A Review and a Framework of Variables for Defining and Characterizing Tinnitus Subphenotypes
Abstract
1. Introduction
2. Materials and Methods
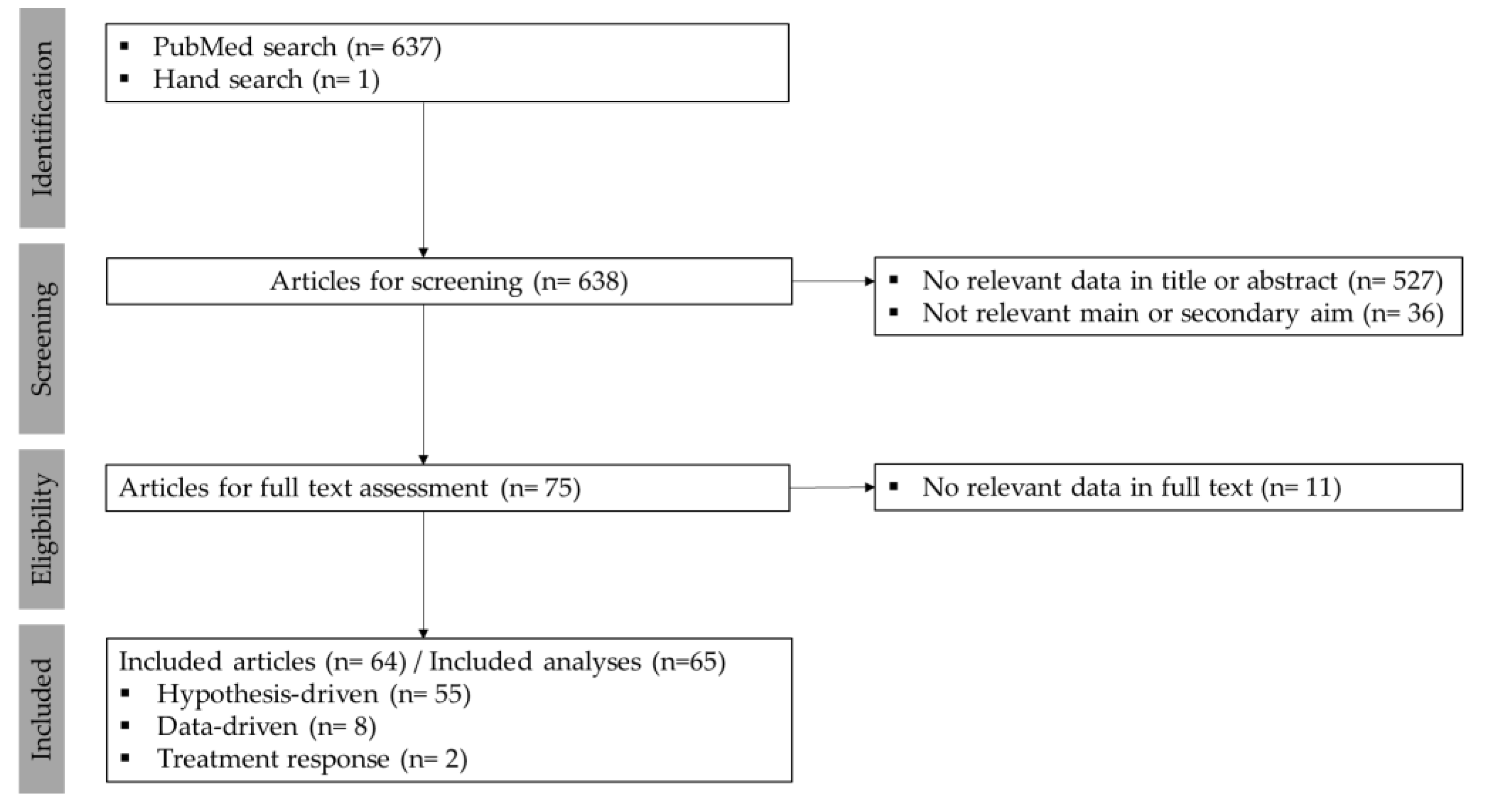
2.1. Search Strategy and Screening Process
2.2. Data Extraction and Synthesis
- Hypothesis-driven: researchers predefined tinnitus subgroups based on one or more variables (hypothesizing that the chosen variables can define phenotypically distinct subgroups) and subsequently compared them against other characteristics. Studies assessing a treatment outcome that compared treatment response across predefined tinnitus subgroups, were also classified as hypothesis-driven.
- Data-driven: analyses used unsupervised learning for defining tinnitus subgroups. The details of the statistical methods used and the identified subgroups were also extracted and charted.
- Treatment response: analyses based tinnitus subgroup definition on the response to a tinnitus treatment (e.g., responders versus non-responders).
3. Results
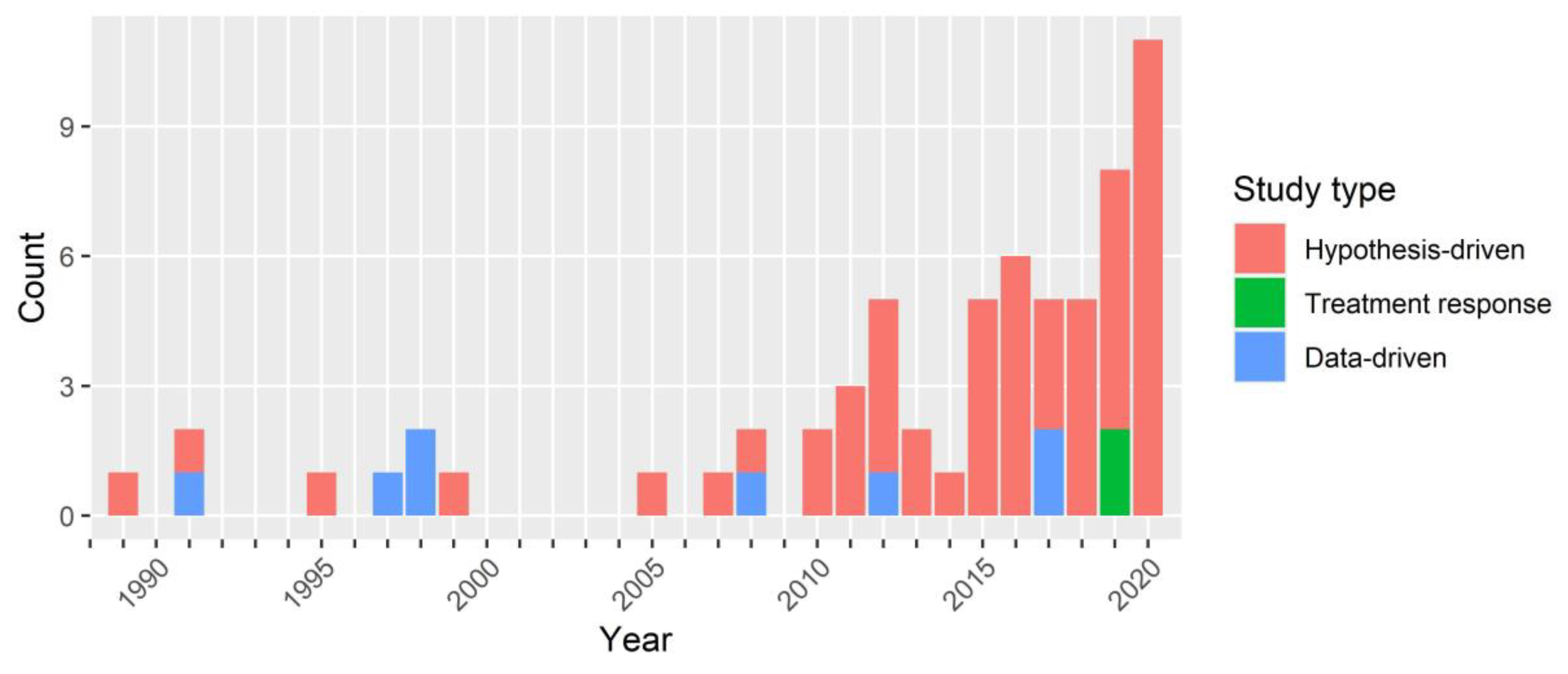
3.1. Included Studies: Overview and Methods
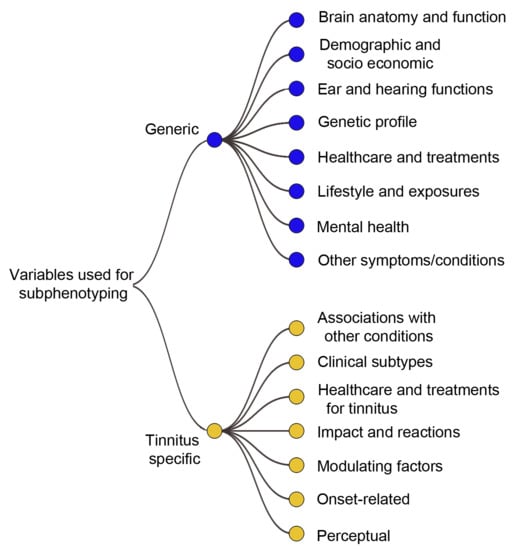
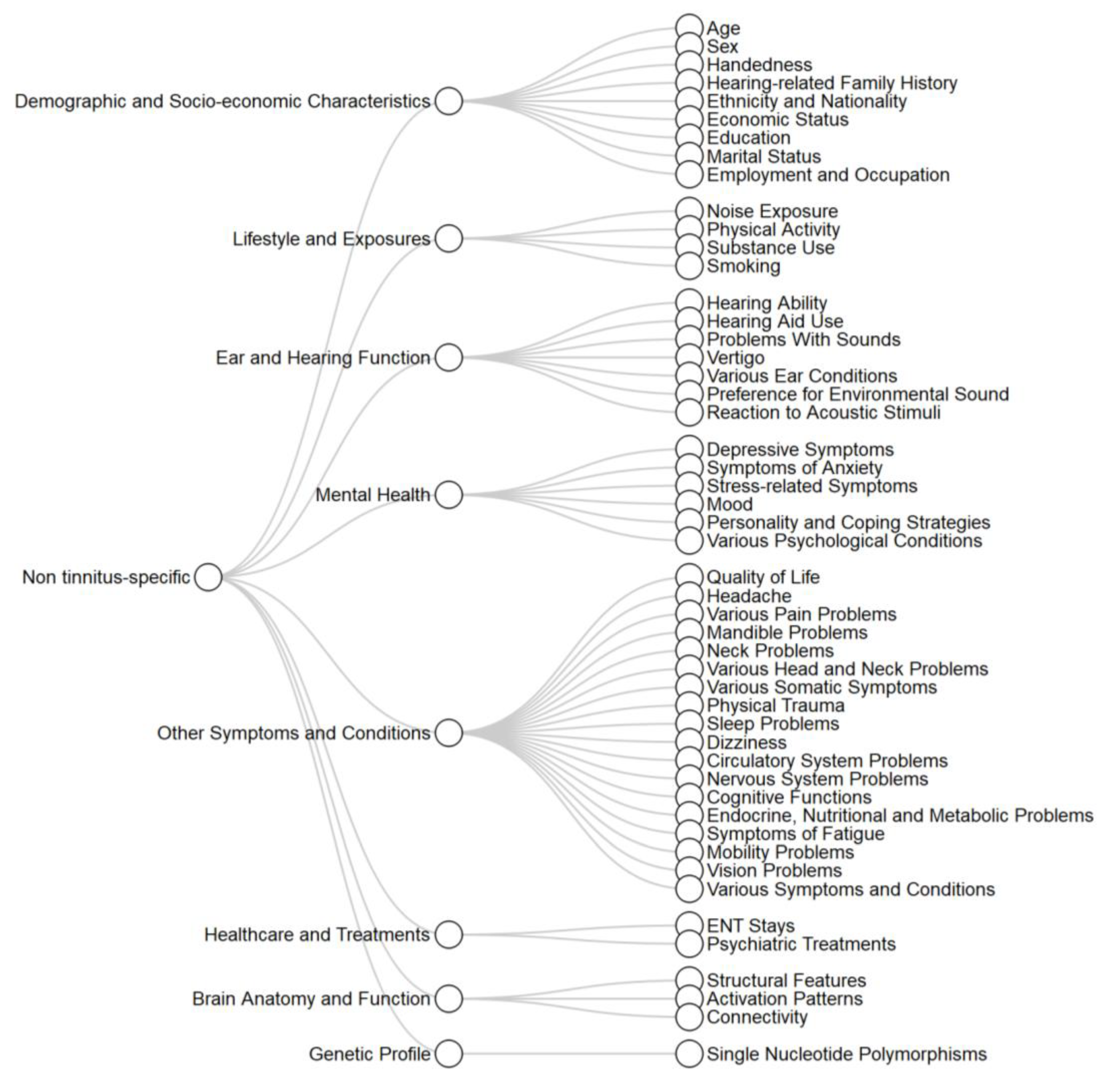
3.2. Conceptual Framework for Categorizing Variables
4. Discussion
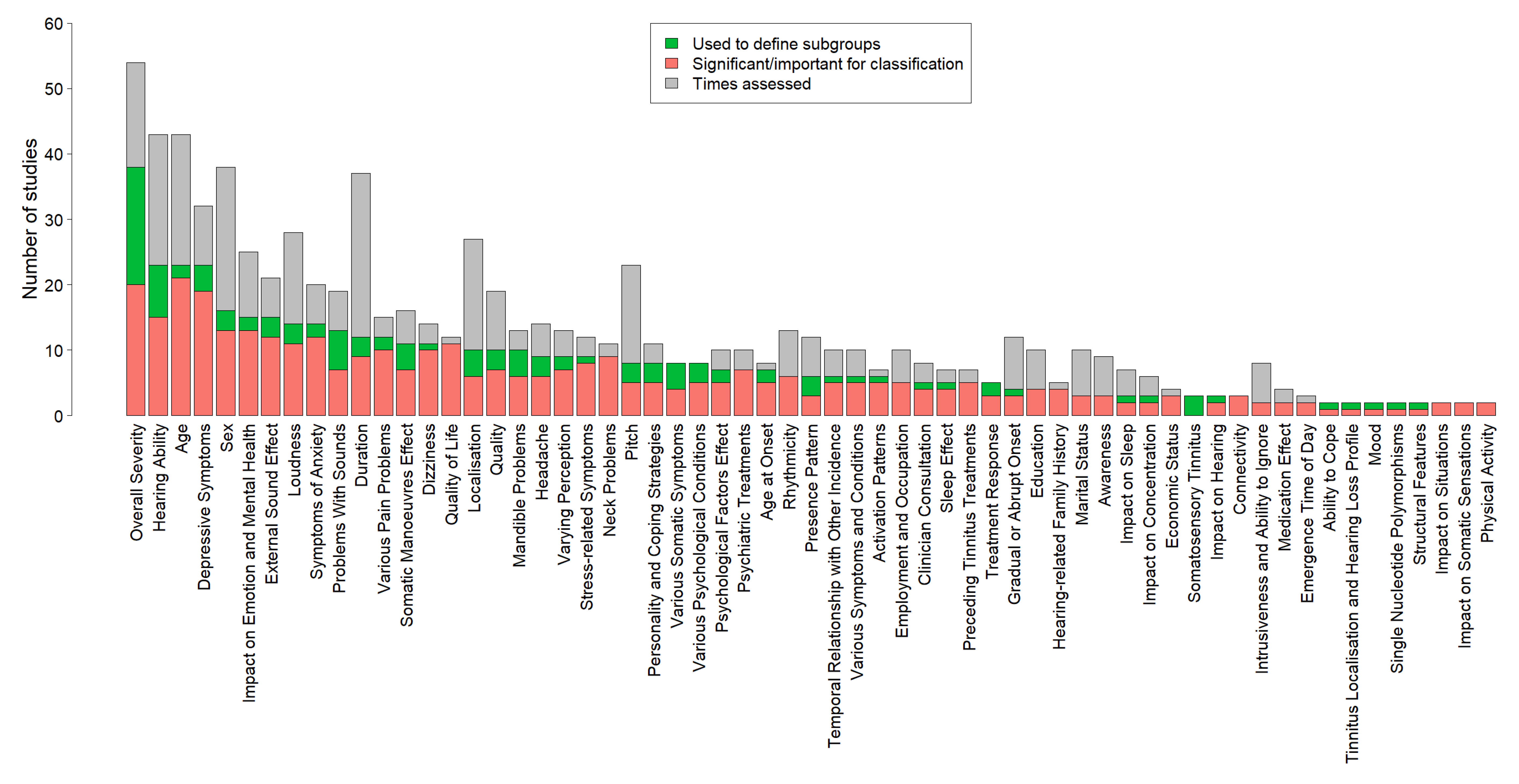
4.1. Overview of Results
4.2. Variables for Tinnitus Phenotyping
4.3. Methodological Considerations
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016, 337, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, C.E.; Wesdorp, F.; van Zanten, G. Socio-Demographic, Health, and Tinnitus Related Variables Affecting Tinnitus Severity. Ear Hear. 2014, 35, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Gallus, S.; Hall, D.A.; Kleinjung, T.; Langguth, B.; Maruotti, A.; Meyer, M.; Norena, A.; Probst, T.; Pryss, R. Towards an understanding of tinnitus heterogeneity. Front. Aging Neurosci. 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; McMahon, C.M.; Rochtchina, E.; Karpa, M.J.; Mitchell, P. Risk factors and impacts of incident tinnitus in older adults. Ann. Epidemiol. 2010, 20, 129–135. [Google Scholar] [CrossRef]
- Nondahl, D.M.; Cruickshanks, K.J.; Wiley, T.L.; Klein, R.; Klein, B.E.; Tweed, T.S. Prevalence and 5-year incidence of tinnitus among older adults: The epidemiology of hearing loss study. J. Am. Acad. Audiol. 2002, 13, 323–331. [Google Scholar] [CrossRef]
- Habtewold, T.D.; Rodijk, L.H.; Liemburg, E.J.; Sidorenkov, G.; Boezen, H.M.; Bruggeman, R.; Alizadeh, B.Z. A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Transl. Psychiatry 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Lötvall, J.; Akdis, C.A.; Bacharier, L.B.; Bjermer, L.; Casale, T.B.; Custovic, A.; Lemanske, R.F.; Wardlaw, A.J.; Wenzel, S.E.; Greenberger, P.A. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011, 127, 355–360. [Google Scholar] [CrossRef]
- Ruscio, J.; Ruscio, A.M. Clarifying boundary issues in psychopathology: The role of taxometrics in a comprehensive program of structural research. J. Abnorm. Psychol. 2004, 113, 24. [Google Scholar] [CrossRef]
- Saria, S.; Goldenberg, A. Subtyping: What it is and its role in precision medicine. IEEE Intell. Syst. 2015, 30, 70–75. [Google Scholar] [CrossRef]
- Cianfrone, G.; Mazzei, F.; Salviati, M.; Turchetta, R.; Orlando, M.P.; Testugini, V.; Carchiolo, L.; Cianfrone, F.; Altissimi, G. Tinnitus Holistic Simplified Classification (THoSC) A New Assessment for Subjective Tinnitus, With Diagnostic and Therapeutic Implications. Ann. Otol. Rhinol. Laryngol. 2015, 124, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Vijendren, A.; Phillips, J. Objective measures of tinnitus: A systematic review. Otol. Neurotol. 2019, 40, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Kleinjung, T.; Landgrebe, M. Tinnitus: The complexity of standardization. Eval. Health Prof. 2011, 34, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Nordberg, A.; Westman, E. Biological subtypes of Alzheimer disease: A systematic review and meta-analysis. Neurology 2020, 94, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Beijers, L.; Wardenaar, K.J.; van Loo, H.M.; Schoevers, R.A. Data-driven biological subtypes of depression: Systematic review of biological approaches to depression subtyping. Mol. Psychiatry 2019, 24, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Green, M.J.; Girshkin, L.; Kremerskothen, K.; Watkeys, O.; Quidé, Y. A systematic review of studies reporting data-driven cognitive subtypes across the psychosis spectrum. Neuropsychol. Rev. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Feczko, E.; Miranda-Dominguez, O.; Marr, M.; Graham, A.M.; Nigg, J.T.; Fair, D.A. The heterogeneity problem: Approaches to identify psychiatric subtypes. Trends Cogn. Sci. 2019, 23, 584–601. [Google Scholar] [CrossRef]
- Pina, A.; Macedo, M.P.; Henriques, R. Clustering Clinical Data in R. In Mass Spectrometry Data Analysis in Proteomics; Humana: New York, NY, USA, 2020; pp. 309–343. [Google Scholar]
- Pierre-Jean, M.; Deleuze, J.-F.; Le Floch, E.; Mauger, F. Clustering and variable selection evaluation of 13 unsupervised methods for multi-omics data integration. Brief. Bioinform. 2019. [Google Scholar] [CrossRef]
- Handl, J.; Knowles, J.; Kell, D.B. Computational cluster validation in post-genomic data analysis. Bioinformatics 2005, 21, 3201–3212. [Google Scholar] [CrossRef]
- Szmulewicz, A.; Millett, C.; Shanahan, M.; Gunning, F.; Burdick, K. Emotional processing subtypes in bipolar disorder: A cluster analysis. J. Affect. Disord. 2020, 266, 194–200. [Google Scholar] [CrossRef]
- Kreuzer, P.M.; Landgrebe, M.; Schecklmann, M.; Staudinger, S.; Langguth, B. The TRI Database Study Group. Trauma-associated tinnitus: Audiological, demographic and clinical characteristics. PLoS ONE 2012, 7, e45599. [Google Scholar] [CrossRef] [PubMed]
- Vielsmeier, V.; Strutz, J.; Kleinjung, T.; Schecklmann, M.; Kreuzer, P.M.; Landgrebe, M. Temporomandibular joint disorder complaints in tinnitus: Further hints for a putative tinnitus subtype. PLoS ONE 2012, 7, e38887. [Google Scholar] [CrossRef]
- Schecklmann, M.; Landgrebe, M.; Langguth, B.; The TRI Database Study Group. Phenotypic characteristics of hyperacusis in tinnitus. PLoS ONE 2014, 9, e86944. [Google Scholar] [CrossRef] [PubMed]
- Vielsmeier, V.; Lehner, A.; Strutz, J.; Steffens, T.; Kreuzer, P.M.; Schecklmann, M.; Landgrebe, M.; Langguth, B.; Kleinjung, T. The Relevance of the High Frequency Audiometry in Tinnitus Patients with Normal Hearing in Conventional Pure-Tone Audiometry. BioMed Res. Int. 2015, 2015, 302515. [Google Scholar] [CrossRef] [PubMed]
- Probst, T.; Pryss, R.C.; Langguth, B.; Spiliopoulou, M.; Landgrebe, M.; Vesala, M.; Harrison, S.; Schobel, J.; Reichert, M.; Stach, M. Outpatient Tinnitus Clinic, Self-Help Web Platform, or Mobile Application to Recruit Tinnitus Study Samples? Front. Aging Neurosci. 2017, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Landgrebe, M.; Schlee, W.; Schecklmann, M.; Vielsmeier, V.; Steffens, T.; Staudinger, S.; Frick, H.; Frick, U. Different Patterns of Hearing Loss among Tinnitus Patients: A Latent Class Analysis of a Large Sample. Front. Neurol. 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Koops, E.A.; Husain, F.T.; van Dijk, P. Profiling intermittent tinnitus: A retrospective review. Int. J. Audiol. 2019, 58, 1–7. [Google Scholar] [CrossRef]
- Niemann, U.; Boecking, B.; Brueggemann, P.; Mebus, W.; Mazurek, B.; Spiliopoulou, M. Tinnitus-related distress after multimodal treatment can be characterized using a key subset of baseline variables. PLoS ONE 2020, 15, e0228037. [Google Scholar] [CrossRef]
- Niemann, U.; Boecking, B.; Brueggemann, P.; Mazurek, B.; Spiliopoulou, M. Gender-Specific Differences in Patients with Chronic Tinnitus—Baseline Characteristics and Treatment Effects. Front. Neurol. 2020, 14, 487. [Google Scholar] [CrossRef]
- Niemann, U.; Brueggemann, P.; Boecking, B.; Mazurek, B.; Spiliopoulou, M. Development and internal validation of a depression severity prediction model for tinnitus patients based on questionnaire responses and socio-demographics. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 1992, 67, 227. [Google Scholar]
- Genitsaridi, E.; Partyka, M.; Gallus, S.; Lopez-Escamez, J.A.; Schecklmann, M.; Mielczarek, M.; Trpchevska, N.; Santacruz, J.L.; Schoisswohl, S.; Riha, C.; et al. Standardised profiling for tinnitus research: The European School for Interdisciplinary Tinnitus Research Screening Questionnaire (ESIT-SQ). Hear Res. 2019, 377, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Fackrell, K.; Li, A.B.; Thavayogan, R.; Smith, S.; Kennedy, V.; Tinoco, C.; Rodrigues, E.D.; Campelo, P.; Martins, T.D. A narrative synthesis of research evidence for tinnitus-related complaints as reported by patients and their significant others. Health Qual. Life Outcomes 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Khan, A. Interactive Collapsible Tree Diagrams Using ‘D3.js’. R Package Version 0.1.7. 2018. Available online: https://cran.r-project.org/web/packages/collapsibleTree/index.html (accessed on 27 November 2020).
- Gohel, D. Functions for Tabular Reporting. R Package Version 0.5.11. 2020. Available online: https://CRAN.R-project.org/package=flextable (accessed on 27 November 2020).
- Al-Swiahb, J.; Park, S.N. Characterization of tinnitus in different age groups: A retrospective review. Noise Health 2016, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Lyttkens, L.; Larsen, H. Distinguishing levels of tinnitus distress. Clin. Otolaryngol. Allied Sci. 1999, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Attias, J.; Shemesh, Z.; Bleich, A.; Solomon, Z.; Bar-Or, G.; Alster, J.; Sohmer, H. Psychological profile of help-seeking and non-help-seeking tinnitus patients. Scand. Audiol. 1995, 24, 13–18. [Google Scholar] [CrossRef]
- Bartels, H.; Middel, B.L.; van der Laan, B.F.; Staal, M.J.; Albers, F.W. The additive effect of co-occurring anxiety and depression on health status, quality of life and coping strategies in help-seeking tinnitus sufferers. Ear Hear. 2008, 29, 947–956. [Google Scholar] [CrossRef]
- Bartels, H.; Pedersen, S.; van der Laan, B.; Staal, M.; Albers, F.; Middel, B. The impact of type D personality on health-related quality of life in tinnitus patients is mainly mediated by anxiety and depression. Otol. Neurotol. 2010, 31, 11–18. [Google Scholar] [CrossRef]
- Boecking, B.; von Sass, J.; Sieveking, A.; Schaefer, C.; Brueggemann, P.; Rose, M.; Mazurek, B. Tinnitus-related distress and pain perceptions in patients with chronic tinnitus–Do psychological factors constitute a link? PLoS ONE 2020, 15, e0234807. [Google Scholar]
- Burkart, M.; Brueggemann, P.; Szczepek, A.; Frank, D.; Mazurek, B. Intermittent tinnitus—An empirical description. HNO 2019, 67, 51–58. [Google Scholar]
- Carpenter-Thompson, J.R.; Schmidt, S.; McAuley, E.; Husain, F.T. Increased frontal response may underlie decreased tinnitus severity. PLoS ONE 2015, 10, e0144419. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Lugo, A.; Edvall, N.K.; Lazar, A.; Lopez-Escamez, J.-A.; Bulla, J.; Uhlen, I.; Hoare, D.J.; Baguley, D.M.; Canlon, B. Association between Hyperacusis and Tinnitus. J. Clin. Med. 2020, 9, 2412. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Congedo, M. The distressed brain: A group blind source separation analysis on tinnitus. PLoS ONE 2011, 6, e24273. [Google Scholar] [CrossRef] [PubMed]
- Edvall, N.K.; Gunan, E.; Genitsaridi, E.; Lazar, A.; Mehraei, G.; Billing, M.; Tullberg, M.; Bulla, J.; Whitton, J.; Canlon, B. Impact of temporomandibular joint complaints on tinnitus-related distress. Front. Neurol. 2019, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, S.I.; Rubinstein, B.; Axelsson, A.; Carlsson, S.G. Psychological dimensions in patients with disabling tinnitus and craniomandibular disorders. Br. J. Audiol. 1991, 25, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Park, J.; Park, S.; Vidal, J.; Ashaikh, H.; Kim, D.; Park, S. Typewriter tinnitus: An investigative comparison with middle ear myoclonic tinnitus and its long-term therapeutic response to carbamazepine. Auris Nasus Larynx 2020, 47, 580–586. [Google Scholar] [CrossRef]
- Heijneman, K.M.; De Kleine, E.; Wiersinga-Post, E.; Van Dijk, P. Can the tinnitus spectrum identify tinnitus subgroups? Noise Health 2013, 15, 101. [Google Scholar] [PubMed]
- Hiller, W.; Goebel, G. When tinnitus loudness and annoyance are discrepant: Audiological characteristics and psychological profile. Audiol. Neurotol. 2007, 12, 391–400. [Google Scholar] [CrossRef]
- Holgers, K.-M.; Zöger, S.; Svedlund, K. Predictive factors for development of severe tinnitus suffering-further characterisation: Factores predictivos para el desarrollo de tinitus severo que sufren una caracterización adicional. Int. J. Audiol. 2005, 44, 584–592. [Google Scholar]
- Jeon, H.W.; Kim, S.Y.; Choi, B.S.; Bae, Y.J.; Koo, J.-W.; Song, J.-J. Pseudo-low frequency hearing loss and its improvement after treatment may be objective signs of significant vascular pathology in patients with pulsatile tinnitus. Otol. Neurotol. 2016, 37, 1344–1349. [Google Scholar]
- Kim, S.I.; Kim, M.G.; Kim, S.S.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Evaluation of tinnitus patients by audiometric configuration. Am. J. Otolaryngol. 2016, 37, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, C.A.; Blanchard, E.B.; Parnes, S.M. Psychological characteristics of individuals high and low in their ability to cope with tinnitus. Psychosom. Med. 1989, 51, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Kanzaki, S.; Oishi, N.; Ogawa, K. Clinical characteristics of patients with tinnitus evaluated with the Tinnitus Sample Case History Questionnaire in Japan: A case series. PLoS ONE 2017, 12, e0180609. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Hund, V.; Landgrebe, M.; Schecklmann, M. Tinnitus Patients with Comorbid Headaches: The Influence of Headache Type and Laterality on Tinnitus Characteristics. Front. Neurol. 2017, 8, 440. [Google Scholar] [CrossRef]
- Lindblad, A.-C.; Hagerman, B.; Rosenhall, U. Noise-induced tinnitus: A comparison between four clinical groups without apparent hearing loss. Noise Health 2011, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.; Edvall, N.K.; Lazar, A.; Mehraei, G.; Lopez-Escamez, J.-A.; Bulla, J.; Uhlen, I.; Canlon, B.; Gallus, S.; Cederroth, C.R. Relationship between headaches and tinnitus in a Swedish study. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Michiels, S.; De Hertogh, W.; Truijen, S.; Van de Heyning, P. Cervical spine dysfunctions in patients with chronic subjective tinnitus. Otol. Neurotol. 2015, 36, 741–745. [Google Scholar] [CrossRef]
- Michiels, S.; Harrison, S.; Vesala, M.; Schlee, W. The Presence of Physical Symptoms in Patients with Tinnitus: International Web-Based Survey. Interact. J. Med. Res. 2019, 8, e14519. [Google Scholar] [CrossRef]
- Milner, R.; Lewandowska, M.; Ganc, M.; Nikadon, J.; Niedziałek, I.; Jędrzejczak, W.W.; Skarżyński, H. Electrophysiological correlates of focused attention on low-and high-distressed tinnitus. PLoS ONE 2020, 15, e0236521. [Google Scholar] [CrossRef]
- Mores, J.T.; Bozza, A.; Magni, C.; Casali, R.L.; do Amaral, M.I.R. Clinical profile and implications of tinnitus in individuals with and without hearing loss. CEP 2019, 13083, 887. [Google Scholar]
- Moring, J.; Bowen, A.; Thomas, J.; Bira, L. The emotional and functional impact of the type of tinnitus sensation. J. Clin. Psychol. Med. Settings 2016, 23, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, R.A.; Bakker, I.; Elvers, H.; Mikolajewska, E.; Michiels, S.; De Hertogh, W.; Samwel, H. Cervicogenic somatosensory tinnitus: An indication for manual therapy plus education? Part 2: A pilot study. Man. Ther. 2016, 23, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.T.; van den Berge, M.J.; Free, R.H.; van der Vliet, A.M.; Knoppel, H.; van Dijk, P.; Hofman, R. The Relation between Tinnitus and a Neurovascular Conflict of the Cochleovestibular Nerve on Magnetic Resonance Imaging. Otol. Neurotol. 2020, 41, e124–e131. [Google Scholar] [CrossRef]
- Ralli, M.; Altissimi, G.; Turchetta, R.; Mazzei, F.; Salviati, M.; Cianfrone, F.; Orlando, M.P.; Testugini, V. Somatosensory tinnitus: Correlation between cranio-cervico-mandibular disorder history and somatic modulation. Audiol. Neurotol. 2016, 21, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Greco, A.; Boccassini, A.; Altissimi, G.; Di Paolo, C.; Falasca, V.; De Virgilio, A.; Polimeni, A.; Cianfrone, G.; de Vincentiis, M. Subtyping patients with somatic tinnitus: Modulation of tinnitus and history for somatic dysfunction help identify tinnitus patients with temporomandibular joint disorders. PLoS ONE 2018, 13, e0202050. [Google Scholar] [CrossRef] [PubMed]
- Sand, P.G.; Langguth, B.; Schecklmann, M.; Kleinjung, T. GDNF and BDNF gene interplay in chronic tinnitus. IJMEG 2012, 3, 245. [Google Scholar]
- Schecklmann, M.; Vielsmeier, V.; Steffens, T.; Landgrebe, M.; Langguth, B.; Kleinjung, T. Relationship between audiometric slope and tinnitus pitch in tinnitus patients: Insights into the mechanisms of tinnitus generation. PLoS ONE 2012, 7, e34878. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Carpenter-Thompson, J.; Husain, F.T. Connectivity of precuneus to the default mode and dorsal attention networks: A possible invariant marker of long-term tinnitus. Neuroimage Clin. 2017, 16, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Zimmerman, B.; Medina, R.O.B.; Carpenter-Thompson, J.R.; Husain, F.T. Changes in gray and white matter in subgroups within the tinnitus population. Brain Res. 2018, 1679, 64–74. [Google Scholar] [CrossRef]
- Simões, J.; Schlee, W.; Schecklmann, M.; Langguth, B.; Farahmand, D.; Neff, P. Big five personality traits are Associated with tinnitus improvement over time. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Song, J.-J.; De Ridder, D.; Schlee, W.; Van de Heyning, P.; Vanneste, S. “Distressed aging”: The differences in brain activity between early-and late-onset tinnitus. Neurobiol. Aging 2013, 34, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-J.; Vanneste, S.; Schlee, W.; Van de Heyning, P.; De Ridder, D. Onset-related differences in neural substrates of tinnitus-related distress: The anterior cingulate cortex in late-onset tinnitus, and the frontal cortex in early-onset tinnitus. Brain Struct. Funct. 2015, 220, 571–584. [Google Scholar] [CrossRef]
- Suzuki, F.A.d.B.; Suzuki, F.A.; Onishi, E.T.; Penido, N.O. Psychoacoustic classification of persistent tinnitus. Braz. J. Otorhinolaryngol. 2018, 84, 583–590. [Google Scholar] [CrossRef]
- Sztuka, A.; Pospiech, L.; Gawron, W.; Dudek, K. DPOAE in estimation of the function of the cochlea in tinnitus patients with normal hearing. Auris Nasus Larynx 2010, 37, 55–60. [Google Scholar] [CrossRef]
- Van der Wal, A.; Luyten, T.; Cardon, E.; Jacquemin, L.; Vanderveken, O.M.; Topsakal, V.; Van de Heyning, P.; De Hertogh, W.; Van Looveren, N.; Van Rompaey, V. Sex Differences in the Response to Different Tinnitus Treatment. Front. Neurol. 2020, 14, 422. [Google Scholar] [CrossRef]
- Vielsmeier, V.; Kleinjung, T.; Strutz, J.; Bürgers, R.; Kreuzer, P.M.; Langguth, B. Tinnitus with temporomandibular joint disorders: A specific entity of tinnitus patients? Otolaryngol. Head Neck Surg. 2011, 145, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.N.; Hu, W.; Li, J.J.; Zhou, J.X.; Zhang, J.P.; Shi, G.F.; He, P.; Li, Z.W.; Li, M. The characteristics of cognitive impairment in subjective chronic tinnitus. Brain Behav. 2018, 8, e00918. [Google Scholar] [CrossRef]
- Ward, J.; Vella, C.; Hoare, D.J.; Hall, D.A. Subtyping Somatic Tinnitus: A Cross-Sectional UK Cohort Study of Demographic, Clinical and Audiological Characteristics. PLoS ONE 2015, 10, e0126254. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kanzaki, S.; Sato, N.; Matsunaga, T.; Muramatsu, M.; Ogawa, K. Single nucleotide polymorphisms in tinnitus patients exhibiting severe distress. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Yüksel, F.; Karataş, D.; Türkdoğan, F.T.; Yüksel, Ö. Increased Atherosclerosis Correlates with Subjective Tinnitus Severity. Indian J. Otolaryngol. Head Neck Surg. 2018, 70, 119–124. [Google Scholar] [CrossRef]
- Andersson, G.; McKenna, L. Tinnitus masking and depression. Audiology 1998, 37, 174–182. [Google Scholar] [CrossRef]
- Newman, C.W.; Wharton, J.A.; Jacobson, G.P. Self-focused and somatic attention in patients with tinnitus. J. Am. Acad. Audiol. 1997, 8, 143–149. [Google Scholar] [PubMed]
- Rizzardo, R.; Savastano, M.; Maron, M.B.; Mangialaio, M.; Salvadori, L. Psychological distress in patients with tinnitus. J. Otolaryngol. 1998, 27, 21–25. [Google Scholar] [PubMed]
- Schecklmann, M.; Lehner, A.; Poeppl, T.B.; Kreuzer, P.M.; Hajak, G.; Landgrebe, M.; Langguth, B. Cluster analysis for identifying sub-types of tinnitus: A positron emission tomography and voxel-based morphometry study. Brain Res. 2012, 1485, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tyler, R.; Coelho, C.; Tao, P.; Ji, H.; Noble, W.; Gehringer, A.; Gogel, S. Identifying tinnitus subgroups with cluster analysis. Am. J. Audiol. 2008, 17, S176–S184. [Google Scholar] [CrossRef][Green Version]
- van den Berge, M.J.C.; Free, R.H.; Arnold, R.; de Kleine, E.; Hofman, R.; van Dijk, J.M.C.; van Dijk, P. Cluster analysis to identify possible subgroups in tinnitus patients. Front. Neurol. 2017, 8, 115. [Google Scholar] [CrossRef]
- Côté, C.; Baril, I.; Morency, C.-È.; Montminy, S.; Couture, M.; Leblond, J.; Roos, M.; Roy, J.-S. Long-Term Effects of a Multimodal Physiotherapy Program on the Severity of Somatosensory Tinnitus and Identification of Clinical Indicators Predicting Favorable Outcomes of the Program. J. Am. Acad. Audiol. 2019, 30, 720–730. [Google Scholar] [CrossRef]
- Kloostra, F.; Arnold, R.; Hofman, R.; Burgerhof, J.; van Dijk, P. Models to predict positive and negative effects of cochlear implantation on tinnitus. Laryngoscope Investig. Otolaryngol. 2019, 4, 138–142. [Google Scholar] [CrossRef]
- Langguth, B.; Goodey, R.; Azevedo, A.; Bjorne, A.; Cacace, A.; Crocetti, A.; Del Bo, L.; De Ridder, D.; Diges, I.; Elbert, T. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog. Res. 2007, 166, 525–536. [Google Scholar] [CrossRef]
- Horne, E.; Tibble, H.; Sheikh, A.; Tsanas, A. Challenges of Clustering Multimodal Clinical Data: Review of Applications in Asthma Subtyping. JMIR Med. Inform. 2020, 8, e16452. [Google Scholar] [CrossRef]
- Niemann, U.; Brueggemann, P.; Boecking, B.; Mebus, W.; Rose, M.; Spiliopoulou, M.; Mazurek, B. Phenotyping chronic tinnitus patients using self-report questionnaire data: Cluster analysis and visual comparison. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. Explainable AI for trees: From local explanations to global understanding. arXiv 2019, arXiv:190504610. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340. [Google Scholar] [CrossRef]
- Assmann, S.F.; Pocock, S.J.; Enos, L.E.; Kasten, L.E. Subgroup analysis and other (mis) uses of baseline data in clinical trials. Lancet 2000, 355, 1064–1069. [Google Scholar] [CrossRef]
- Simoes, J.P.; Neff, P.; Schoisswohl, S.; Bulla, J.; Schecklmann, M.; Harrison, S.S.; Vesala, M.; Langguth, B.; Schlee, W. Towards personalized tinnitus treatment: An exploratory study based on internet crowdsensing. Front. Public Health 2019, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Feczko, E.; Balba, N.; Miranda-Dominguez, O.; Cordova, M.; Karalunas, S.; Irwin, L.; Demeter, D.; Hill, A.; Langhorst, B.; Painter, J.G. Subtyping cognitive profiles in autism spectrum disorder using a functional random forest algorithm. Neuroimage 2018, 172, 674–688. [Google Scholar] [CrossRef] [PubMed]
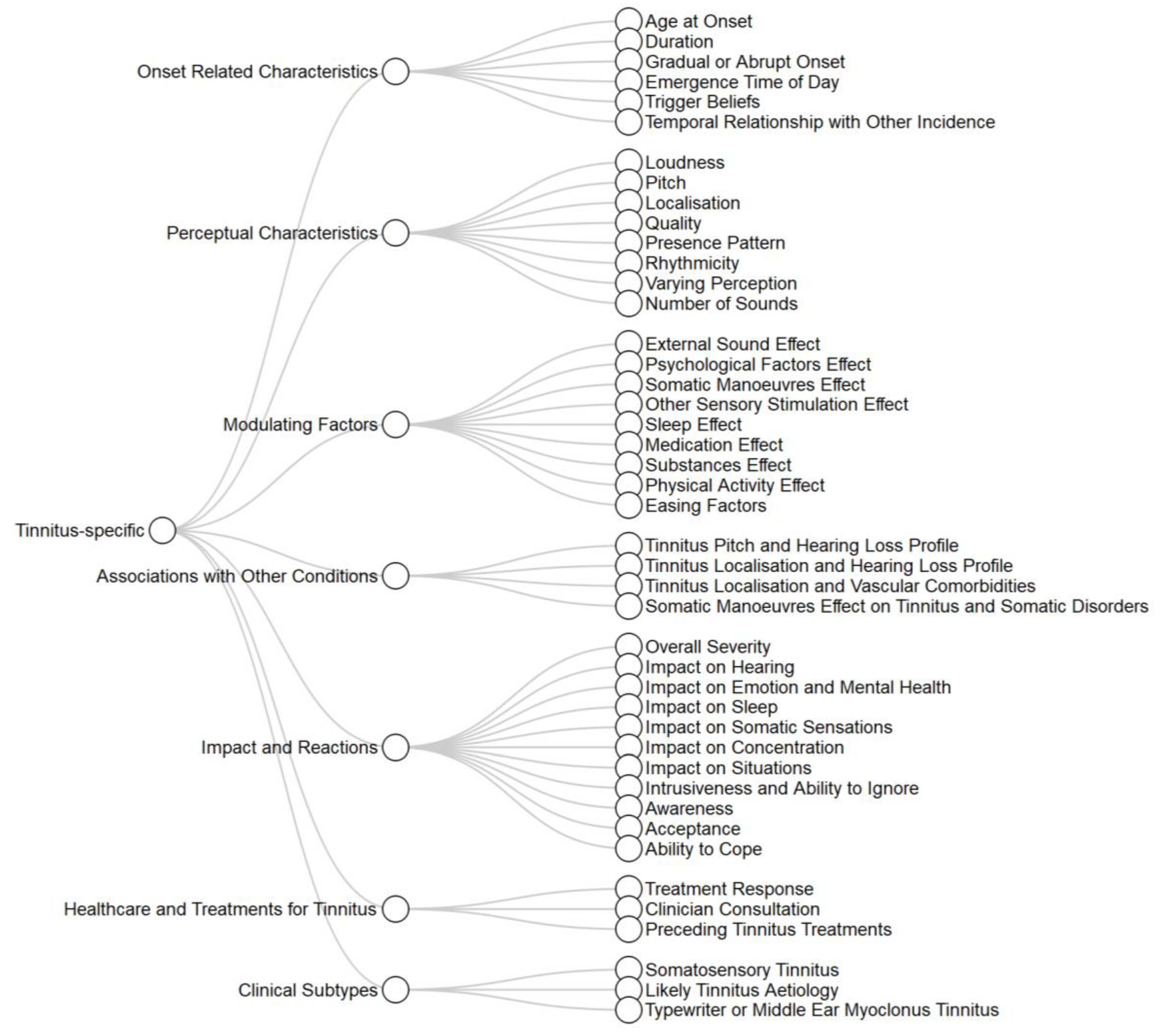
| Studies | Variables for Subgrouping | N | Method for Subgrouping (Method for Selection of Number of Groups) | Validation | Group Descriptions |
|---|---|---|---|---|---|
| Erlandsson, Rubinstein [50] | Mood characteristics | 42 | Cluster analysis with k-means—distance measure not specified (prespecified three groups) | Comparison of subgroups against variables not used for subgrouping (hearing ability, age, anxiety, various tinnitus characteristics) | 3 groups: 1. Low mood, higher tinnitus impact, anxiety, and hearing asymmetry. 2. Moderate mood. 3. High mood. |
| Newman, Wharton [87] | Personality and somatic symptoms (self-focused attention, somatic attention) | 51 | Cluster analysis minimizing squared distances from cluster means (selection among 4 groupings based on clinical interpretation) | Comparison of subgroups against variables not used for subgrouping (tinnitus impact, depressive symptomatology, tinnitus psychoacoustic characteristics) | 2 groups: 1. Lower score on both self-attention and somatic attention measures. 2. More internally directed, higher score on the attention measures. On average more depressed, with greater emotional distress due to tinnitus, and with greater perceived tinnitus handicap. |
| Andersson and McKenna [86] | Depressive symptoms, hearing ability (PTA), tinnitus loudness, external sound effect on tinnitus (MML) | 30 | Hierarchical cluster analysis with Ward’s method and Euclidean distances (not specified) | Comparison of subgroups against variables not used for subgrouping (age, tinnitus duration and severity) | 3 groups: 1. Low depression, average loudness, slightly above average MML and PTA. 2. High value only in depression, the youngest. 3. High values in all variables, the oldest. |
| Rizzardo, Savastano [88] | Personality and other mental health characteristics including symptoms of anxiety and depression | 84 | Hierarchical cluster analysis with complete linkage—distance measure not specified (not specified) | Comparison of subgroups against variables not used for subgrouping (demographics, clinical characteristics) | 3 groups: 1. Higher scores for depression, anxiety, neuroticism, and hypochondriasis, more often somatic symptoms and psychotropic drug use, less ENT stays, higher tinnitus distress, and lesser degree of denial. 2. Normal psychological tests apart from marked denial. 3. One patient. |
| Tyler, Coelho [90] | Age, hearing function, symptoms of anxiety, depression, and stress, somatic symptoms, tinnitus duration, perceptual characteristics, modulating factors, and severity (not all variables specified) | 153 | 2-step cluster analysis (SPSS) (tested solutions with 4–6 clusters; chose 4 because it resulted in about equal group sizes) | None | 4 groups: 1. Loud, persistent and distressing tinnitus, highest anxiety and depression scores, with loudness hyperacusis. 2. Varying tinnitus pitch and loudness, worse in noise. 3. Copers, tinnitus not influenced by touch. 4. Copers, tinnitus worse in quiet and better in noise. |
| Schecklmann, Lehner [89] | Three analyses using different variables: A. Tinnitus severity, duration, and localisation B. Grey matter volume (MRI) C. Brain glucose metabolism (PET) | 44 | Hierarchical cluster analysis with Ward’s method and Euclidean distances (forced to 2 groups in order to have sample sizes with sufficient statistical power) | Comparison of subgroups against variables not used for subgrouping (age, sex, hearing function, and tinnitus duration, localisation and severity) | 2 groups from each of the three analyses: A. Unilateral versus mainly bilateral tinnitus. B. Higher versus lower grey matter volume in medial superior prefrontal, cingulate, temporal, insular, orbital frontal, temporal, pre- and post-central and thalamic areas. C. Higher versus lower glucose metabolism in middle and superior temporal, precuneus, and superior parietal areas. |
| van den Berge, Free [91] | Two analyses using different variables: A. The variables with the highest loading on each of 8 components from a principal components analysis on 30 variables (depression or anxiety symptoms, hearing ability, tinnitus onset-related and perceptual characteristics, modulating factors, and impact) B. 11 selected variables: Sex, hearing function, tinnitus perceptual characteristics, modulating factors, and severity | A. 976, B. 761 | 2-step cluster analysis (SPSS) (probably silhouette measure for selection of number of groups) | Silhouette measure | A. 4 groups 1. Tinnitus not easily influenced, higher hearing asymmetry, lower depression score. 2. Gradual onset of tinnitus, easily negatively influenced by loud sounds and sleep deprivation. 3. Tinnitus less loud with loud sounds, no effect from sleep deprivation or nap. 4. Acute onset tinnitus, tinnitus easily negatively influenced by loud sounds or sleep deprivation. B. 3 groups 1. Tinnitus not easily influenced, preference for noisy environments, tinnitus mostly unilateral, low tinnitus severity scores, lower depression scores, sounds not often uncomfortably loud. 2. Mainly males, tinnitus worse by stress, loud sounds and movement of head and neck, preference for noisy environments, sometimes sounds are uncomfortably loud, most with no or slight HL, bilateral tinnitus with variable loudness. 3. Tinnitus worse by loud sounds and stress, prefer silent environment, often find sounds uncomfortably loud, tinnitus often bilateral, most with no, slight, or asymmetric HL, variable loudness. |
| Langguth, Landgrebe [27] | Hearing function at 7 frequencies (0.125, 0.250, 0.5, 1, 2, 4, and 8 kHz) for each ear (categorically coded as normal, mild/moderate loss, severe/profound loss, or no data) | 2838 | Latent class analysis (tested 2–12 groups and used BIC to determine optimal solution) | Comparison of subgroups against variables not used for subgrouping (demographics, tinnitus severity, depressive symptomatology, and various tinnitus characteristics) | 8 groups: 1. Lacking audiometry: phenotypic characteristics similar to average. 2. Bilateral high frequency HL: more males, more often tonal tinnitus. 3. Near normal hearing: the youngest, youngest age at onset, lowest tinnitus severity, more often tonal tinnitus. 4. Bilateral medium-high frequency (2 kHz and above) HL: more males, gradual tinnitus onset. 5. Severe pantonal HL: the oldest, more females, older age at onset, highest tinnitus severity, highest depression scores, gradual tinnitus onset. 6. Left-sided pantonal medium HL: more females, older age at onset, more often abrupt tinnitus onset, left-sided tinnitus. 7. Right-sided pantonal severe HL: more often abrupt tinnitus onset and right-sided tinnitus, less often pulsatile tinnitus. 8. Left-sided pantonal severe HL: more often left sided and constant tinnitus. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genitsaridi, E.; Hoare, D.J.; Kypraios, T.; Hall, D.A. A Review and a Framework of Variables for Defining and Characterizing Tinnitus Subphenotypes. Brain Sci. 2020, 10, 938. https://doi.org/10.3390/brainsci10120938
Genitsaridi E, Hoare DJ, Kypraios T, Hall DA. A Review and a Framework of Variables for Defining and Characterizing Tinnitus Subphenotypes. Brain Sciences. 2020; 10(12):938. https://doi.org/10.3390/brainsci10120938
Chicago/Turabian StyleGenitsaridi, Eleni, Derek J. Hoare, Theodore Kypraios, and Deborah A. Hall. 2020. "A Review and a Framework of Variables for Defining and Characterizing Tinnitus Subphenotypes" Brain Sciences 10, no. 12: 938. https://doi.org/10.3390/brainsci10120938
APA StyleGenitsaridi, E., Hoare, D. J., Kypraios, T., & Hall, D. A. (2020). A Review and a Framework of Variables for Defining and Characterizing Tinnitus Subphenotypes. Brain Sciences, 10(12), 938. https://doi.org/10.3390/brainsci10120938