Spontaneous Ultrasonic Vocalization Transmission in Adult, Male Long–Evans Rats Is Age-Dependent and Sensitive to EtOH Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ultrasonic Vocalization Recordings
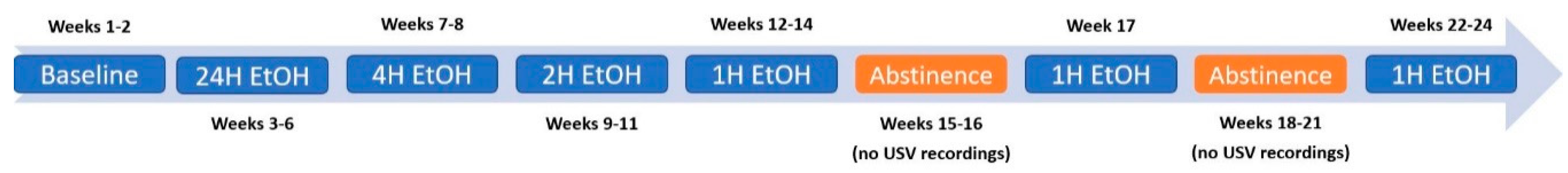
2.3. EtOH Consumption Timeline
2.4. WAAVES Automated Analysis of USVs
2.5. Validation Process for WAAVES Automation
2.6. Statistical Analyses of USV Counts and Acoustic Characteristics
2.6.1. Linear Mixed Models
2.6.2. Linear Regression
3. Results
3.1. Rate of USV Emissions
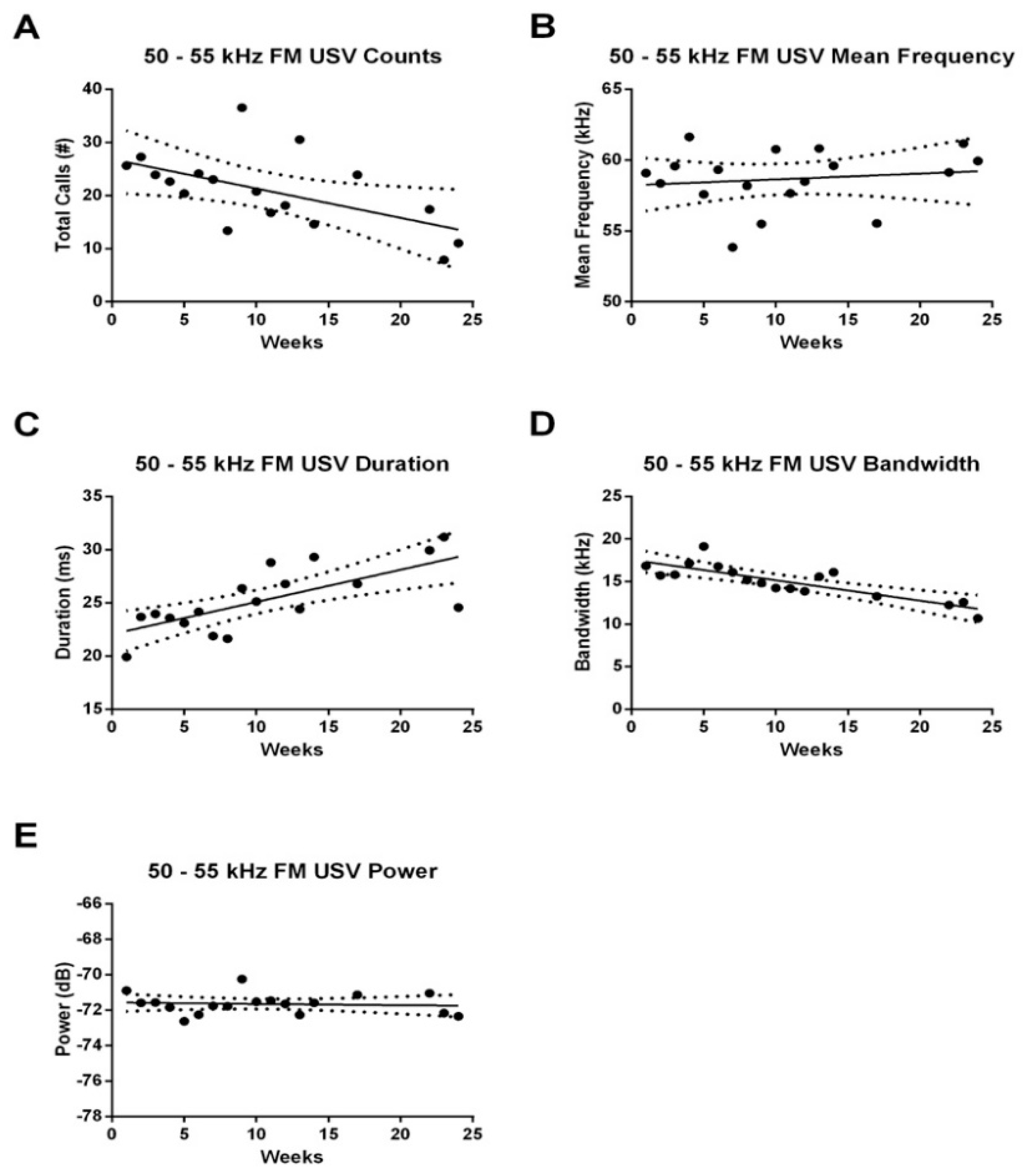
3.2. Effect of Age on USV Emissions
3.2.1. 50–55 kHz FM USVs
3.2.2. 22–28 kHz FM USVs
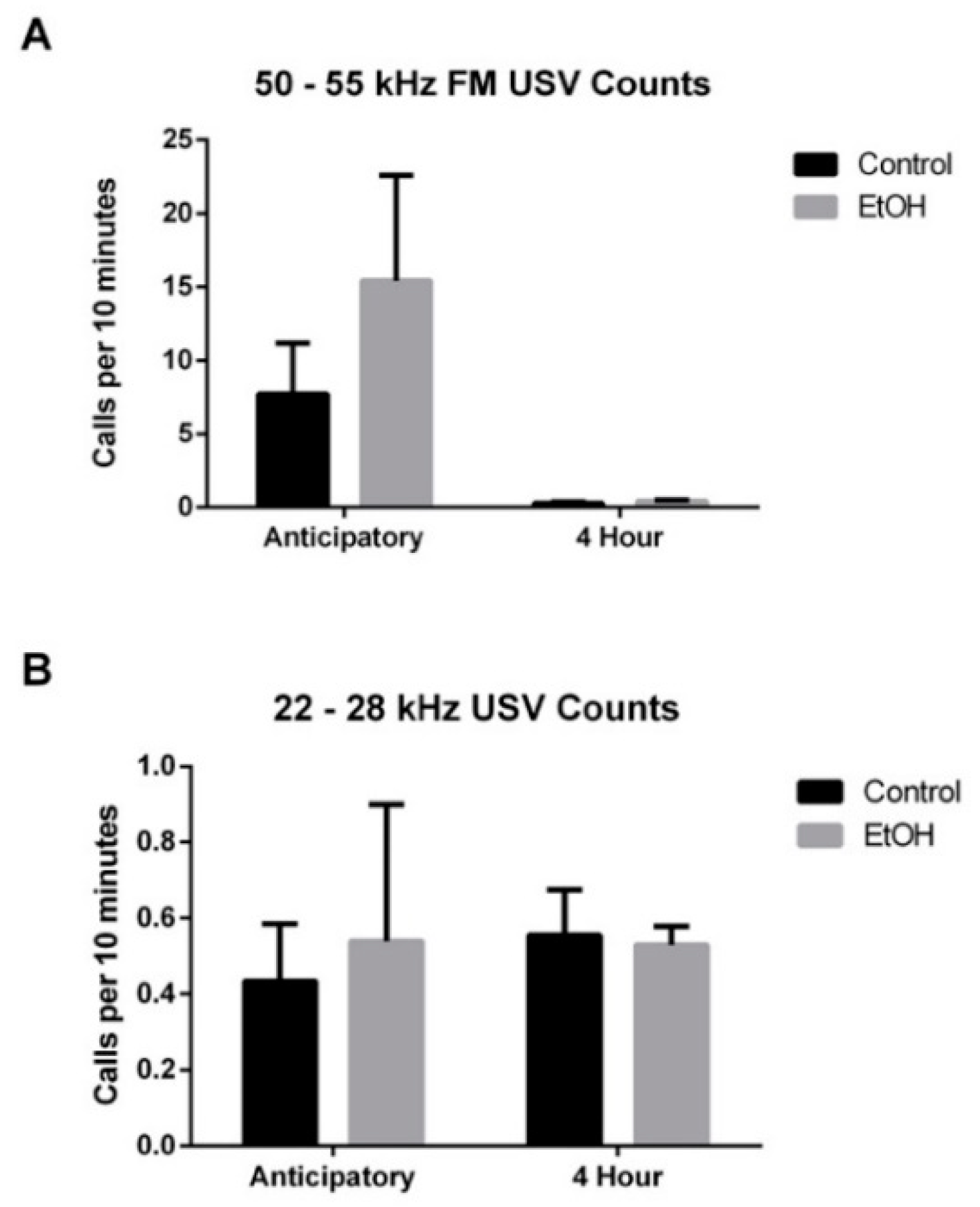
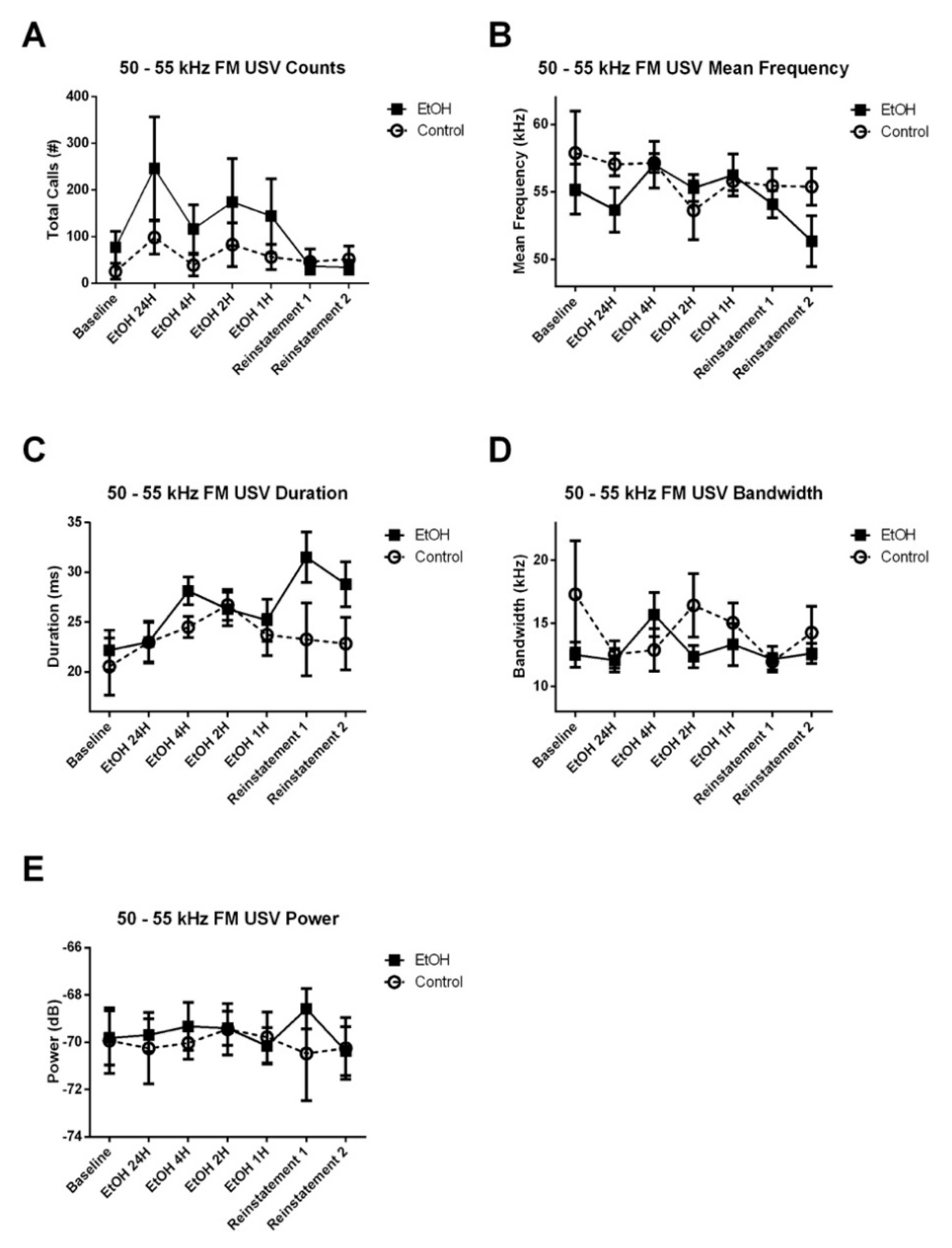
3.3. EtOH Modulation of USV Emissions
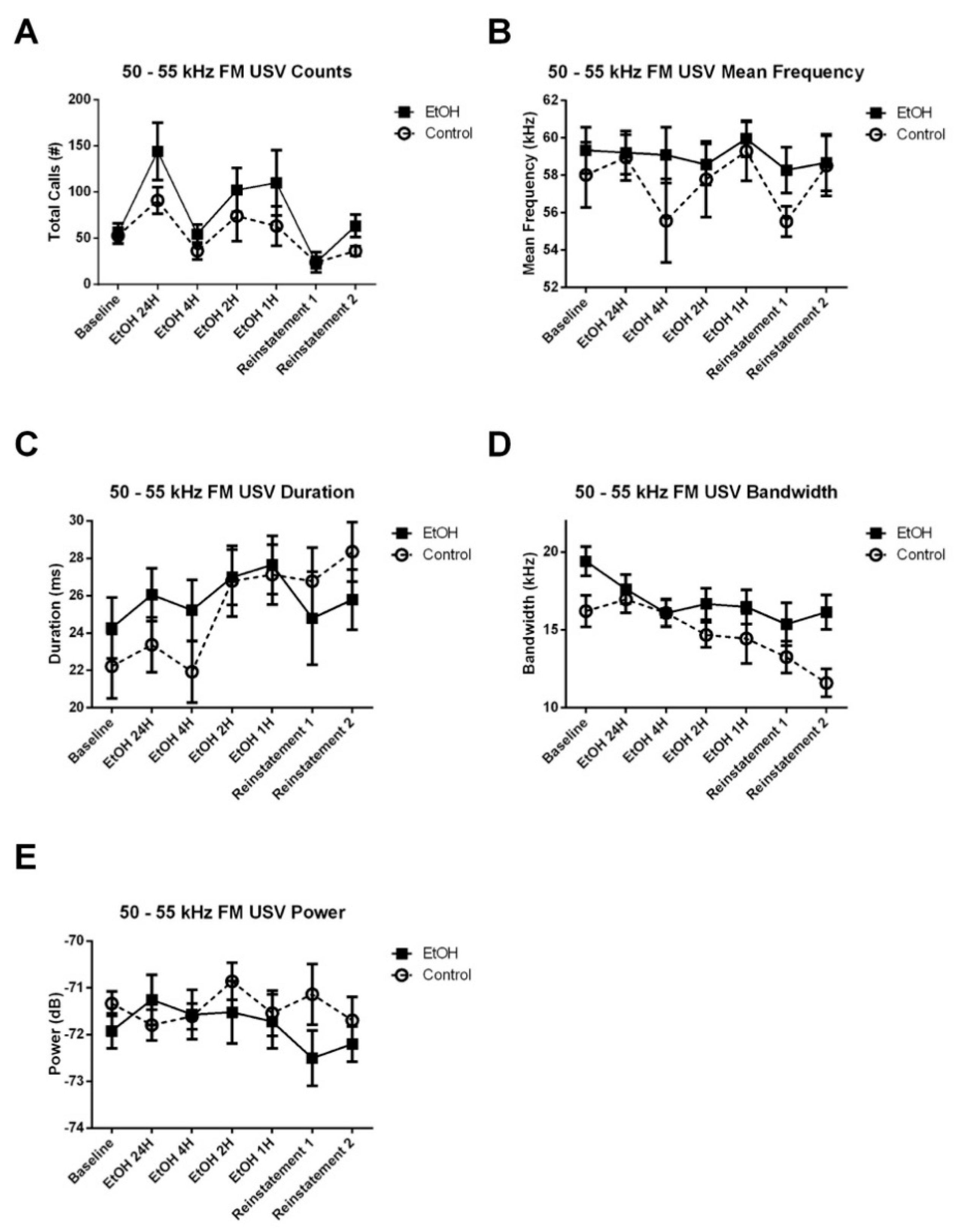
3.3.1. 50–55 kHz FM USVs
3.3.2. 22–28 kHz USVs
3.4. Validation of USV Automated Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nyby, J.; Whitney, G. Ultrasonic communication of adult myomorph rodents. Neurosci. Biobehav. Rev. 1978, 2, 1–14. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Pharmacology of Ultrasonic Vocalizations in adult Rats: Significance, Call Classification and Neural Substrate. Curr. Neuropharmacol. 2015, 13, 180–192. [Google Scholar] [CrossRef]
- Litvin, Y.; Blanchard, D.C.; Blanchard, R.J. Rat 22 kHz ultrasonic vocalizations as alarm cries. Behav. Brain Res. 2017, 182, 166–172. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Pharmacological and behavioral characteristics of 22kHz alarm calls in rats. Neurosci. Biobehav. Rev. 2011, 25, 611–617. [Google Scholar] [CrossRef]
- Wöhr, M.; Borta, A.; Schwarting, R.K.W. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: A dose-response study in the rat. Neurobiol. Learn. Mem. 2005, 84, 228–240. [Google Scholar] [CrossRef]
- Simola, N.; Fenu, S.; Costa, G.; Pinna, A.; Plumitallo, A.; Morelli, M. Pharmacological characterization of 50-kHz ultrasonic vocalizations in rats: Comparison of the effects of different psychoactive drugs and relevance in drug-induced reward. Neuropharmacology 2012, 63, 224–234. [Google Scholar] [CrossRef]
- Panksepp, J.; Burgdorf, J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: Effects of social housing and genetic variables. Behav. Brain Res. 2000, 115, 25–38. [Google Scholar] [CrossRef]
- Burgdorf, J.; Panksepp, J.; Moskal, J.R. Frequency-modulated 50kHz ultrasonic vocalizations: A tool for uncovering the molecular substrates of positive affect. Neurosci. Biobehav. Rev. 2011, 35, 1831–1836. [Google Scholar] [CrossRef]
- Kroes, R.A.; Burgdorf, J.; Otto, N.J.; Panksepp, J.; Moskal, J.R. Social defeat, a paradigm of depression in rats that elicits 22-kHz vocalizations, preferentially activates the cholinergic signaling pathway in the periaqueductal gray. Behav. Brain Res. 2007, 182, 290–300. [Google Scholar] [CrossRef]
- Machold, R.P. Loss of rostral brainstem cholinergic activity results in decreased ultrasonic vocalization behavior and altered sensorimotor gating. Behav. Brain Res. 2013, 256, 51–55. [Google Scholar] [CrossRef]
- Scardochio, T.; Clarke, P.B.S. Inhibition of 50-kHz ultrasonic vocalizations by dopamine receptor subtype-selective agonists and antagonists in adult rats. Psychopharmacology 2013, 226, 589–600. [Google Scholar] [CrossRef]
- Scardochio, T.; Trujillo-Pisanty, I.; Conover, K.; Shizgal, P.; Clarke, P.B.S. The Effects of Electrical and Optical Stimulation of Midbrain Dopaminergic Neurons on Rat 50-kHz Ultrasonic Vocalizations. Front. Behav. Neurosci. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Ciucci, M.R.; Ahrens, A.M.; Ma, S.T.; Kane, J.R.; Windham, E.B.; Woodlee, M.T.; Schallert, T. Reduction of dopamine synaptic activity: Degradation of 50-kHz ultrasonic vocalization in rats. Behav. Neurosci. 2009, 123, 328–336. [Google Scholar] [CrossRef]
- Johnson, A.M.; Grant, L.M.; Schallert, T.; Ciucci, M.R. Changes in rat 50-kHz ultrasonic vocalizations during dopamine denervation and aging: Relevance to neurodegeneration. Curr. Neuropharmacol. 2015, 13, 211–219. [Google Scholar] [CrossRef]
- Ahrens, A.M.; Nobile, C.W.; Page, L.E.; Maier, E.Y.; Duvauchelle, C.L.; Schallert, T. Individual differences in the conditioned and unconditioned rat 50-kHz ultrasonic vocalizations elicited by repeated amphetamine exposure. Psychopharmacology 2013, 229, 687–700. [Google Scholar] [CrossRef]
- Maier, E.Y.; Abdalla, M.; Ahrens, A.M.; Schallert, T.; Duvauchelle, C.L. The missing variable: Ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology 2012, 19, 1141–1152. [Google Scholar] [CrossRef]
- Weiss, F.; Lorang, M.T.; Bloom, F.E.; Koob, G.F. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J. Pharmacol. Exp. Ther. 1993, 267, 250–258. [Google Scholar]
- Engleman, E.; McBride, W.J.; Wilber, A.A.; Shaikh, S.R.; Eha, R.D.; Lumeng, L.; Li, T.K.; Murphy, J.M. Reverse microdialysis of a dopamine uptake inhibitor in the nucleus accumbens of alcohol-preferring rats: Effects on dialysate dopamine levels and ethanol intake. Alcohol. Clin. Exp. Res. 2000, 24, 795–801. [Google Scholar] [CrossRef]
- Hutchison, K.E.; Swift, R.; Rohsenow, D.J.; Monti, P.M.; Davidson, D.; Almeida, A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology 2001, 155, 27–34. [Google Scholar] [CrossRef]
- Panocka, I.; Pompei, P.; Massi, M. Suppression of alcohol preference in rats induced by risperidone, a serotonin 5-HT2 and dopamine D2 receptor antagonist. Brain Res. Bull. 1993, 31, 595–599. [Google Scholar] [CrossRef]
- Tiihonen, J.; Hallikainen, T.; Lachman, H.; Saito, T.; Volavka, J.; Kauhanen, J.; Salonen, J.T.; Ryynänen, O.P.; Koulu, M.; Karvonen, M.K.; et al. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Mol. Psychiatry 1999, 4, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Voisey, J.; Swagell, C.D.; Hughes, I.P.; Lawford, B.R.; Young, R.M.D.; Morris, C.P. A novel SNP in COMT is associated with alcohol dependence but not opiate or nicotine dependence: A case control study. Behav. Brain Funct. 2011, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.T.; Noronha, A.; Goldman, D.; Koob, G.F. Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology 2017. [Google Scholar] [CrossRef] [PubMed]
- Löf, E.; Olausson, P.; DeBejczy, A.; Stomberg, R.; McIntosh, J.M.; Taylor, J.R.; Söderpalm, B. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology 2007, 195, 333–343. [Google Scholar] [CrossRef]
- Ericson, M.; Molander, A.; Löf, E.; Engel, J.A.; Söderpalm, B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2003, 467, 85–93. [Google Scholar] [CrossRef]
- Ericson, M.; Sama, M.A.; Yeh, H.H. Acute ethanol exposure elevates muscarinic tone in the septohippocampal system. J. Neurophysiol. 2009, 103, 290–296. [Google Scholar] [CrossRef]
- Ericson, M.; Blomqvist, O.; Engel, J.A.; Söderpalm, B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur. J. Pharmacol. 1998, 358, 189–196. [Google Scholar] [CrossRef]
- Erwin, B.L.; Slaton, R.M. Varenicline in the Treatment of Alcohol Use Disorders. Ann. Pharmacother. 2014, 48, 1445. [Google Scholar] [CrossRef]
- Wang, J.C.; Hinrichs, A.L.; Stock, H.; Budde, J.; Allen, R.; Bertelsen, S.; Kwon, J.M.; Wu, W.; Dick, D.M.; Rice, J.; et al. Evidence of common and specific genetic effects: Association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum. Mol. Genet. 2004, 13, 1903–1911. [Google Scholar] [CrossRef]
- Jung, M.H.; Park, B.L.; Lee, B.-C.; Ro, Y.; Park, R.; Shin, H.D.; Bae, J.S.; Kang, T.-C.; Choi, I.-G. Association of CHRM2 polymorphisms with severity of alcohol dependence. Genes Brain Behav. 2010, 10, 253–256. [Google Scholar] [CrossRef]
- Wang, J.C.; Grucza, R.; Cruchaga, C.; Hinrichs, A.L.; Bertelsen, S.; Budde, J.P.; Fox, L.; Goldstein, E.; Reyes, O.; Saccone, N.; et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol. Psychiatry 2009, 14, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Ehringer, M.; Clegg, H.V.; Collins, A.C.; Corley, R.P.; Crowley, T.; Hewitt, J.K.; Hopfer, C.; Krauter, K.; Lessem, J.; Rhee, S.H.; et al. Association of the neuronal nicotinic receptor β2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Joslyn, G.; Brush, G.; Robertson, M.; Smith, T.L.; Kalmijn, J.; Schuckit, M.; White, R.L. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 20368–20373. [Google Scholar] [CrossRef] [PubMed]
- Reno, J.M.; Thakore, N.; Gonzales, R.; Schallert, T.; Bell, R.L.; Maddox, W.T.; Duvauchelle, C.L. Alcohol-preferring P rats emit spontaneous 22-28 kHz ultrasonic vocalizations that are altered by acute and chronic alcohol experience. Alcohol. Clin. Exp. Res. 2015, 39, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Thakore, N.; Reno, J.M.; Gonzales, R.A.; Schallert, T.; Bell, R.L.; Maddox, W.T.; Duvauchelle, C.L. Alcohol enhances unprovoked 22–28kHz USVs and suppresses USV mean frequency in High Alcohol Drinking (HAD-1) male rats. Behav. Brain Res. 2016, 302, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Thakore, N.; Reno, J.M.; Bell, R.L.; Maddox, W.T.; Schallert, T.; Duvauchelle, C.L. Alcohol-Naïve USVs Distinguish Male HAD-1 from LAD-1 Rat Strains. Alcoholism 2017. [Google Scholar] [CrossRef]
- Reno, J.M.; Thakore, N.; Cormack, L.K.; Schallert, T.; Bell, R.L.; Maddox, W.T.; Duvauchelle, C.L. Negative Affect-Associated USV Acoustic Characteristics Predict Future Excessive Alcohol Drinking and Alcohol Avoidance in Male P and NP Rats. Alcohol. Clin. Exp. Res. 2017, 1–12. [Google Scholar] [CrossRef]
- Mittal, N.; Maddox, W.T.; Schallert, T.; Duvauchelle, C.L. Rodent ultrasonic vocalizations as biomarkers of future alcohol use: A predictive analytic approach. Cogn. Affect. Behav. Neurosci. 2017. [Google Scholar] [CrossRef]
- Wöhr, M.; Houx, B.; Schwarting, R.K.W.; Spruijt, B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol. Behav. 2008, 93, 766–776. [Google Scholar] [CrossRef]
- Reno, J.M.; Marker, B.; Cormack, L.K.; Schallert, T.; Duvauchelle, C.L. Automating ultrasonic vocalization analyses: The WAAVES program. J. Neurosci. Methods 2013, 219, 155–161. [Google Scholar] [CrossRef]
- Reno, J.M.; Duvauchelle, C.L. Response to: Making WAAVES in the vocalization community: How big is the splash? J. Neurosci. Methods 2014, 221, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Tests in Linear Mixed Effects Models Version. Cran. URL 2016. Available online: https://CRAN.R-project.org/package=lmerTest (accessed on 26 June 2016).
- Ma, S.T.; Maier, E.Y.; Ahrens, A.M.; Schallert, T.; Duvauchelle, C.L. Repeated intravenous cocaine experience: Development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav. Brain Res. 2010, 212, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.Y.; Ahrens, A.M.; Ma, S.T.; Schallert, T.; Duvauchelle, C.L. Cocaine deprivation effect: Cue abstinence over weekends boosts anticipatory 50-kHz ultrasonic vocalizations in rats. Behav. Brain Res. 2010, 214, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.L.; Malavar, J.C.; George, O.; Koob, G.F.; Vendruscolo, L.F. Anticipatory 50 kHz ultrasonic vocalizations are associated with escalated alcohol intake in dependent rats. Behav. Brain Res. 2014, 271, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Basken, J.N.; Connor, N.P.; Ciucci, M.R. Effect of aging on ultrasonic vocalizations and laryngeal sensorimotor neurons in rats. Exp. Brain Res. 2012, 219, 351–361. [Google Scholar] [CrossRef]
- Priddy, B.M.; Carmack, S.A.; Thomas, L.C.; Vendruscolo, J.C.M.; Koob, G.F.; Vendruscolo, L.F. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol. Biochem. Behav. 2017, 152, 61–67. [Google Scholar] [CrossRef]
- Rahman, S.; Engleman, E.A.; Bell, R.L. Nicotinic receptor modulation to treat alcohol and drug dependence. Front. Neurosci. 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Prasad, P.; Ambekar, A.; Vaswani, M. Dopamine D2 receptor polymorphisms and susceptibility to alcohol dependence in Indian males: A preliminary study. BMC Med. Genet. 2010, 11, 24. [Google Scholar] [CrossRef]
- Vengeliene, V.; Bilbao, A.; Molander, A.; Spanagel, R. Neuropharmacology of alcohol addiction. Br. J. Pharmacol. 2008, 154, 299–315. [Google Scholar] [CrossRef]
- Yoshimoto, K.; McBride, W.J.; Lumeng, L.; Li, T.K. Ethanol enhances the release of dopamine and serotonin in the nucleus accumbens of HAD and LAD lines of rats. Alcohol. Clin. Exp. Res. 1992, 16, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Wood, P.L.; Kroes, R.A.; Moskal, J.R.; Panksepp, J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behav. Brain Res. 2007, 182, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Van Gaalen, M.M.; Schwarting, R.K. Affective communication in rodents. Behav. Pharmacol. 2015, 26, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Duvauchelle, C.L.; Maddox, W.T.; Reno, J.M.; Thakore, N.; Mittal, N.; Cormack, L.K.; Bell, R.L.; Gonzales, R.; Schallert, T. Alcohol-Preferring Rats and 22-kHz Negative-Affect. Ultrasonic Vocalizations; Brudzynski, S., Ed.; Elsevier Publishing: San Diego, CA, USA, 2018; Volume 25, pp. 401–411. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, N.; Maddox, W.T.; Schallert, T.; Duvauchelle, C.L. Spontaneous Ultrasonic Vocalization Transmission in Adult, Male Long–Evans Rats Is Age-Dependent and Sensitive to EtOH Modulation. Brain Sci. 2020, 10, 890. https://doi.org/10.3390/brainsci10110890
Mittal N, Maddox WT, Schallert T, Duvauchelle CL. Spontaneous Ultrasonic Vocalization Transmission in Adult, Male Long–Evans Rats Is Age-Dependent and Sensitive to EtOH Modulation. Brain Sciences. 2020; 10(11):890. https://doi.org/10.3390/brainsci10110890
Chicago/Turabian StyleMittal, Nitish, W. Todd Maddox, Timothy Schallert, and Christine L. Duvauchelle. 2020. "Spontaneous Ultrasonic Vocalization Transmission in Adult, Male Long–Evans Rats Is Age-Dependent and Sensitive to EtOH Modulation" Brain Sciences 10, no. 11: 890. https://doi.org/10.3390/brainsci10110890
APA StyleMittal, N., Maddox, W. T., Schallert, T., & Duvauchelle, C. L. (2020). Spontaneous Ultrasonic Vocalization Transmission in Adult, Male Long–Evans Rats Is Age-Dependent and Sensitive to EtOH Modulation. Brain Sciences, 10(11), 890. https://doi.org/10.3390/brainsci10110890




