 0.290 |
| Female, n (%) | 132 (65.0%) | 27 (55.1%) | 0.196 |
| Disease duration (years), mean ± SD (median) | 18.7 ± 12.5 (17.6) | 16.1 ± 12.0 (17.2) | 0.304 |
| Chronic episode (>2 years), n (%) | 66 (62.3%) | 14 (46.7%) | 0.125 |
| Number of MDE resistant to ≥2 treatments, mean ± SD (median) | 1.3 ± 1.0 (1.0) | 1.2 ± 0.7 (1.0) | 0.655 |
| Number of MDE with psychotic characteristics, mean ± SD (median) | 0.1 ± 0.5 (0.0) | 0.2 ± 0.4 (0.0) | 0.585 |
| MADRS, mean ± SD (median) | 28.4 ± 6.9 (28) | 31.4 ± 6.8 (32) | 0.007 |
| Baseline MADRS ≥28, n (%) | 107 (52.7%) | 36 (73.5%) | 0.009 |
| Results | |||
| Number of hospitalisations for depression, mean ± SD (median) | |||
| Between baseline and 6 months | 0.4 ± 1.4 (0) | 2.8 ± 6.5 (1) | <0.001 |
| Between 6 months and 1 year | 0.4 ± 1.9 (0) | 1.2 ± 1.8 (1) | 0.030 |
| At least one hospitalisation for depression, n (%) | |||
| Between baseline and 6 months | 24 (21.2%) | 34 (69.4%) | <0.001 |
| Between 6 months and 1 year | 11 (13.4%) | 24 (58.5%) | <0.001 |
| Number of hospitalisations outside depression, mean ± SD (median) | |||
| Between baseline and 6 months | 0.1 ± 0.3 (0) | 0.2 ± 0.5 (0) | 0.207 |
| Between 6 months and 1 year | 0.1 ± 0.4 (0) | 0.2 ± 0.6 (0) | 0.698 |
| At least one hospitalisation outside depression, n (%) | |||
| Between baseline and 6 months | 16 (14.2%) | 5 (10.2%) | 0.491 |
| Between 6 months and 1 year | 8 (9.8%) | 4 (9.8%) | 0.999 |
| At least one ER visit during first year of follow-up, n (%) | 8 (9.8%) | 9 (22.0%) | 0.065 |
| At least one ambulance ride during first year of follow-up, n (%) | 4 (4.9%) | 4 (9.8%) | 0.301 |
| Utility values, mean ± SD (median) | |||
| Baseline | 0.49 ± 0.26 (0.51) | 0.48 ± 0.33 (0.53) | 0.799 |
| 6 months | 0.51 ± 0.33 (0.53) | 0.55 ± 0.31 (0.58) | 0.416 |
| 1 year | 0.59 ± 0.32 (0.67) | 0.60 ± 0.30 (0.56) | 0.876 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yrondi, A.; Bennabi, D.; Haffen, E.; Quelard, D.; Samalin, L.; Maruani, J.; Allauze, E.; Pierre, D.; Bougerol, T.; Camus, V.; et al. Treatment-Resistant Depression in a Real-World Setting: First Interim Analysis of Characteristics, Healthcare Resource Use, and Utility Values of the FondaMental Cohort. Brain Sci. 2020, 10, 962. https://doi.org/10.3390/brainsci10120962
Yrondi A, Bennabi D, Haffen E, Quelard D, Samalin L, Maruani J, Allauze E, Pierre D, Bougerol T, Camus V, et al. Treatment-Resistant Depression in a Real-World Setting: First Interim Analysis of Characteristics, Healthcare Resource Use, and Utility Values of the FondaMental Cohort. Brain Sciences. 2020; 10(12):962. https://doi.org/10.3390/brainsci10120962
Chicago/Turabian StyleYrondi, Antoine, Djamila Bennabi, Emmanuel Haffen, Delphine Quelard, Ludovic Samalin, Julia Maruani, Etienne Allauze, Damien Pierre, Thierry Bougerol, Vincent Camus, and et al. 2020. "Treatment-Resistant Depression in a Real-World Setting: First Interim Analysis of Characteristics, Healthcare Resource Use, and Utility Values of the FondaMental Cohort" Brain Sciences 10, no. 12: 962. https://doi.org/10.3390/brainsci10120962
APA StyleYrondi, A., Bennabi, D., Haffen, E., Quelard, D., Samalin, L., Maruani, J., Allauze, E., Pierre, D., Bougerol, T., Camus, V., D’Amato, T., Doumy, O., Holtzmann, J., Lançon, C., Moliere, F., Moirand, R., Nieto, I., Richieri, R. M., Horn, M., ... Aouizerate, B. (2020). Treatment-Resistant Depression in a Real-World Setting: First Interim Analysis of Characteristics, Healthcare Resource Use, and Utility Values of the FondaMental Cohort. Brain Sciences, 10(12), 962. https://doi.org/10.3390/brainsci10120962







