Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome
Abstract
1. Introduction
1.1. Background
1.2. Measurement of mGluR5s in the Living Human Brain
[18F]FPEB
2. Materials and Methods
2.1. Participants
2.1.1. Recruiting Sites
2.1.2. Inclusion Criteria
2.1.3. Exclusion Criteria
2.1.4. Institute for Neurodegenerative Disorders (IND)
2.1.5. Johns Hopkins University (JHU)
2.2. Assessments
2.2.1. Institute for Neurodegenerative Disorders (IND)
2.2.2. Johns Hopkins University (JHU)
2.3. Procedures
2.3.1. Magnetic Resonance Imaging (MRI)
IND
JHU
2.3.2. Positron Emission Tomography (PET)
IND
JHU
2.3.3. Comparisons and Contrasts of Cohorts from the IND and JHU
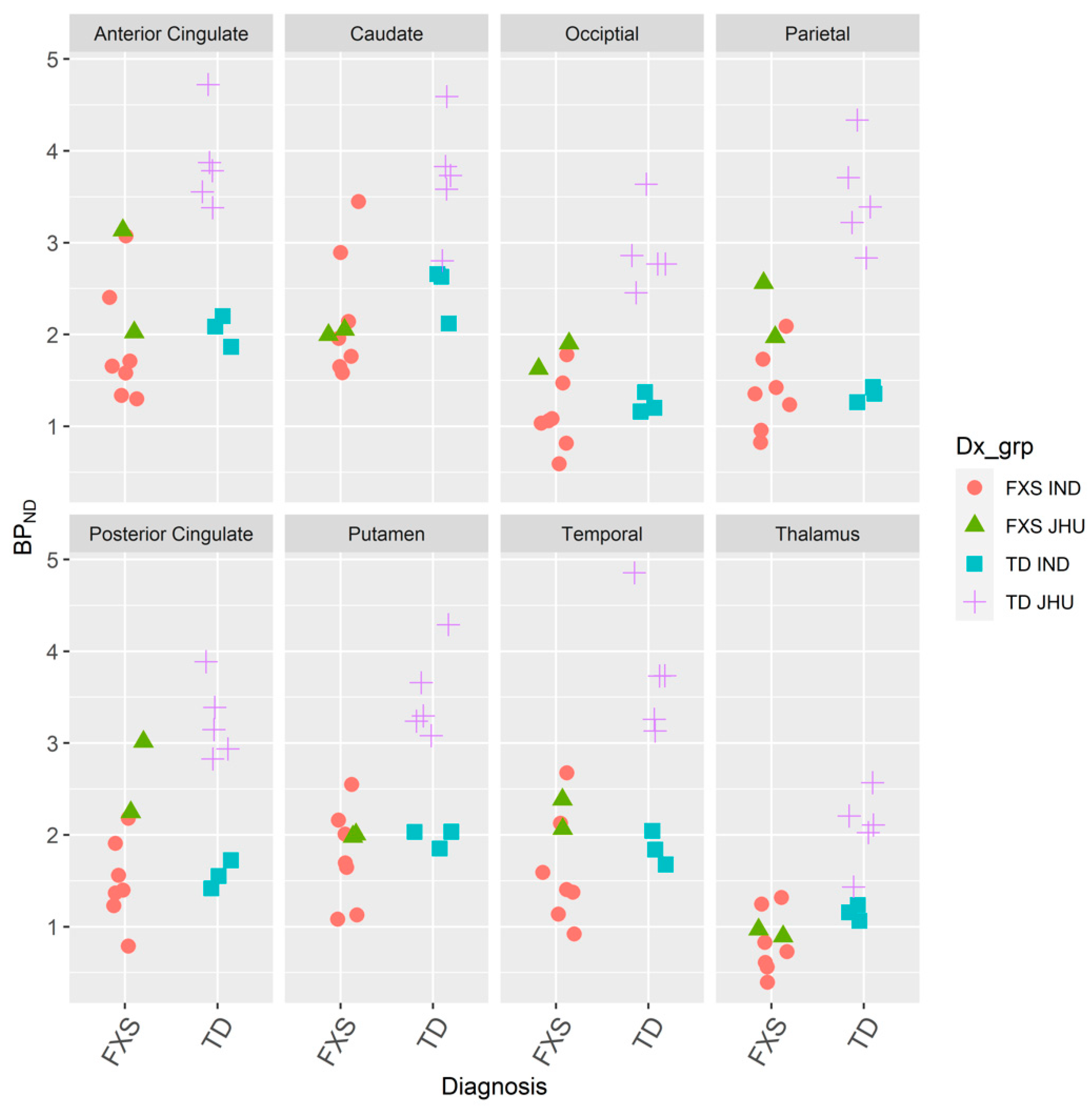
3. Results
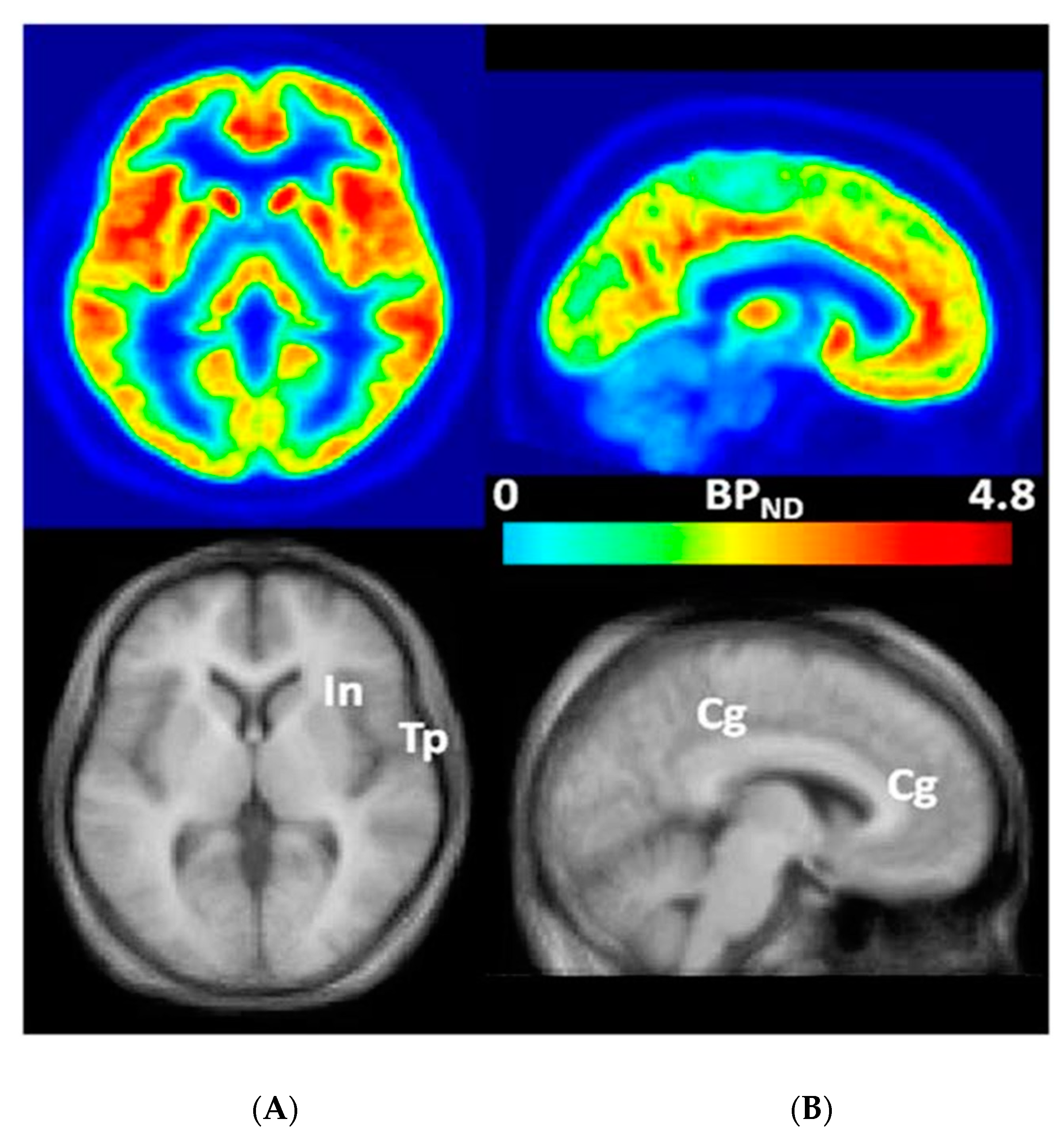
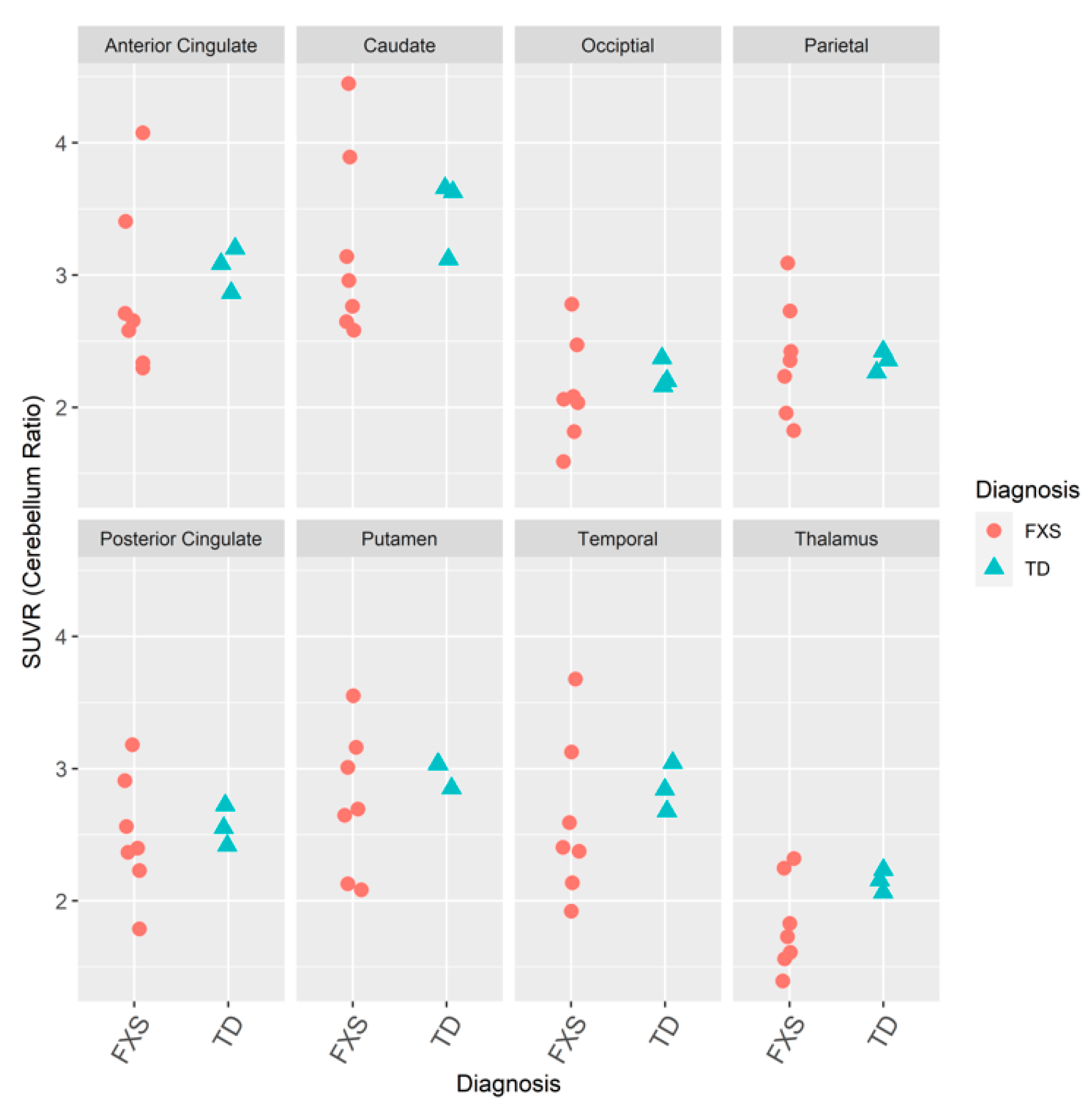
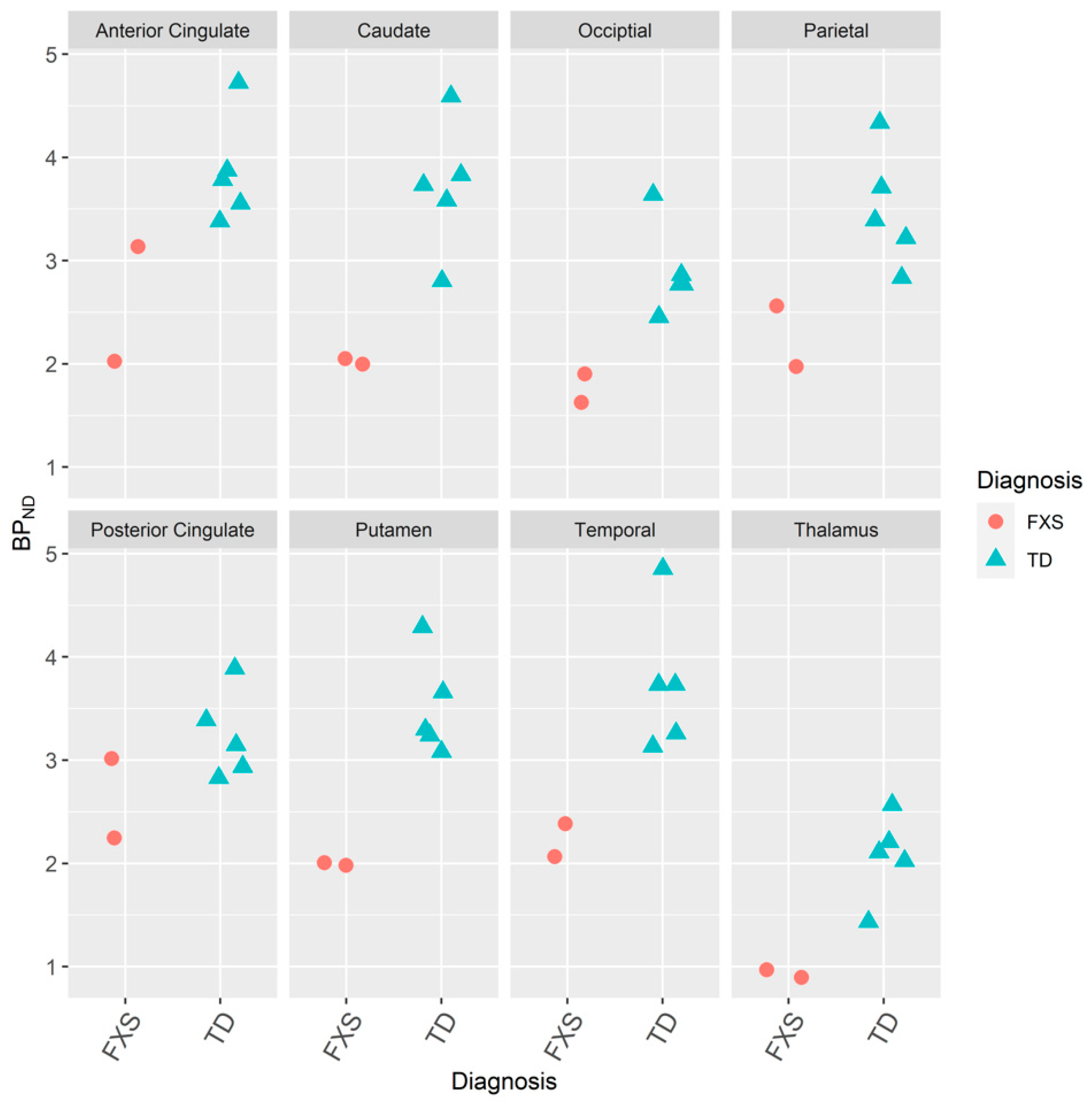
3.1. mGluR5s in Humans with FXS
3.1.1. IND
3.1.2. JHU
3.1.3. IND and JHU
4. Discussion
4.1. mGluR5s in Humans with FXS
4.1.1. Feasibility of a Complex Protocol of MRI and PET Scans in Participants with FXS
Adults
Adolescents and Children
4.2. mGluR5 Measurement in Men with FXS
4.3. Avoiding Effects of Diurnal Variations of mGluR5s
4.4. Limitations and Future Studies
Multimodal Imaging
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [18F]FPEB | 3-[18F]fluoro-5-(2- pyridinylethynyl)benzonitrile |
| ASD | Autism spectrum disorder |
| BPND | Non-displaceable binding potential |
| CDC | Centers for Disease Control and Prevention |
| DTI | Diffusion tensor imaging |
| ERK | Extracellular signal-regulated kinase |
| ERP | Event-related brain potential |
| FORWARD | Fragile X Online Registry With Accessible Research Database of the National Fragile X Foundation (NFXF) |
| FMR1 | Fragile X mental retardation 1 |
| FMRP | Fragile X Mental Retardation Protein |
| FXS | Fragile X syndrome |
| ID | Intellectual disability |
| LTD | Long-term depression |
| MAPK | Mitogen-activated protein kinase |
| MBq | Megabecquerel |
| mCi | Millicurie |
| mGluR5 | Metabotropic glutamate receptor subtype 5 |
| MRI | Magnetic resonance imaging |
| mTOR | Mammalian target of rapamycin (mTOR) |
| NAM | Negative allosteric modulator |
| NFXF | National Fragile X Foundation |
| PET | Positron emission tomography |
| PET/MRI | Positron emission tomography/magnetic resonance imaging |
| rs-FMRI | Resting-state functional magnetic resonance imaging |
| RTGA | Reference tissue graphical analysis |
| SPM | Statistical parametric mapping |
| TACs | Time activity curves |
| TD | Typical development |
| VOIs | Volumes of interest |
| (period) | Missing data |
References
- Bardoni, B.; Schenck, A.; Mandel, J.-L. The Fragile X Mental Retardation Protein. Brain Res. Bull. 2001, 56, 375–382. [Google Scholar] [CrossRef]
- Brasic, J.R.; Farhadi, F.; Elshourbagy, T. Autism Spectrum Disorder. Medscape Drugs & Diseases. Updated 18 March 2020. Available online: http://emedicine.medscape.com/article/912781-overview (accessed on 21 November 2020).
- Budimirovic, D.B.; Berry-Kravis, E.; Erickson, C.A.; Hall, S.S.; Hessl, D.; Reiss, A.L.; King, M.K.; Abbeduto, L.; Kaufmann, W.E. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J. Neurodev. Disord. 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Kaufmann, W.E. What can we learn about autism from studying fragile X syndrome? Dev. Neurosci. 2011, 33, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Subramanian, M. Neurobiology of autism and intellectual disability: Fragile X syndrome. In Neurobiology of Disease, 2nd ed.; Johnston, M.V., Ed.; Oxford University Press: New York, NY, USA, 2016; pp. 375–384. [Google Scholar]
- Hagerman, R.J.; Des-Portes, V.; Gasparini, F.; Jacquemont, S.; Gomez-Mantilla, B. Translating molecular advances in fragile X syndrome into therapy: A review. J. Clin. Psychiatry 2014, 75, e294–e307. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Berry-Kravis, E.; Kaufmann, W.E.; Ono, M.Y.; Tartaglia, N.; Lachiewicz, A.; Kronk, R.; Delahunty, C.; Hessl, D.; Visootsak, J.; et al. Advances in the treatment of fragile X syndrome. Pediatrics 2009, 123, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Capone, G.; Clarke, M.; Budimirovic, D.B. Autism in genetic intellectual disability: Insights into idiopathic autism. In Autism: Current Theories and Evidence; Zimmerman, A.W., Ed.; The Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 81–108. [Google Scholar]
- Pretto, D.I.; Kumar, M.; Cao, Z.; Cunningham, C.L.; Durbin-Johnson, B.; Qi, L.; Berman, R.; Noctor, S.C.; Hagerman, R.J.; Pessah, I.N.; et al. Reduced excitatory amino acid transporter 1 and metabotropic glutamate receptor 5 expression in the cerebellum of fragile X mental retardation gene 1 premutation carriers with fragile X-associated tremor/ataxia syndrome. Neurobiol. Aging 2014, 35, 1189–1197. [Google Scholar] [CrossRef]
- Ascano, M., Jr.; Mukherjee, N.; Bandaru, P.; Miller, J.B.; Nusbaum, J.D.; Corcoran, D.L.; Langlois, C.; Munschauer, M.; Dewell, S.; Hafner, M.; et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 2012, 492, 382–386. [Google Scholar] [CrossRef]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.S.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Schlageter, A.; Filopovic-Sadic, S.; Protic, D.D.; Bram, E.; Mahone, E.M.; Nicholson, K.; Culp, K.; Javanmardi, K.; Kemppainnen, J.; et al. A genotype-phenotype study of high-resolution FMR1 nucleic acid and protein analyses in fragile X patients with neurobehavioral assessments. Brain Sci. 2020, 10, 694. [Google Scholar] [CrossRef]
- Bagni, C.; Zukin, R.S. A synaptic perspective of fragile X syndrome and autism spectrum disorders. Neuron 2019, 101, 1070–1088. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- D’Antoni, S.; Spatuzza, M.; Bonaccorso, C.M.; Musumeci, S.A.; Ciranna, L.; Nicoletti, F.; Huber, K.M.; Catania, M.V. Dysregulation of group-1 metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with intellectual disability and autism. Neurosci. Biobehav. Rev. 2014, 46 (Pt 2), 228–241. [Google Scholar] [CrossRef]
- Hajós, M. Portraying inhibition of metabotropic glutamate receptor 5 in fragile X mice. Biol. Psychiatry 2014, 75, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Hinton, V.J.; Brown, W.T.; Wisniewski, K.; Rudelli, R.D. Analysis of neocortex in three males with the fragile X syndrome. Am. J. Med. Genet. 1991, 41, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Moser, H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 2000, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Lohith, T.G.; Osterweil, E.K.; Fujita, M.; Jenko, K.J.; Bear, M.F.; Innis, R.B. Is metabotropic glutamate receptor 5 upregulated in prefrontal cortex in fragile X syndrome? Mol. Autism 2013, 4, 15. [Google Scholar] [CrossRef]
- Jong, Y.-J.I.; Harmon, S.K.; O’Malley, K.L. Location and cell-type-specific bias of metabotropic glutamate receptor, mGlu5, negative allosteric modulators. ACS Chem. Neurosci. 2019, 10, 4558–4570. [Google Scholar] [CrossRef]
- DeLorenzo, C.; Gallezot, J.-D.; Gardus, J.; Yang, J.; Planeta, B.; Nabulsi, N.; Ogden, R.T.; Labaree, D.C.; Huang, Y.H.; Mann, J.J.; et al. In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C]ABP688 and [18F]FPEB. J. Cereb. Blood Flow Metab. 2017, 37, 2716–2727. [Google Scholar] [CrossRef]
- Ansari, M.S.; Jones, C.K.; Felts, A.S.; Lindsley, C.W.; Alagille, D.; Tamagnan, G.D.; Kessler, R.M.; Baldwin, R.M. One pot synthesis of [18F]FPEB in a semi-automated module. J. Labelled Comp. Radiopharm. 2009, 52 (Suppl. 1), 318. [Google Scholar]
- Barret, O.; Tamagnan, G.; Batis, J.; Jennings, D.; Zubal, G.; Russel, D.; Marek, K.; Seibyl, J. Quantitation of glutamate mGluR5 receptor with 18F-FPEB PET in humans. Neuroimage 2010, 52 (Suppl. 1), S202. [Google Scholar] [CrossRef]
- Brasic, J.R.; Syed, A.B.; Farhadi, F.; Wong, D.F. PET Scanning in Autism Spectrum Disorder. Medscape Drugs & Diseases. Updated 16 April 2020. Available online: http://emedicine.medscape.com/article/1155568-overview (accessed on 21 November 2020).
- Fatemi, S.H.; Wong, D.F.; Brašić, J.R.; Kuwabara, H.; Mathur, A.; Folsom, T.D.; Jacob, S.; Realmuto, G.M.; Pardo, J.V.; Lee, S. Metabotropic glutamate receptor 5 tracer [18F]-FPEB displays increased binding potential in postcentral gyrus and cerebellum of male individuals with autism: A pilot PET study. Cerebellum Ataxias 2018, 5, 3. [Google Scholar] [CrossRef]
- Leurquin-Sterk, G.; Postnov, A.; Celen, S.; de Laat, B.; Bormans, G.; Van Laere, K.J. Kinetic modeling and longterm test-retest of 18F-FPEB mGluR5 PET in healthy volunteers. J. Nucl. Med. 2015, 56 (Suppl. 3), 49. [Google Scholar]
- Leurquin-Sterk, G.; Postnov, A.; de Laat, B.; Casteels, C.; Celen, S.; Crunelle, C.L.; Bormans, G.; Koole, M.; Van Laere, K. Kinetic modeling and long-term test-retest reproducibililty of the mGluR5 PET tracer 18F-FPEB in human brain. Synapse 2016, 70, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M.; Lim, K.; Labaree, D.; Lin, S.-F.; McCarthy, T.J.; Seibyl, J.P.; Tamagnan, G.; Huang, Y.; Carson, R.E.; Ding, Y.-S.; et al. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [18F]FPEB in bolus and bolus plus-constant-infusion studies in humans. J. Cereb. Blood Flow Metab. 2013, 33, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.; Planeta-Wilson, B.; Lim, K.; Lin, S.F.; Najafzadeh, S.; McCarthy, T.; Ding, Y.S.; Carson, R.E.; Morris, E.D.; Williams, W.A. Test-retest evaluation of [F-18] FPEB, a PET tracer for the mGluR5 receptors in humans. J. Cereb. Blood Flow Metab. 2012, 32 (Suppl. 1), S122–S123. [Google Scholar]
- Wong, D.F.; Waterhouse, R.; Kuwabara, H.; Kim, J.; Brašić, J.R.; Chamroonrat, W.; Stabins, M.; Holt, D.P.; Dannals, R.F.; Hamill, T.G.; et al. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: A first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J. Nucl. Med. 2013, 54, 388–396. [Google Scholar] [CrossRef]
- Brašić, J.R.; Mathur, A.K.; Budimirovic, D.B. The urgent need for molecular imaging to confirm target engagement for clinical trials of fragile X syndrome and other subtypes of autism spectrum disorder. Arch. Neurosci. 2019, 6, e91831. [Google Scholar] [CrossRef]
- Innis, R.B.; Cunninghamm, V.J.; Delforge, J.; Fujita, M.; Gjedde, A.; Gunn, R.N.; Holden, J.; Houle, S.; Huang, S.C.; Ichise, M.; et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007, 27, 1533–1539. [Google Scholar] [CrossRef]
- The Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, London, UK. Statistical Parametric Mapping (SPM). 2020. Available online: http://www.fil.ion.ucl.ac.uk/spm/ (accessed on 21 November 2020).
- Russell, D.; Jennings, D.; Tamagnan, G.; Seibyl, J.; Koren, A.; Zubal, G.; Marek, K. Evaluation of novel radiotracers targeting non-dopaminergic striatal biomarkers in HD: 18F-FPEB and PET imaging for metabotropic glutamate receptor type 5 (mGluR5) expression in healthy subjects and subjects with Huntington disease (HD). Neurotherapeutics 2010, 7, 142. [Google Scholar] [CrossRef]
- Russell, D.S.; Jennings, D.L.; Tamagnan, G.; Alagilles, D.; Carson, R.E.; Barret, O.; Batis, J.; Koren, A.; Zubal, G.; Seibyl, J.P.; et al. Evaluation of novel radiotracers targeting non-dopaminergic striatal biomarkers in HD: [18F] FPEB and PET imaging for metabotrophic glutamate receptor type 5 (mGluR5) in healthy subjects and subjects with Huntington’s disease (HD). Mov. Disord. 2010, 25 (Suppl. 2), S391–S392. [Google Scholar] [CrossRef]
- Russell, D.; Tamagnan, G.; Barrett, O.; Seibyl, J.; Marek, K. Evaluation of mGluR5 in early Parkinson’s disease using 18F-FPEB PET imaging. Mov. Disord. 2010, 25 (Suppl. 2), S383–S384. [Google Scholar]
- Wang, J.Q.; Tueckmantel, W.; Zhu, A.J.; Pellegrino, D.; Brownell, A.L. Synthesis and preliminary biological evaluation of 3-[F-18]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse 2007, 61, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Seibyl, J.; Russell, D.; Jennings, D.; Marek, K. Neuroimaging over the course of Parkinson’s disease: From early detection of the at-risk patient to improving pharmacotherapy of later-stage disease. Semin. Nucl. Med. 2012, 42, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Des Portes, V.; Hagerman, R.; Jacquemont, S.; Charles, P.; Visootsak, J.; Brinkman, M.; Rerat, K.; Koumaras, B.; Zhu, L.; et al. Mavoglurant in fragile X syndrome: Results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med. 2016, 8, 321ra5. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Hagerman, R.; Visootsak, J.; Budimirovic, D.; Kaufmann, W.E.; Cherubini, M.; Zarevics, P.; Walton-Bowen, K.; Wang, P.; Bear, M.F.; et al. Arbaclofen in fragile X syndrome: Results of phase 3 trials. J. Neurodev. Disord. 2017, 9, 3. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Hessl, D.; Coffey, S.; Hervey, C.; Schneider, A.; Yuhas, J.; Hutchison, J.; Snape, M.; Tranfaglia, M.; Nguyen, D.V.; et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. 2009, 46, 266–271. [Google Scholar] [CrossRef]
- Berry-Kravis, E.M.; Hessl, D.; Rathmell, B.; Zarevics, P.; Cherubini, M.; Walton-Bowen, K.; Mu, Y.; Nguyen, D.V.; Gonzalez-Heydrich, J.; Wang, P.P.; et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: A randomized, controlled, phase 2 trial. Sci. Transl. Med. 2012, 4, 152ra127. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Hessl, D.; Abbeduto, L.; Reiss, A.L.; Beckel-Mitchener, A.; Urv, T.K. Outcome measures for clinical trials in fragile X syndrome. J. Dev. Behav. Pediatr. 2013, 34, 508–522. [Google Scholar] [CrossRef]
- Berry-Kravis, E.M.; Lindemann, L.; Jønch, A.E.; Apostol, G.; Bear, M.F.; Carpenter, R.L.; Crawley, J.N.; Curie, A.; Des Portes, V.; Hossain, F.; et al. Drug development for neurodevelopmental disorders: Lessons learned from fragile X syndrome. Nat. Rev. Drug Discov. 2018, 17, 280–299. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Duy, P.Q. Neurobehavioral features and targeted treatments in fragile X syndrome: Current insights and future directions. Engrami 2015, 37, 5–19. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Duy, P.Q. Challenges in translating therapeutic frontiers in clinical trials: Where are we now and what’s next? Madridge J. Neuroscience 2016, 1, e1–e3. [Google Scholar] [CrossRef]
- Duy, P.Q.; Budimirovic, D.B. Fragile X syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials. Transl. Neurosci. 2017, 8, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.A.; Davenport, M.H.; Schaefer, T.L.; Wink, L.K.; Pedapati, E.V.; Sweeney, J.A.; Fitzpatrick, S.E.; Brown, W.T.; Budimirovic, D.; Hagerman, R.J.; et al. Fragile X targeted pharmacotherapy: Lessons learned and future directions. J. Neurodev. Disord. 2017, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Jønch, A.E.; Jacquemont, S. Reflections on clinical trials in fragile X syndrome. In Fragile X Syndrome: From Genetics to Targeted Treatment; Willemsen, R., Kooy, R.F., Eds.; Academic: London, UK, 2017; pp. 419–441. [Google Scholar]
- Ligsay, A.; Hagerman, R.; Berry-Kravis, E. Overview of targeted double-blind, placebo-controlled clinical trials in fragile X syndrome. In Fragile X Syndrome: From Genetics To Targeted Treatment; Willemsen, R., Kooy, R.F., Eds.; Academic: London, UK, 2017; pp. 401–418. [Google Scholar]
- Ligsay, A.; Van Dijck, A.; Nguyen, D.V.; Lozano, R.; Chen, Y.; Bickel, E.S.; Hessl, D.; Schneider, A.; Angkustsir, K.; Tassone, F.; et al. A randomized double-blind, placebo controlled trial of ganaxolone in children and adolescents with fragile X syndrome. J. Neurodev. Disord. 2017, 9, 26. [Google Scholar] [CrossRef]
- Russell, D. A PET Brain Imaging Study of mGluR5 in Subjects with Neuropsychiatric Conditions (FPEB). ClinicalTrials.gov Identifier: NCT00870974 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT00870974 (accessed on 21 November 2020).
- World Medical Association. Declaration of Helsinki: Medical Research Involving Human Subjects. 2013. Available online: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/ (accessed on 21 November 2020).
- International Committee of Medical Journal Editors (ICMJE). Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. 2019. Available online: http://www.icmje.org/icmje-recommendations.pdf (accessed on 21 November 2020).
- Brasic, J.R.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Mathur, A.; Slifer, K.; Sedlak, T.; Martin, S.D.; Brinson, Z.; et al. Dataset of Reduced cerebral expression of metabotropic glutamate receptor subtype 5 in men with fragile X syndrome. Available online: https://doi.org/10.5281/zenodo.4279744 (accessed on 23 November 2020).
- Deb, S.; Hare, M.; Prior, L.; Bhaumick, S. Dementia Screening Questionnaire for Individuals with Intellectual Disabilities. Br. J. Psychiatry 2007, 190, 440–444. [Google Scholar] [CrossRef]
- Roid, G.H. Stanford-Binet Intelligence Scales, 5th ed.; (SB-5); Western Psychological Services (WPS): Torrance, CA, USA, 2003. [Google Scholar]
- Hessl, D.; Nguyen, D.V.; Green, C.; Chavez, A.; Tassone, F.; Hagerman, R.J.; Senturk, D.; Schneider, A.; Lightbody, A.; Reiss, A.L.; et al. A solution to limitations of cognitive testing in children with intellectual disabilities: The case of fragile X syndrome. J. Neurodev. Disord. 2009, 1, 33–45. [Google Scholar] [CrossRef]
- Sparrow, S.S.; Cicchetti, D.V.; Saulnier, C.A. Vineland Adaptive Behavior Scales, 3rd ed.; (Vineland-3); Pearson: San Antonio, TX, USA, 2020. [Google Scholar]
- Brašić, J.R.; Zhou, Y.; Musachio, J.L.; Hilton, J.; Fan, H.; Crabb, A.; Endres, C.J.; Reinhardt, M.J.; Dogan, A.S.; Alexander, M.; et al. Single photon emission computed tomography experience with (S)5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine in the living human brain of smokers and nonsmokers. Synapse 2009, 63, 339–358. [Google Scholar] [CrossRef]
- Wienhard, K.; Dahlbom, M.; Eriksson, L.; Michel, C.; Bruckbauer, T.; Pietrzyk, U.; Heiss, W.-D. The ECAT EXACT HR: Performance of a new high resolution positron scanner. J. Comput. Assist. Tomogr. 1994, 18, 110–118. [Google Scholar] [CrossRef]
- Cox, A.D.; Virues-Ortega, J.; Julio, F.; Martin, T.L. Establishing motion control in children with autism and intellectual disability: Applications for anatomical and functional MRI. J. Appl. Behav. Anal. 2017, 50, 8–26. [Google Scholar] [CrossRef]
- Slifer, K.J.; Cataldo, M.F.; Cataldo, M.D.; Llorente, A.M.; Gerson, A.C. Behavior analysis of motion control for pediatric neuroimaging. J. Appl. Behav. Anal. 1993, 26, 469–470. [Google Scholar] [CrossRef]
- Slifer, K.J.; Koontz, K.L.; Cataldo, F. Operant-contingency-based preparation of children for functional magnetic resonance imaging. J. Appl. Behav. Anal. 2002, 35, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Brašić, J.R.; Bibat, G.; Kumar, A.; Zhou, Y.; Hilton, J.; Yablonski, M.E.; Dogan, A.S.; Guevara, M.R.; Stephane, M.; Johnston, M.; et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse 2012, 66, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Brašić, J.R.; Cascella, N.; Kumar, A.; Zhou, Y.; Hilton, J.; Raymont, V.; Crabb, A.; Guevara, M.R.; Horti, A.G.; Wong, D.F. Positron emission tomography (PET) experience with 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) in the living human brain of smokers with paranoid schizophrenia. Synapse 2012, 66, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Cheng, J.-C.; Blinder, S.; Camborde, M.-L.; Sossi, V. Statistical dynamic image reconstruction in state-of-the-art high-resolution PET. Phys. Med. Biol. 2005, 50, 4887–4912. [Google Scholar] [CrossRef] [PubMed]
- Sossi, V.; de Jong, H.W.A.M.; Barker, W.C.; Bloomfield, P.; Burbar, Z.; Camborde, M.-L.; Comtat, C.; Eriksson, L.A.; Houle, S.; Keator, D.; et al. The second generation HRRT—A multi-centre scanner performance investigation. IEEE Nucl. Sci. Symp. Conf. Ref. 2005, 4, 2195–2199. [Google Scholar]
- Fischl, B.; van der Kouwe, A.; Destrieux, C.; Halgren, E.; Ségonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef]
- Hoopes, A. (Ed.) FreeSurfer Download and Install. 2020. Available online: https://surfer.nmr.mgh.harvard.edu/fswiki/DownloadAndInstall (accessed on 21 November 2020).
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011, 56, 907–922. [Google Scholar] [CrossRef]
- Woolrich, M.W.; Jbabdi, S.; Patenaude, B.; Chappell, M.; Makni, S.; Behrens, T.; Beckmann, C.; Jenkinson, M.; Smith, S.M. Bayesian analysis of neuroimaging data in FSL. Neuroimage 2009, 45 (Suppl. 1), S173–S186. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. High-dimensional image warping. In Human Brain Function, 2nd ed.; Frackowiak, R.S.J., Ashburner, J., Penny, W.D., Zeki, S., Friston, K.J., Frith, C., Dolan, R., Price, C.J., Eds.; Academic: Waltham, MA, USA, 2004; pp. 656–673. [Google Scholar]
- Ashburner, J.; Friston, K.J. Rigid body registration. In Human Brain Function, 2nd ed.; Frackowiak, R.S.J., Ashburner, J., Penny, W.D., Zeki, S., Friston, K.J., Frith, C., Dolan, R., Price, C.J., Eds.; Academic: Waltham, MA, USA, 2004; pp. 635–654. [Google Scholar]
- Logan, J.; Volkow, N.D.; Wang, G.J.; Ding, Y.S.; Alexoff, D.L. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cerebral. Blood Flow Metab. 1996, 16, 834–840. [Google Scholar] [CrossRef]
- Logan, J.; Alexoff, D.; Fowler, J.S. The use of alternative forms of graphical analysis to balance bias and precision in PET images. J. Cereb. Blood Flow Metab. 2011, 31, 535–546. [Google Scholar] [CrossRef]
- Carson, R.E. Tracer kinetic modeling in PET. In Positron Emission Tomography: Basic Science and Clinical Practice; Valk, P.E., Bailey, D.L., Townsend, D.W., Maisey, M.N., Eds.; Springer: London, UK, 2003; pp. 147–179. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https:www.R-project-org (accessed on 29 September 2020).
- Catana, C. Principles of simultaneous PET/MR imaging. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 231–243. [Google Scholar] [CrossRef]
- Trivedi, R.R.; Bhattacharyya, S. Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5). Biochem. Biophys. Res. Commun. 2012, 427, 185–190. [Google Scholar] [CrossRef]
- Castañeda, T.R.; de Prado, B.M.; Prieto, D.; Mora, F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: Modulation by light. J. Pineal Res. 2004, 36, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Fuller, P.M.; Gooley, J.J.; Saper, C.B. Neurobiology of the sleep-wake cycle: Sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythm. 2006, 21, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Yuan, S.; Zheng, Z.; Liu, T.; Lin, L. Effects of endogenous melatonin on glutatmate and GABA rhythms in the striatum of unilateral 6-hydroxydopamine-lesioned rats. Neuroscience 2015, 286, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Brasic, J.; Budimirovic, D.; Mathur, A.; Nandi, A.; Mahone, E.; Slifer, K.; Brinson, Z.; Sedlak, T.; Ryan, M.; Landa, R.; et al. Metabotropic Glutamate Receptor Subtype 5 Function in Fragile X Syndrome. J. Nucl. Med. 2020, 61 (Suppl. 1), 1552. Available online: http://jnm.snmjournals.org/content/61/supplement_1/1552.abstract?sid=7a58a0b9-ec27-42f3-b06e-7ded1f2dc2c8 (accessed on 21 November 2020).
- Darnell, R.B. The genetic control of stoichiometry underlying autism. Annu. Rev. Neurosci. 2020, 43, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Jacquemont, S.; Pacini, L.; Jønch, A.E.; Cencelli, G.; Rozenberg, I.; He, Y.; D’Andrea, L.; Pedini, G.; Eldeeb, M.; Willemsen, R.; et al. Protein synthesis levels are increased in a subset of individuals with fragile X syndrome. Hum. Mol. Genet. 2018, 27, 2039–2051, Erratum in: Hum. Mol. Genet. 2018, 27. [Google Scholar] [CrossRef]
- Schmidt, K.C.; Loutaev, I.; Quezado, Z.; Sheeler, C.; Smith, C.B. Regional rates of brain protein synthesis are unaltered in dexmedetomidine sedated young men with fragile X syndrome: A L-[1-11C]leucine PET study. Neurobiol. Dis. 2020, 143, 104978. [Google Scholar] [CrossRef]
- Razak, K.A.; Dominick, K.C.; Erickson, C.A. Developmental studies in fragile X syndrome. J. Neurodev. Disord. 2020, 12, 13. [Google Scholar] [CrossRef]
- Li, W.; Kutas, M.; Gray, J.A.; Hagerman, R.H.; Olichney, J.M. The role of glutamate in language and language disorders—Evidence from ERP and pharmacological studies. Neurosci. Biobehav. Rev. 2020, 119, 217–241. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.N.; Harrigan, T.P.; Brasic, J.R. A low-cost quantitative continuous measurement of movements in the extremities of people with Parkinson’s disease. MethodsX 2019, 6, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Zafarullah, M.; Tassone, F. Molecular biomarkers in fragile X syndrome. Brain Sci. 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed]
| Format | Time to Repetition (TR) (ms) | Time to Echo (TE) (ms) | Thickness (mm) | Number of Slices |
|---|---|---|---|---|
| T1 sagittal | 500 | 8 | 5.0 | 21 |
| T1 SPGR recalled acquisition in the steady-state axial | 35 | 6 | 1.5 | 124 |
| T2 oblique | 5900 | 95 | 5.0 | 27 |
| DTI | 12,100 | 88 | 2.0 | 72 |
| Region | Term | df | Sum of Squares | F Statistic | p-Value |
|---|---|---|---|---|---|
| Anterior Cingulate | Diagnosis | 1 | 5.69 | 15.1 | 0.00165 |
| Source | 1 | 5.93 | 15.7 | 0.00141 | |
| Caudate | Diagnosis | 1 | 4.93 | 10.6 | 0.00569 |
| Source | 1 | 1.22 | 2.62 | 0.128 | |
| Occipital | Diagnosis | 1 | 4.37 | 23.1 | 0.000279 |
| Source | 1 | 4.92 | 26 | 0.000163 | |
| Parietal | Diagnosis | 1 | 5.32 | 18.9 | 0.000675 |
| Source | 1 | 8.58 | 30.4 | 0.000076 | |
| Posterior Cingulate | Diagnosis | 1 | 3.18 | 17.6 | 0.000906 |
| Source | 1 | 7.04 | 38.9 | 2.18E-05 | |
| Putamen | Diagnosis | 1 | 5.4 | 18.4 | 0.000753 |
| Source | 1 | 3.1 | 10.6 | 0.00583 | |
| Temporal | Diagnosis | 1 | 7.07 | 17.9 | 0.000834 |
| Source | 1 | 5.92 | 15 | 0.00169 | |
| Thalamus | Diagnosis | 1 | 3.32 | 23.7 | 0.000249 |
| Source | 1 | 1.06 | 7.55 | 0.0157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brašić, J.R.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Mathur, A.; Slifer, K.; Sedlak, T.; Martin, S.D.; Brinson, Z.; et al. Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome. Brain Sci. 2020, 10, 899. https://doi.org/10.3390/brainsci10120899
Brašić JR, Nandi A, Russell DS, Jennings D, Barret O, Mathur A, Slifer K, Sedlak T, Martin SD, Brinson Z, et al. Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome. Brain Sciences. 2020; 10(12):899. https://doi.org/10.3390/brainsci10120899
Chicago/Turabian StyleBrašić, James R., Ayon Nandi, David S. Russell, Danna Jennings, Olivier Barret, Anil Mathur, Keith Slifer, Thomas Sedlak, Samuel D. Martin, Zabecca Brinson, and et al. 2020. "Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome" Brain Sciences 10, no. 12: 899. https://doi.org/10.3390/brainsci10120899
APA StyleBrašić, J. R., Nandi, A., Russell, D. S., Jennings, D., Barret, O., Mathur, A., Slifer, K., Sedlak, T., Martin, S. D., Brinson, Z., Vyas, P., Seibyl, J. P., Berry-Kravis, E. M., Wong, D. F., & Budimirovic, D. B. (2020). Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome. Brain Sciences, 10(12), 899. https://doi.org/10.3390/brainsci10120899