Preterm Birth Impedes Structural and Functional Development of Cerebellar Purkinje Cells in the Developing Baboon Cerebellum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Slice Preparation
2.3. Electrophysiology
2.4. Immunohistochemistry
2.5. Quantification
3. Results
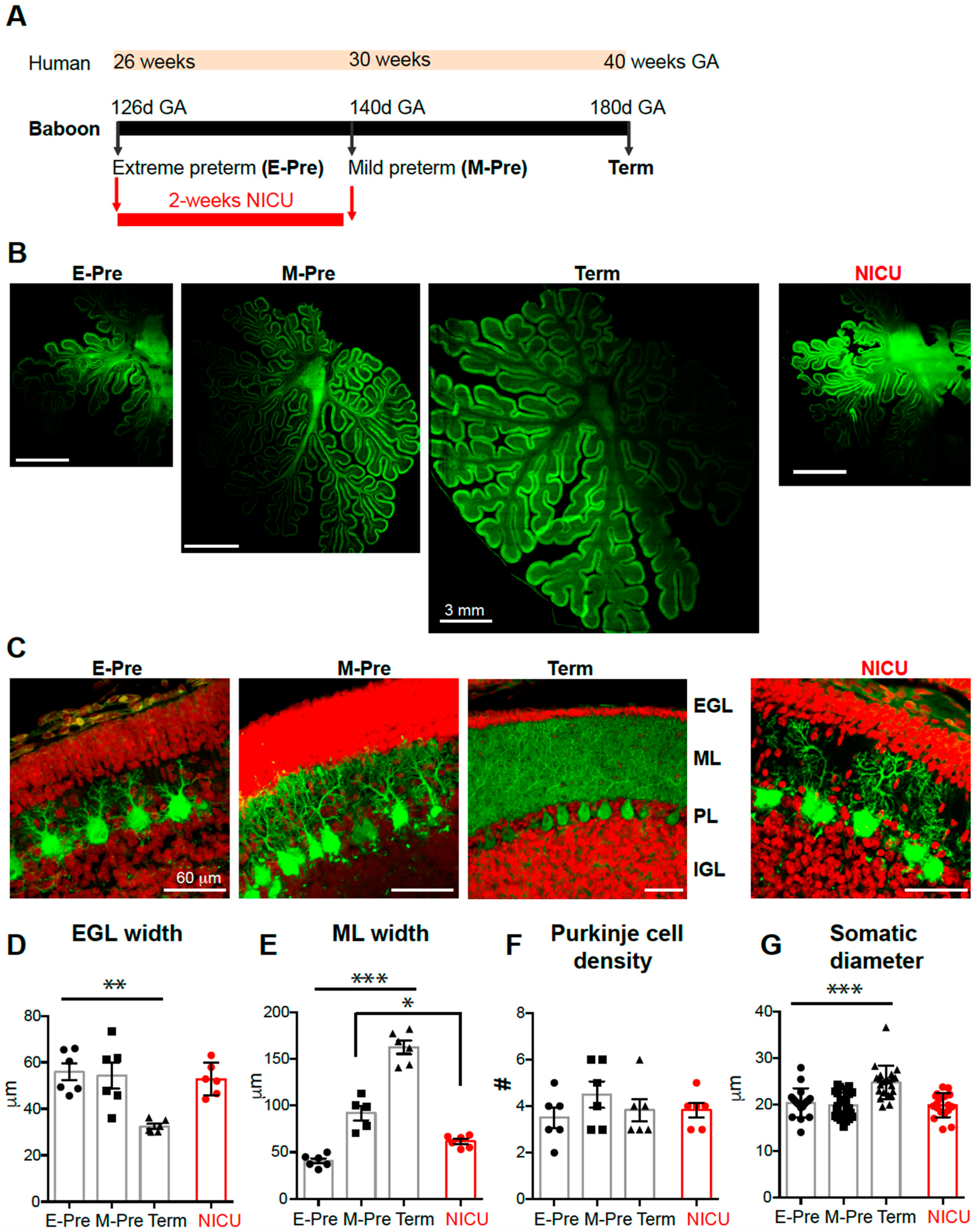
3.1. Morphological Development of the Cerebellum Occurs during Late Gestation and in the NICU
3.2. Preterm Birth Followed by NICU Experience Impacts Morphological Purkinje Cell Development
3.3. NICU Experience Influences the Late-Gestation Refinement of Intrinsic Electrophysiological Properties of Purkinje Cells
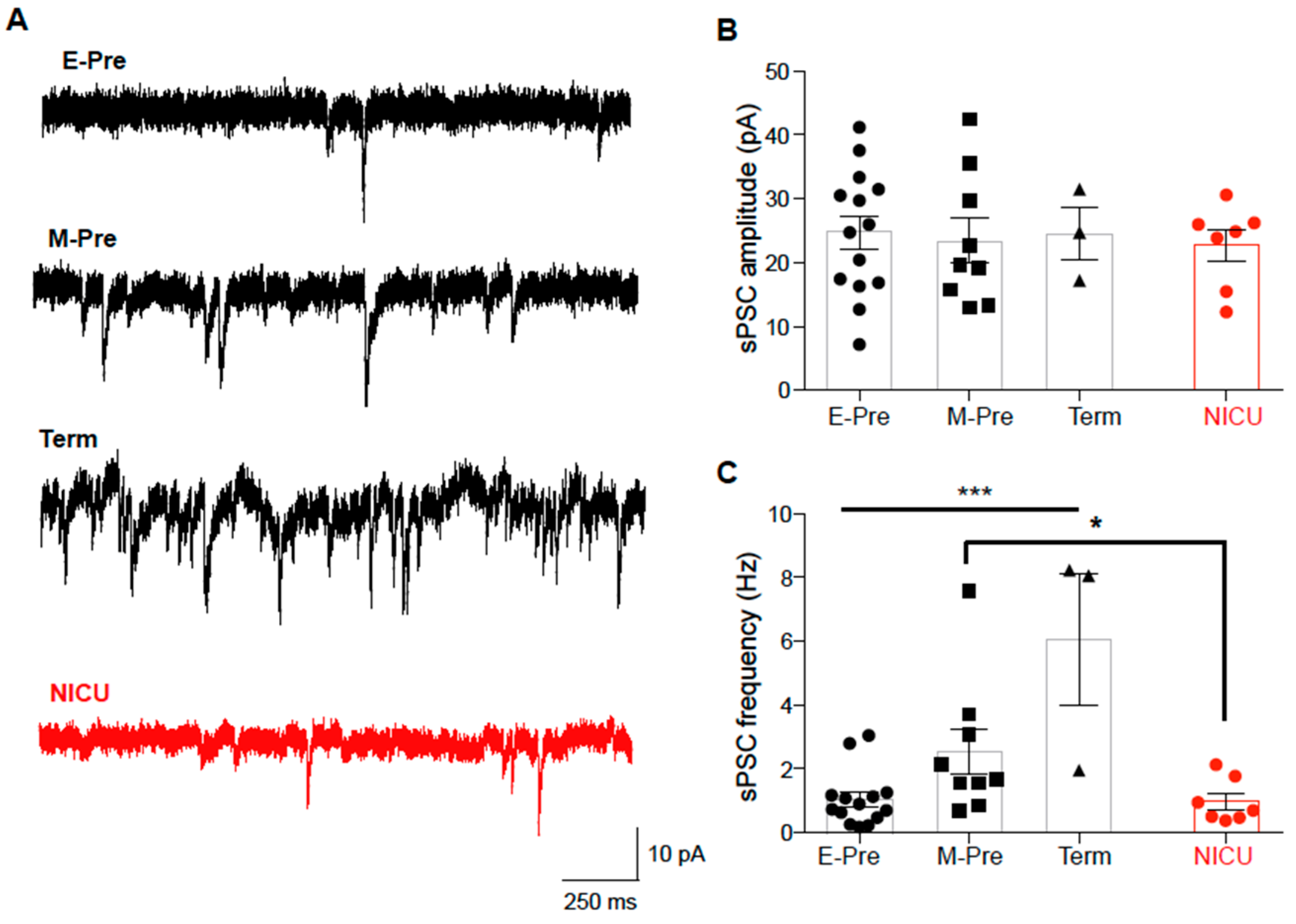
3.4. Synaptic Input to Purkinje Cells is Increased throughout Development and Impeded by NICU Experience
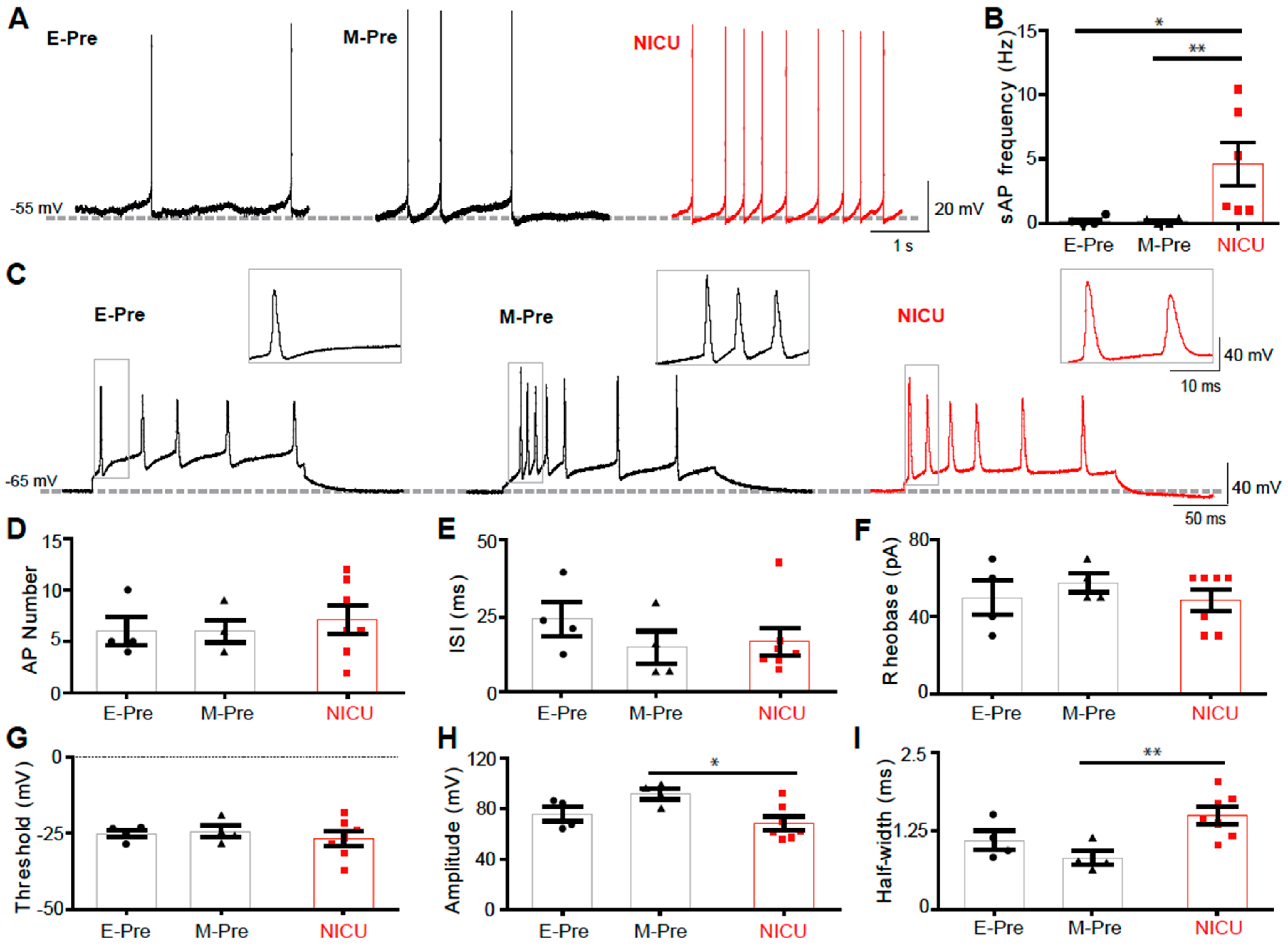
3.5. DCN Neurons of NICU-Experienced Animals have Altered AP Firing Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K.; Drake, P. Births: Final Data for 2017. Natl. Vital. Stat. Rep. 2018, 67, 1–50. [Google Scholar] [PubMed]
- Limperopoulos, C.; Soul, J.S.; Gauvreau, K.; Hüppi, P.S.; Warfield, S.K.; Bassan, H.; Robertson, R.; Volpe, J.J.; Du Plessis, A.J. Late Gestation Cerebellar Growth Is Rapid and Impeded by Premature Birth. Pediatrics 2005, 115, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Limperopoulos, C.; Bizzarro, M.J.; Raskind, C.; Baltimore, R.S.; Gallagher, P.G. Cerebellar Hemorrhage in the Preterm Infant: Ultrasonographic Findings and Risk Factors. Pediatrics 2005, 116, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; He, J.; Curati, W.L.; Dubowitz, L.M.S.; Cowan, F.M.; Bydder, G.M. Cerebellar infarction and atrophy in infants and children with a history of premature birth. Pediatr. Radiol. 1997, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Allin, M.; Matsumoto, H.; Santhouse, A.; Nosarti, C.; Alasady, M.H.S.; Stewart, A.L.; Rifkin, L.; Murray, R. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain 2001, 124, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, A.; Brugger, P.C.; Boltshauser, E.; Zoder, G.; Sterniste, W.; Birnbacher, R.; Prayer, D. Disruption of cerebellar development: Potential complication of extreme prematurity. Am. J. Neuroradiol. 2005, 26, 1659–1667. [Google Scholar] [PubMed]
- Volpe, J.J. Cerebellum of the Premature Infant: Rapidly Developing, Vulnerable, Clinically Important. J. Child Neurol. 2009, 24, 1085–1104. [Google Scholar] [CrossRef]
- Parker, J.; Mitchell, A.; Kalpakidou, A.; Walshe, M.; Jung, H.-Y.; Nosarti, C.; Santosh, P.; Rifkin, L.; Wyatt, J.; Murray, R.; et al. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain 2008, 131, 1344–1351. [Google Scholar] [CrossRef]
- Morrison, M.E.; Mason, C.A. Granule Neuron Regulation of Purkinje Cell Development: Striking a Balance Between Neurotrophin and Glutamate Signaling. J. Neurosci. 1998, 18, 3563–3573. [Google Scholar] [CrossRef]
- Telgkamp, P.; Raman, I.M. Depression of Inhibitory Synaptic Transmission between Purkinje Cells and Neurons of the Cerebellar Nuclei. J. Neurosci. 2002, 22, 8447–8457. [Google Scholar] [CrossRef]
- Person, A.L.; Raman, I.M. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nat. Cell Biol. 2012, 481, 502–505. [Google Scholar] [CrossRef]
- Barron, T.; Saifetiarova, J.; Bhat, M.A.; Kim, J.H. Myelination of Purkinje axons is critical for resilient synaptic transmission in the deep cerebellar nucleus. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Rees, S.; Loeliger, M.M.; Munro, K.M.; Shields, A.; Dalitz, P.A.; Dieni, S.; Thomson, M.A.; Coalson, J.; Inder, T. Cerebellar Development in a Baboon Model of Preterm Delivery. J. Neuropathol. Exp. Neurol. 2009, 68, 605–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loeliger, M.; Inder, T.E.; Shields, A.; Dalitz, P.; Cain, S.; Yoder, B.; Rees, S. High-frequency oscillatory ventilation is not associated with increased risk of neuropathology compared with positive pressure ventilation: A preterm primate model. Pediatr. Res. 2009, 66, 545–550. [Google Scholar] [CrossRef][Green Version]
- Haldipur, P.; Bharti, U.; Alberti, C.; Sarkar, C.; Gulati, G.; Iyengar, S.; Gressens, P.; Mani, S. Preterm Delivery Disrupts the Developmental Program of the Cerebellum. PLoS ONE 2011, 6, e23449. [Google Scholar] [CrossRef]
- McKay, B.E.; Turner, R.W. Physiological and morphological development of the rat cerebellar Purkinje cell. J. Physiol. 2005, 567, 829–850. [Google Scholar] [CrossRef]
- Blanco, C.L.; McGill-Vargas, L.L.; McCurnin, D.; Quinn, A.R. Hyperglycemia increases the risk of death in extremely preterm baboons. Pediatr. Res. 2013, 73, 337–343. [Google Scholar] [CrossRef]
- Volpe, J.J. Dysmaturation of Premature Brain: Importance, Cellular Mechanisms, and Potential Interventions. Pediatr. Neurol. 2019, 95, 42–66. [Google Scholar] [CrossRef]
- Haldipur, P.; Aldinger, K.A.; Bernardo, S.; Deng, M.; Timms, A.E.; Overman, L.M.; Winter, C.; Lisgo, S.; Razavi, F.; Silvestri, E.; et al. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 2019, 366, 454–460. [Google Scholar] [CrossRef]
- Zecevic, N.; Rakic, P. Differentiation of Purkinje cells and their relationship to other components of developing cerebellar cortex in man. J. Comp. Neurol. 1976, 167, 27–47. [Google Scholar] [CrossRef]
- Haldipur, P.; Bharti, U.; Govindan, S.; Sarkar, C.; Iyengar, S.; Gressens, P.; Mani, S. Expression of Sonic Hedgehog During Cell Proliferation in the Human Cerebellum. Stem Cells Dev. 2012, 21, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-H.; Lin, C.-Y.; Wang, J.; Sims, P.A.; Pan, M.-K.; Liou, J.-Y.; Lee, D.; Tate, W.J.; Kelly, G.C.; Louis, E.D.; et al. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol. 2017, 133, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-K.; Li, Y.-S.; Wong, S.-B.; Ni, C.-L.; Wang, Y.-M.; Liu, W.-C.; Lu, L.-Y.; Lee, J.-C.; Cortes, E.P.; Vonsattel, J.-P.G.; et al. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci. Transl. Med. 2020, 12, eaay1769. [Google Scholar] [CrossRef]
- Altman, J.; Anderson, W.J. Experimental reorganization of the cerebellar cortex. I. Morphological effects of elimination of all microneurons with prolonged x-irradiation started at birth. J. Comp. Neurol. 1972, 146, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P.; Sidman, R.L. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J. Comp. Neurol. 1973, 152, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.A. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 1999, 9, 445–448. [Google Scholar] [CrossRef]
- Tremblay, S.; Pai, A.; Richter, L.; Vafaei, R.; Potluri, P.; Ellegood, J.; Lerch, J.; Goldowitz, D. Systemic inflammation combined with neonatal cerebellar haemorrhage aggravates long-term structural and functional outcomes in a mouse model. Brain Behav. Immun. 2017, 66, 257–276. [Google Scholar] [CrossRef]
- Messerschmidt, A.; Prayer, D.; Brugger, P.C.; Boltshauser, E.; Zoder, G.; Sterniste, W.; Pollak, A.; Weber, M.A.; Birnbacher, R. Preterm birth and disruptive cerebellar development: Assessment of perinatal risk factors. Eur. J. Paediatr. Neurol. 2008, 12, 455–460. [Google Scholar] [CrossRef]
- Tam, E.W.; Miller, S.P.; Studholme, C.; Chau, V.; Glidden, D.; Poskitt, K.J.; Ferriero, D.M.; Barkovich, A.J. Differential Effects of Intraventricular Hemorrhage and White Matter Injury on Preterm Cerebellar Growth. J. Pediatr. 2011, 158, 366–371. [Google Scholar] [CrossRef]
- Rees, S.; Stringer, M.; Just, Y.; Hooper, S.B.; Harding, R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Dev. Brain Res. 1997, 103, 103–118. [Google Scholar] [CrossRef]
- Mallard, E.C.; Rees, S.; Stringer, M.; Cock, M.L.; Harding, R. Effects of Chronic Placental Insufficiency on Brain Development in Fetal Sheep. Pediatr. Res. 1998, 43, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Zonouzi, M.; Scafidi, J.; Li, P.; McEllin, B.; Edwards, J.; DuPree, J.L.; Harvey, L.; Sun, D.; Hübner, C.A.; Cull-Candy, S.G.; et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 2015, 18, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Sathyanesan, A.; Kundu, S.; Abbah, J.; Gallo, V. Neonatal brain injury causes cerebellar learning deficits and Purkinje cell dysfunction. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Noguchi, K.K.; Walls, K.C.; Wozniak, D.F.; Olney, J.W.; Roth, K.A.; Farber, N.B. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008, 15, 1582–1592. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barron, T.; Kim, J.H. Preterm Birth Impedes Structural and Functional Development of Cerebellar Purkinje Cells in the Developing Baboon Cerebellum. Brain Sci. 2020, 10, 897. https://doi.org/10.3390/brainsci10120897
Barron T, Kim JH. Preterm Birth Impedes Structural and Functional Development of Cerebellar Purkinje Cells in the Developing Baboon Cerebellum. Brain Sciences. 2020; 10(12):897. https://doi.org/10.3390/brainsci10120897
Chicago/Turabian StyleBarron, Tara, and Jun Hee Kim. 2020. "Preterm Birth Impedes Structural and Functional Development of Cerebellar Purkinje Cells in the Developing Baboon Cerebellum" Brain Sciences 10, no. 12: 897. https://doi.org/10.3390/brainsci10120897
APA StyleBarron, T., & Kim, J. H. (2020). Preterm Birth Impedes Structural and Functional Development of Cerebellar Purkinje Cells in the Developing Baboon Cerebellum. Brain Sciences, 10(12), 897. https://doi.org/10.3390/brainsci10120897




