Amygdala Low-Frequency Stimulation Reduces Pathological Phase-Amplitude Coupling in the Pilocarpine Model of Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Status Epilepticus Induction and Video Monitoring
2.3. Stereotaxic Procedures
2.4. EEG/Local Field Potential (LFP) Recording
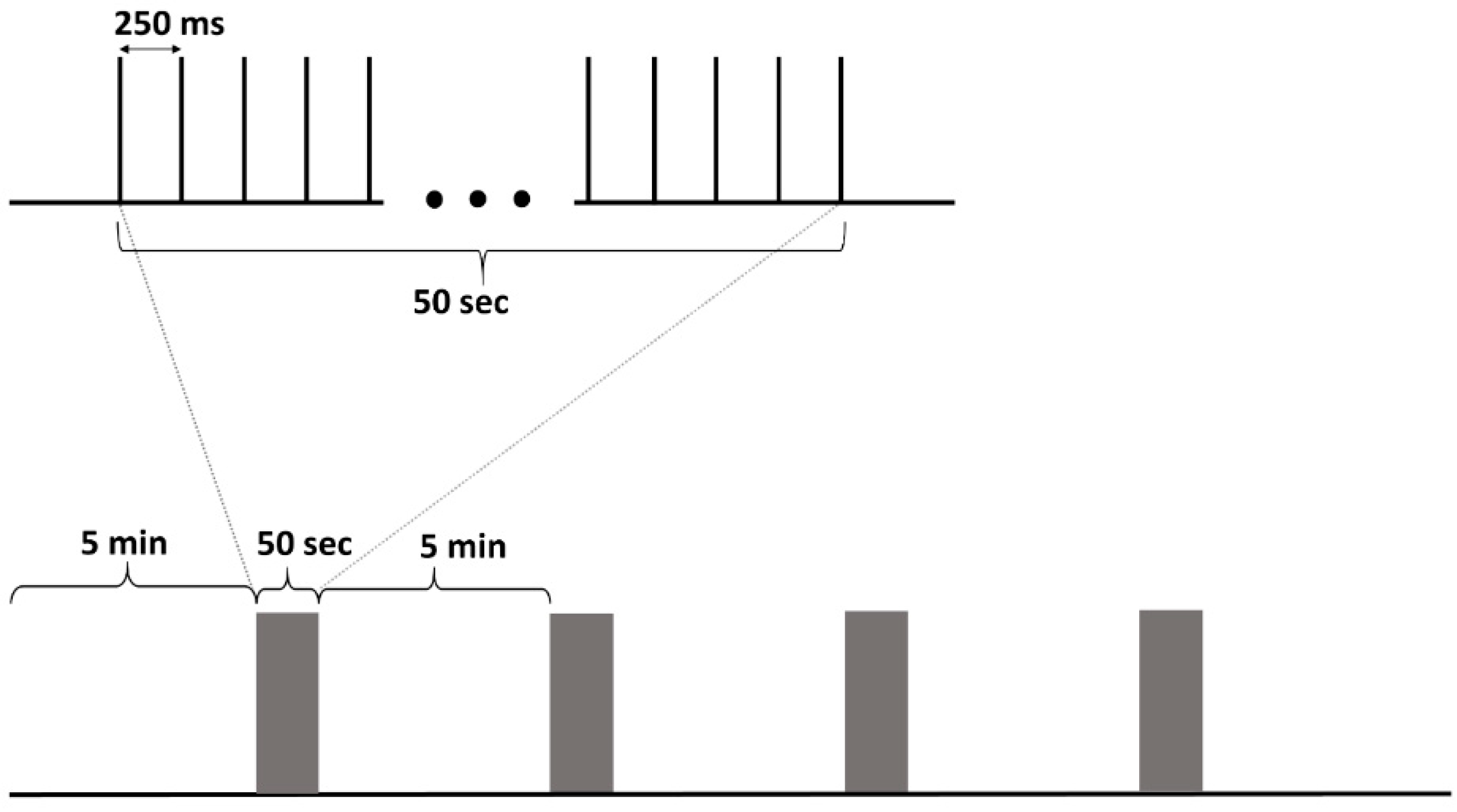
2.5. Stimulation Protocol
2.6. Histology
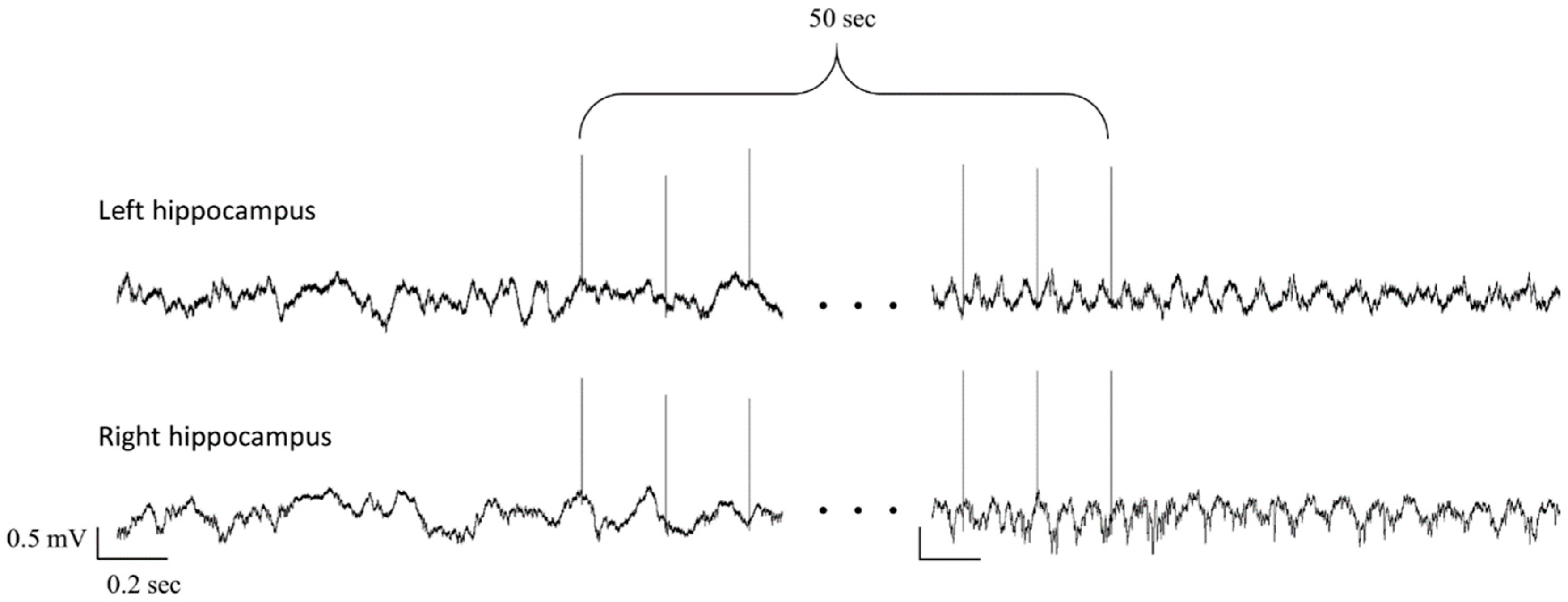
2.7. Interictal Discharges Analysis
2.8. Spectral Analysis
2.9. Phase-Amplitude Coupling (PAC)
2.10. Statistical Analysis
3. Results
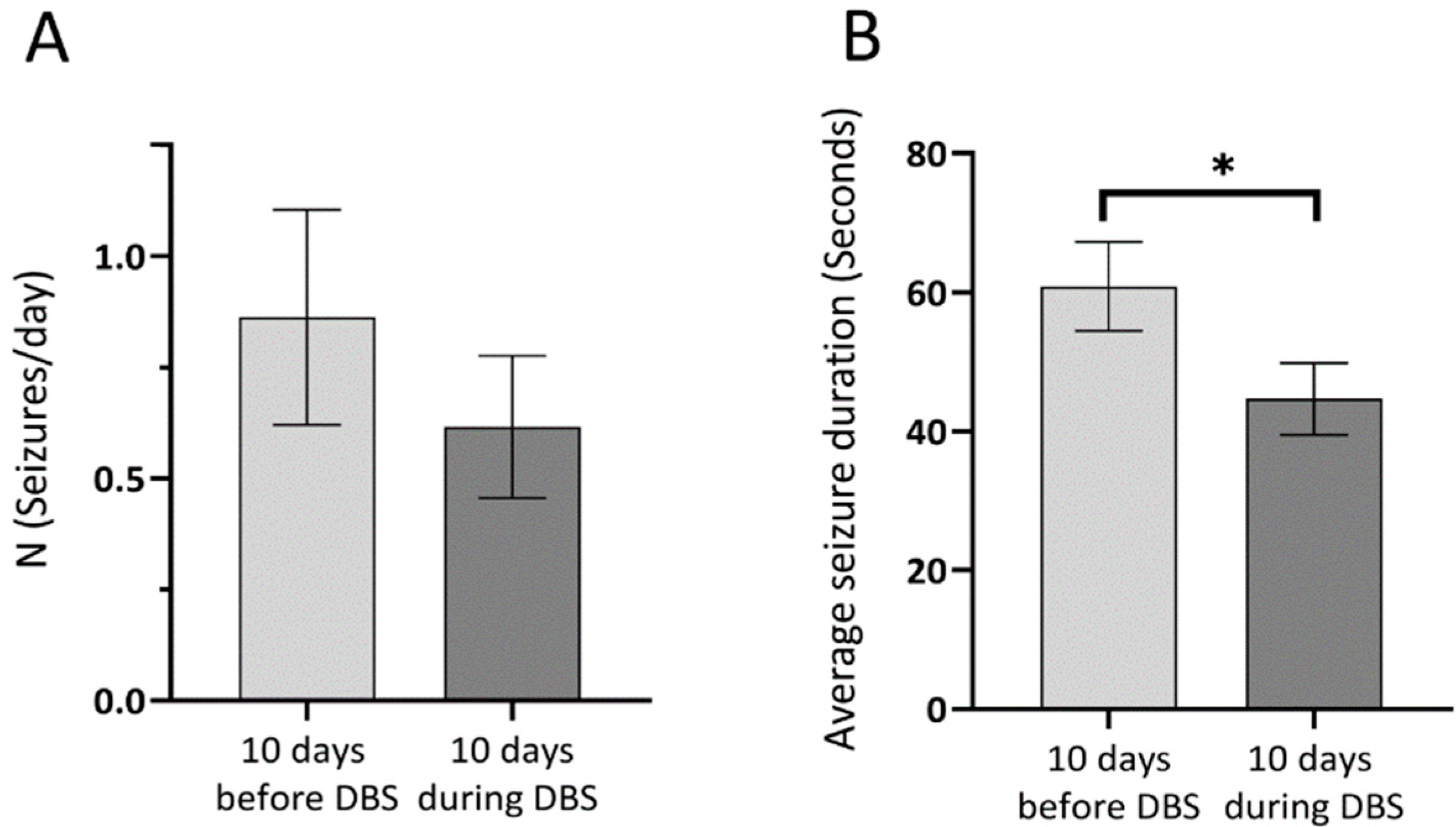
3.1. Seizure Rate and Duration
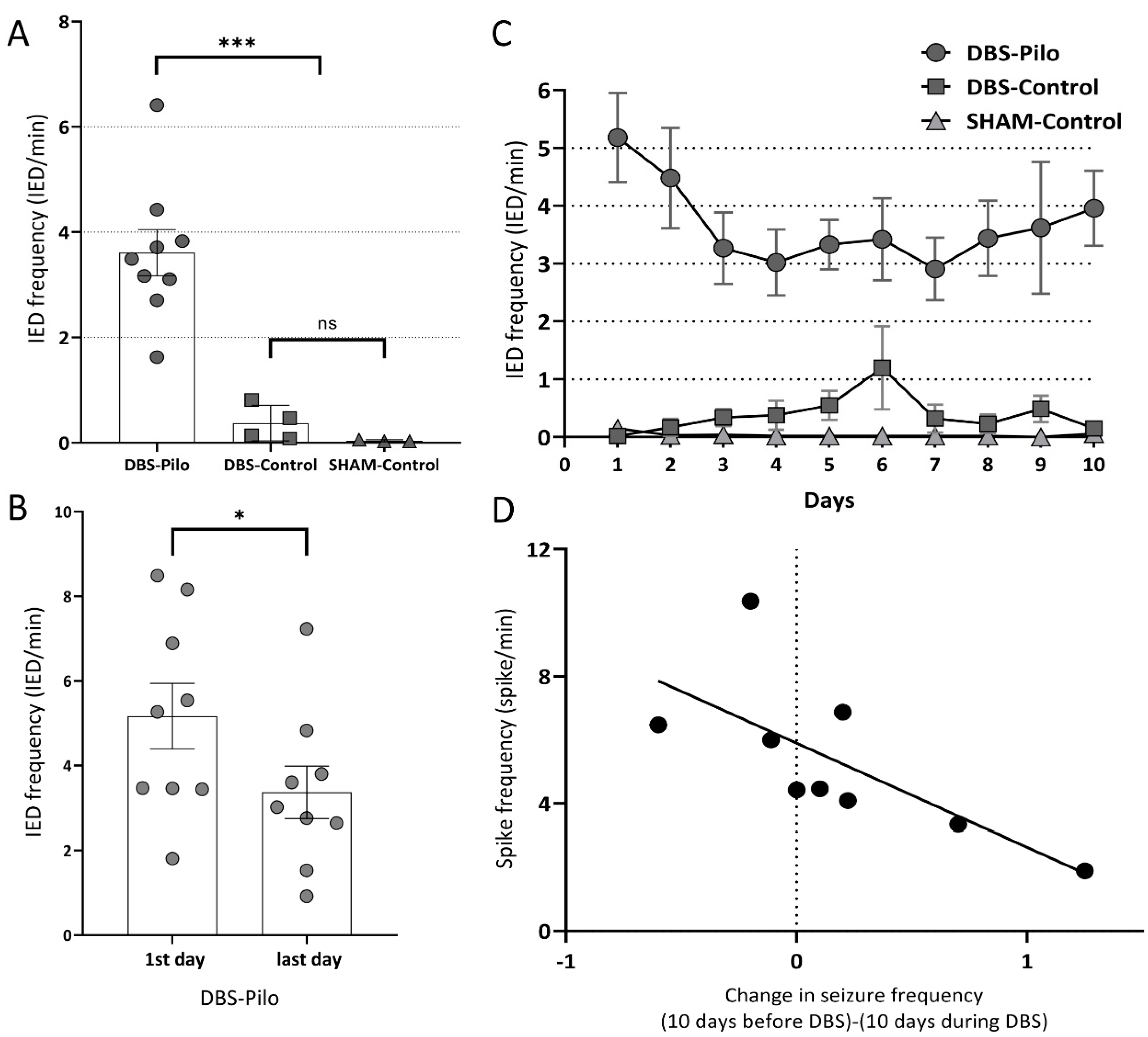
3.2. Interictal Epileptiform Discharges
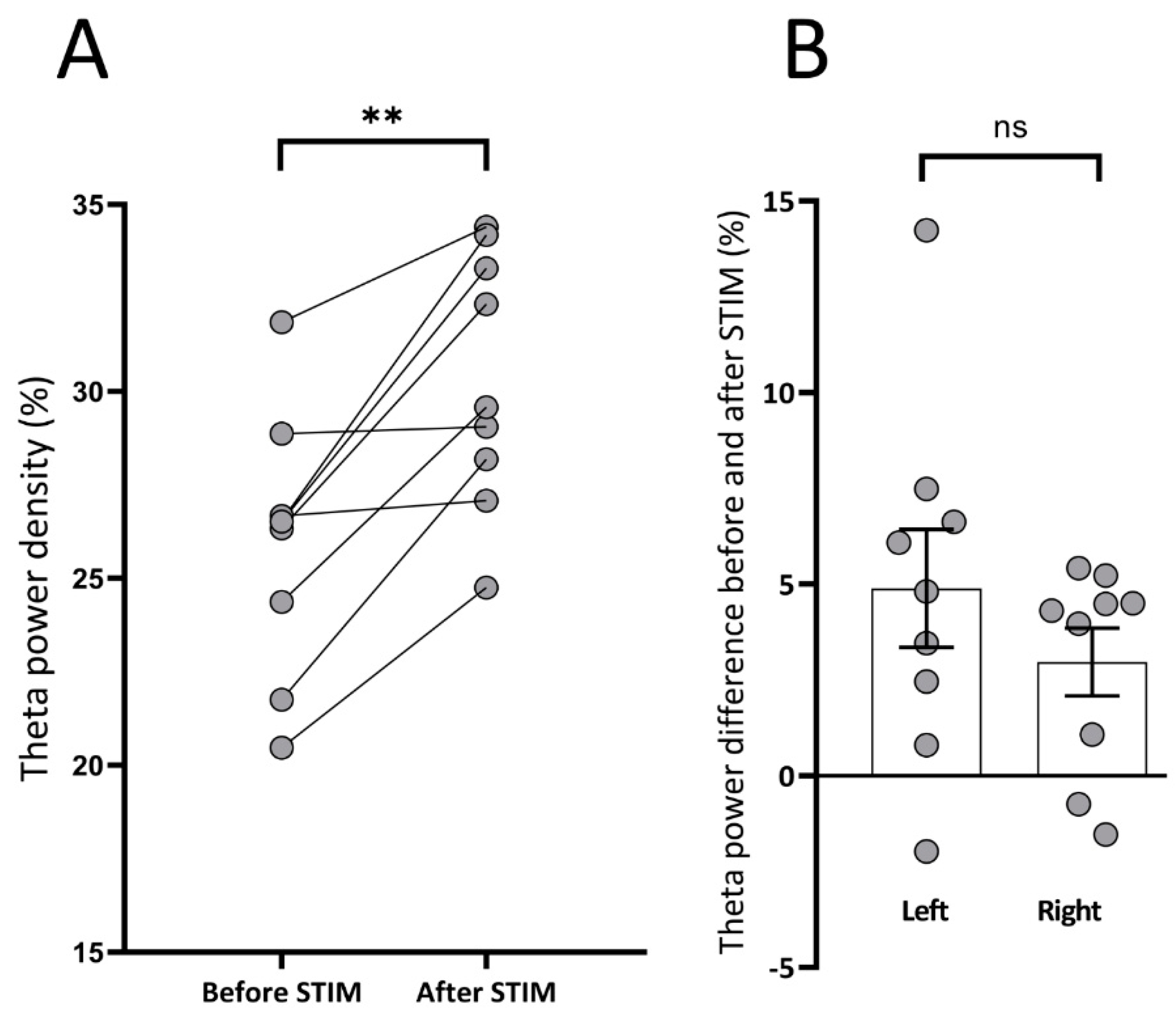
3.3. Spectral Analysis: Theta Power Density
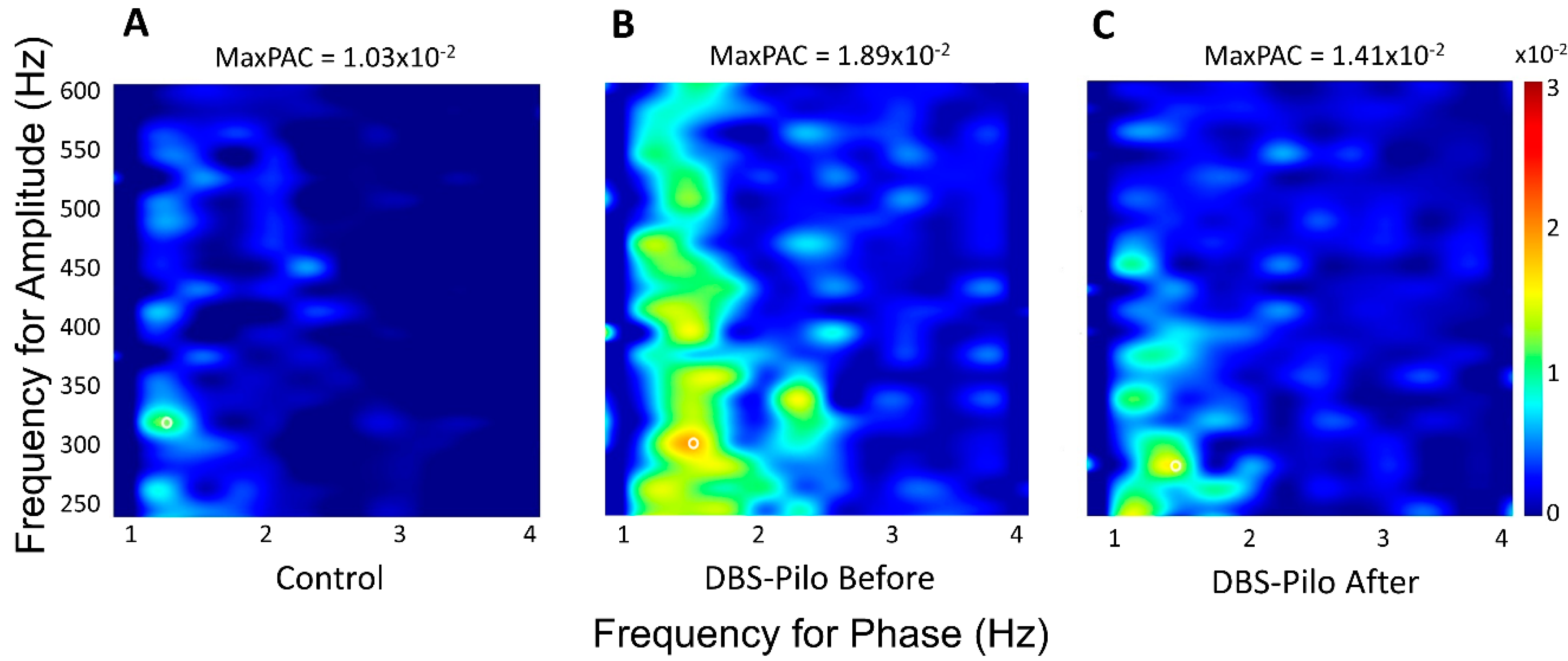
3.4. Phase-Amplitude Coupling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BLA | Basolateral Amygdala |
| DBS | Deep Brain Stimulation |
| EEG | Electroencephalography |
| GABA | Gamma-Aminobutyric Acid |
| HFO | High-Frequency Oscillation |
| HFS | High-Frequency Stimulation |
| IED | Interictal Epileptiform Discharge |
| LFP | Local Field Potential |
| LFS | Low-Frequency Stimulation |
| PAC | Phase-Amplitude Coupling |
| Pilo | Pilocarpine |
| PTZ | Pentylenetetrazole |
| SD | Standard Deviation |
| SE | Status Epilepticus |
| SEM | Standard Error of the Mean |
| SRSs | Spontaneous Recurrent Seizures |
| tPAC | Time-Resolved Phase-Amplitude Coupling |
| TLE | Temporal Lobe Epilepsy |
References
- Téllez-Zenteno, J.F.; Hernández-Ronquillo, L. A Review of the Epidemiology of Temporal Lobe Epilepsy. Epilepsy Res. Treat. 2012, 2012, 630853. [Google Scholar] [CrossRef] [PubMed]
- Engel, J., Jr. Mesial Temporal Lobe Epilepsy: What Have We Learned? Neuroscientist 2001, 7, 340–352. [Google Scholar] [CrossRef] [PubMed]
- French, J.A. Refractory epilepsy: Clinical overview. Epilepsia 2007, 48, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Schmidt, D. New horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res. 2006, 69, 183–272. [Google Scholar] [CrossRef] [PubMed]
- Centeno, R.S.; Yacubian, E.M.; Sakamoto, A.C.; Ferraz, A.F.P.; Junior, H.C.; Cavalheiro, S. Pre-surgical evaluation and surgical treatment in children with extratemporal epilepsy. Child’s Nerv. Syst. 2006, 22, 945–959. [Google Scholar] [CrossRef]
- Spencer, S.S. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002, 1, 375–382. [Google Scholar] [CrossRef]
- Zangiabadi, N.; Ladino, L.D.; Sina, F.; Orozco-Hernández, J.P.; Carter, A.; Téllez-Zenteno, J.F. Deep brain stimulation and drug-resistant epilepsy: A review of the literature. Front. Neurol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eastin, T.M.; Lopez-Gonzalez, M.A. Stimulation and neuromodulation in the treatment of epilepsy. Brain Sci. 2018, 8, 2. [Google Scholar] [CrossRef]
- Kwon, C.S.; Ripa, V.; Al-Awar, O.; Panov, F.; Ghatan, S.; Jetté, N. Epilepsy and neuromodulation—Randomized controlled trials. Brain Sci. 2018, 8, 69. [Google Scholar] [CrossRef]
- Wichmann, T.; DeLong, M.R. Deep Brain Stimulation for Neurologic and Neuropsychiatric Disorders. Neuron 2006, 52, 197–204. [Google Scholar] [CrossRef]
- Klinger, N.V.; Mittal, S. Clinical efficacy of deep brain stimulation for the treatment of medically refractory epilepsy. Clin. Neurol. Neurosurg. 2016, 140, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Salanova, V.; Witt, T.; Worth, R.; Henry, T.R.; Gross, R.E.; Nazzaro, J.M.; Labar, D.; Sperling, M.R.; Sharan, A.; Sandok, E.; et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015, 84, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Tykocki, T.; Mandat, T.; Kornakiewicz, A.; Koziara, H.; Nauman, P. Deep brain stimulation for refractory epilepsy. Arch. Med. Sci. 2012, 8, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, M.; Fomenko, A.; Lozano, A.M.; Kiening, K.L. Cellular, molecular, and clinical mechanisms of action of deep brain stimulation—A systematic review on established indications and outlook on future developments. EMBO Mol. Med. 2019, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cukiert, A.; Cukiert, C.M.; Burattini, J.A.; Mariani, P.P.; Bezerra, D.F. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: A prospective, controlled, randomized, double-blind study. Epilepsia 2017, 58, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Choi, S.J.; Joo, E.Y.; Seo, D.W.; Hong, S.B.; Lee, J.I.; Hong, S.C.; Lee, S.; Shon, Y.M. The Role of Anterior Thalamic Deep Brain Stimulation as an Alternative Therapy in Patients with Previously Failed Vagus Nerve Stimulation for Refractory Epilepsy. Stereotact. Funct. Neurosurg. 2019, 97, 176–182. [Google Scholar] [CrossRef]
- Hirsch, E.; Danober, L.; Simler, S.; De Vasconcelos, A.P.; Maton, B.; Nehlig, A.; Marescaux, C.; Vergnes, M. The amygdala is critical for seizure propagation from brainstem to forebrain. Neuroscience 1997, 77, 975–984. [Google Scholar] [CrossRef]
- Akirav, I.; Richter-Levin, G. Mechanisms of amygdala modulation of hippocampal plasticity. J. Neurosci. 2002, 22, 9912–9921. [Google Scholar] [CrossRef]
- Gaito, J.; Nobrega, J.N.; Gaito, S.T. Interference Effect of 3 Hz Brain Stimulation on Kindling Behavior Induced by 60 Hz Stimulation. Epilepsia 1980, 21, 73–84. [Google Scholar] [CrossRef]
- Ghotbedin, Z.; Janahmadi, M.; Mirnajafi-Zadeh, J.; Behzadi, G.; Semnanian, S. Electrical low frequency stimulation of the kindling site preserves the electrophysiological properties of the rat hippocampal CA1 pyramidal neurons from the destructive effects of amygdala kindling: The basis for a possible promising epilepsy therapy. Brain Stimul. 2013, 6, 515–523. [Google Scholar] [CrossRef]
- Fisher, R.; Salanova, V.; Witt, T.; Worth, R.; Henry, T.; Gross, R.; Oommen, K.; Osorio, I.; Nazzaro, J.; Laber, D.; et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010, 51, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Wyckhuys, T.; Boon, P.; Raedt, R.; Van Nieuwenhuyse, B.; Vonck, K.; Wadman, W. Suppression of hippocampal epileptic seizures in the kainate rat by Poisson distributed stimulation. Epilepsia 2010, 51, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Schrader, L.M.; Stern, J.M.; Wilson, C.L.; Fields, T.A.; Salamon, N.; Nuwer, M.R.; Vespa, P.M.; Fried, I. Low frequency electrical stimulation through subdural electrodes in a case of refractory status epilepticus. Clin. Neurophysiol. 2006, 117, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Ikeda, A.; Satow, T.; Takeshita, K.; Takayama, M.; Matsuhashi, M.; Matsumoto, R.; Ohara, S.; Mikuni, N.; Takahashi, J.; et al. Low-frequency electric cortical stimulation has an inhibitory effect on epileptic focus in mesial temporal lobe epilepsy. Epilepsia 2002, 43, 491–495. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; de Castro Medeiros, D.; de Souza e Rezende, G.H.; Moraes, M.F.D.; Cota, V.R. Temporally unstructured electrical stimulation to the amygdala suppresses behavioral chronic seizures of the pilocarpine animal model. Epilepsy Behav. 2014, 36, 159–164. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Maciel, R.M.; Moraes, M.F.D.; Rosa Cota, V. Asynchronous, bilateral, and biphasic temporally unstructured electrical stimulation of amygdalae enhances the suppression of pentylenetetrazole-induced seizures in rats. Epilepsy Res. 2018, 146, 1–8. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Drabowski, B.M.B.; Rodrigues, S.M.A.F.; Maciel, R.M.; Moraes, M.F.D.; Cota, V.R. Seizure suppression by asynchronous non-periodic electrical stimulation of the amygdala is partially mediated by indirect desynchronization from nucleus accumbens. Epilepsy Res. 2019, 154, 107–115. [Google Scholar] [CrossRef]
- Cota, V.R.; de Castro Medeiros, D.; da Páscoa Vilela, M.R.S.; Doretto, M.C.; Moraes, M.F.D. Distinct patterns of electrical stimulation of the basolateral amygdala influence pentylenetetrazole seizure outcome. Epilepsy Behav. 2009, 14, 26–31. [Google Scholar] [CrossRef]
- Kile, K.B.; Tian, N.; Durand, D.M. Low frequency stimulation decreases seizure activity in a mutation model of epilepsy. Epilepsia 2010, 51, 1745–1753. [Google Scholar] [CrossRef]
- Gharib, A.; Sayyahi, Z.; Komaki, A.; Barkley, V.; Sarihi, A.; Mirnajafi-Zadeh, J. The role of 5-HT1A receptors of hippocampal CA1 region in anticonvulsant effects of low-frequency stimulation in amygdala kindled rats. Physiol. Behav. 2018, 196, 119–125. [Google Scholar] [CrossRef]
- Ghafouri, S.; Fathollahi, Y.; Semnanian, S.; Shojaei, A.; Mirnajafi-Zadeh, J. Effects of low frequency stimulation on spontaneous inhibitory and excitatory post-synaptic currents in hippocampal CA1 pyramidal cells of kindled rats. Cell J. 2016, 18, 547–555. [Google Scholar] [PubMed]
- Ghafouri, S.; Fathollahi, Y.; Semnanian, S.; Shojaei, A.; Asgari, A.; Amini, A.E.; Mirnajafi-Zadeh, J. Deep brain stimulation restores the glutamatergic and GABAergic synaptic transmission and plasticity to normal levels in kindled rats. PLoS ONE 2019, 14, e0224834. [Google Scholar] [CrossRef] [PubMed]
- Dell, K.L.; Cook, M.J.; Maturana, M.I. Deep Brain Stimulation for Epilepsy: Biomarkers for Optimization. Curr. Treat. Options Neurol. 2019, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Jefferys, J.G.R.; Avoli, M. Interictal Epileptiform Discharges in Partial Epilepsy: Complex Neurobiological Mechanisms Based on Experimental and Clinical Evidence, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M., Olsen, R., Delgado-Escueta, A., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012.
- Bettus, G.; Wendling, F.; Guye, M.; Valton, L.; Régis, J.; Chauvel, P.; Bartolomei, F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008, 81, 58–68. [Google Scholar] [CrossRef]
- Miller, J.W.; Turner, G.M.; Gray, B.C. Anticonvulsant effects of the experimental induction of hippocampal theta activity. Epilepsy Res. 1994, 18, 195–204. [Google Scholar] [CrossRef]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef]
- Alvarado-Rojas, C.; Valderrama, M.; Fouad-Ahmed, A.; Feldwisch-Drentrup, H.; Ihle, M.; Teixeira, C.A.; Sales, F.; Schulze-Bonhage, A.; Adam, C.; Dourado, A.; et al. Slow modulations of high-frequency activity (40-140 Hz) discriminate preictal changes in human focal epilepsy. Sci. Rep. 2014, 4, 4545. [Google Scholar] [CrossRef]
- Zhang, R.; Ren, Y.; Liu, C.; Xu, N.; Li, X.; Cong, F.; Ristaniemi, T.; Wang, Y.P. Temporal-spatial characteristics of phase-amplitude coupling in electrocorticogram for human temporal lobe epilepsy. Clin. Neurophysiol. 2017, 128, 1707–1718. [Google Scholar] [CrossRef]
- Salimpour, Y.; Anderson, W.S. Cross-frequency coupling based neuromodulation for treating neurological disorders. Front. Neurosci. 2019, 13, 125. [Google Scholar] [CrossRef]
- Turski, W.A.; Cavalheiro, E.A.; Bortolotto, Z.A.; Mello, L.M.; Schwarz, M.; Turski, L. Seizures produced by pilocarpine in mice: A behavioral, electroencephalographic and morphological analysis. Brain Res. 1984, 32, 237–253. [Google Scholar] [CrossRef]
- Blair, R.E.; Deshpande, L.S.; Holbert, W.H.; Churn, S.B.; DeLorenzo, R.J. Age-dependent mortality in the pilocarpine model of status epilepticus. Neurosci. Lett. 2009, 453, 233–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clifford, D.B.; Olney, J.W.; Maniotis, A.; Collins, R.C.; Zorumski, C.F. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience 1987, 23, 953–968. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.G.; Avoli, M.; Biomediche, S.; Emilia, R.; Ba, B.; Kingdom, U.; Sperimentale, M.; et al. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Lüttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. (Eds.) The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: London, UK, 2007; ISBN 9780125476126. [Google Scholar]
- Kane, N.; Acharya, J.; Benickzy, S.; Caboclo, L.; Finnigan, S.; Kaplan, P.W.; Shibasaki, H.; Pressler, R.; van Putten, M.J.A.M. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Pract. 2017, 2, 170–185. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.; Pantazis, D.; Leahy, R. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Samiee, S.; Baillet, S. Time-resolved phase-amplitude coupling in neural oscillations. Neuroimage 2017, 159, 270–279. [Google Scholar] [CrossRef]
- Chauvière, L.; Rafrafi, N.; Thinus-Blanc, C.; Bartolomei, F.; Esclapez, M.; Bernard, C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J. Neurosci. 2009, 29, 5402–5410. [Google Scholar] [CrossRef]
- Shuman, T.; Amendolara, B.; Golshani, P. Theta Rhythmopathy in TLE Cognitive Disability. Epilepsy Curr. 2017, 17, 107–111. [Google Scholar] [CrossRef]
- Kitchigina, V.F. Alterations of Coherent Theta and Gamma Network Oscillations as an Early Biomarker of Temporal Lobe Epilepsy and Alzheimer’s Disease. Front. Integr. Neurosci. 2018, 12, 36. [Google Scholar] [CrossRef]
- Asgari, A.; Semnanian, S.; Atapour, N.; Shojaei, A.; Moradi, H.; Mirnajafi-Zadeh, J. Combined sub-threshold dosages of phenobarbital and low-frequency stimulation effectively reduce seizures in amygdala-kindled rats. Neurol. Sci. 2014, 35, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Shahpari, M.; Mirnajafi-Zadeh, J.; Seyed, S.M.; Yadollahpour, A. Effect of low-frequency electrical stimulation parameters on its anticonvulsant action during rapid perforant path kindling in rat. Epilepsy Res. 2012, 99, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Velasco, A.L. Electrical brain stimulation for epilepsy. Nat. Rev. Neurol. 2014, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-N.; Lee, C.-Y.; Lee, S.-T.; Tu, P.-H.; Chang, B.-L.; Lee, C.-H.; Cheng, M.-Y.; Chang, C.-W.; Tseng, W.-E.J.; Hsieh, H.-Y.; et al. Low and High Frequency Hippocampal Stimulation for Drug-Resistant Mesial Temporal Lobe Epilepsy. Neuromodul. Technol. Neural Interface 2016, 19, 365–372. [Google Scholar] [CrossRef]
- Mina, F.; Benquet, P.; Pasnicu, A.; Biraben, A.; Wendling, F. Modulation of epileptic activity by deep brain stimulation: A model-based study of frequency-dependent effects. Front. Comput. Neurosci. 2013, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Jalilifar, M.; Yadollahpour, A.; Moazedi, A.A.; Ghotbeddin, Z. Low Frequency Electrical Stimulation Either Prior to or after Rapid Kindling Stimulation Inhibits the Kindling-Induced Epileptogenesis. BioMed Res. Int. 2017, 2017, 8623. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, B.; Raedt, R.; Delbeke, J.; Wadman, W.J.; Boon, P.; Vonck, K. In search of optimal DBS paradigms to treat epilepsy: Bilateral versus unilateral hippocampal stimulation in a rat model for temporal lobe epilepsy. Brain Stimul. 2015, 8, 192–199. [Google Scholar] [CrossRef]
- Jiruska, P.; de Curtis, M.; Jefferys, J.G.R.; Schevon, C.A.; Schiff, S.J.; Schindler, K. Synchronization and desynchronization in epilepsy: Controversies and hypotheses. J. Physiol. 2013, 591, 787–797. [Google Scholar] [CrossRef]
- Li, H.; Fan, W.; Yang, J.; Song, S.; Liu, Y.; Lei, P.; Shrestha, L.; Mella, G.; Chen, W.; Xu, H. Asymmetry in cross-hippocampal connectivity in unilateral mesial temporal lobe epilepsy. Epilepsy Res. 2015, 118, 14–21. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 4, 18024. [Google Scholar] [CrossRef] [PubMed]
- Karoly, P.J.; Freestone, D.R.; Boston, R.; Grayden, D.B.; Himes, D.; Leyde, K.; Seneviratne, U.; Berkovic, S.; O’Brien, T.; Cook, M.J. Interictal spikes and epileptic seizures: Their relationship and underlying rhythmicity. Brain 2016, 139, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Kudlacek, J.; Hlinka, J.; Chvojka, J.; Hadrava, M.; Kumpost, V.; Powell, A.D.; Janca, R.; Maturana, M.I.; Karoly, P.J.; et al. Loss of neuronal network resilience precedes seizures and determines the ictogenic nature of interictal synaptic perturbations. Nat. Neurosci. 2018, 21, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Ikeda, A.; Matsumoto, R.; Begum, T.; Usui, K.; Yamamoto, J.; Matsuhashi, M.; Takayama, M.; Mikuni, N.; Takahashi, J.; et al. Electric stimulation on human cortex suppresses fast cortical activity and epileptic spikes. Epilepsia 2004, 45, 787–791. [Google Scholar] [CrossRef]
- Yamamoto, J.; Ikeda, A.; Kinoshita, M.; Matsumoto, R.; Satow, T.; Takeshita, K.; Matsuhashi, M.; Mikuni, N.; Miyamoto, S.; Hashimoto, N.; et al. Low-frequency electric cortical stimulation decreases interictal and ictal activity in human epilepsy. Seizure 2006, 15, 520–527. [Google Scholar] [CrossRef]
- Arcot Desai, S.; Tcheng, T.K.; Morrell, M.J. Quantitative electrocorticographic biomarkers of clinical outcomes in mesial temporal lobe epileptic patients treated with the RNS® system. Clin. Neurophysiol. 2019, 130, 1364–1374. [Google Scholar] [CrossRef]
- Pitkänen, A.; Pikkarainen, M.; Nurminen, N.; Ylinen, A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 2000, 911, 369–391. [Google Scholar] [CrossRef]
- Carrington, C.A.; Gilby, K.L.; Mcintyre, D.C. Effect of Focal Low-frequency Stimulation on Amygdala-kindled Afterdischarge Thresholds and Seizure Profiles in Fast- and Slow-kindling Rat Strains. Epilepsia 2007, 48, 1604–1613. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Cheng, H.; Guo, Y.; Xu, C.; Wang, S.; Zhang, J.; Ding, M.; Chen, Z. Low-frequency stimulation inhibits epileptogenesis by modulating the early network of the limbic system as evaluated in amygdala kindling model. Brain Struct. Funct. 2013, 219, 1685–1696. [Google Scholar] [CrossRef]
- Arabadzisz, D.; Antal, K.; Parpan, F.; Emri, Z.; Fritschy, J.M. Epileptogenesis and chronic seizures in a mouse model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. Exp. Neurol. 2005, 194, 76–90. [Google Scholar] [CrossRef]
- Colom, L.V.; Garcıa-Hernandez, A.; Castaneda, M.T.; Perez-Cordova, M.G.; Garrido-Sanabria, E.R. Septo-Hippocampal Networks in Chronically Epileptic Rats: Potential Antiepileptic Effects of Theta Rhythm Generation. J. Neurophysiol. 2006, 95, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.W.; Özkan, M.; Burgess, A.P.; Prokic, E.J.; Wafford, K.A.; O’Neill, M.J.; Greenhill, S.D.; Stanford, I.M.; Woodhall, G.L. Phase-amplitude coupled persistent theta and gamma oscillations in rat primary motor cortex in vitro. Neuropharmacology 2017, 119, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Dugladze, T.; Vida, I.; Tort, A.B.; Gross, A.; Otahal, J.; Heinemann, U.; Kopell, N.J.; Gloveli, T. Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA 2007, 104, 17530–17535. [Google Scholar] [CrossRef] [PubMed]
- Izadi, A.; Pevzner, A.; Lee, D.J.; Ekstrom, A.D.; Shahlaie, K.; Gurkoff, G.G. Medial septal stimulation increases seizure threshold and improves cognition in epileptic rats. Brain Stimul. 2019, 12, 735–742. [Google Scholar] [CrossRef]
- Lee, D.J.; Izadi, A.; Melnik, M.; Seidl, S.; Echeverri, A.; Shahlaie, K.; Gurkoff, G.G. Stimulation of the medial septum improves performance in spatial learning following pilocarpine-induced status epilepticus. Epilepsy Res. 2017, 130, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.; Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef]
- Buzsáki, G.; Mizuseki, K. The log-dynamic brain: How skewed distributions affect network operations. Nat. Rev. Neurosci. 2014, 15, 264–278. [Google Scholar] [CrossRef]
- Tort, A.B.L.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef]
- Jefferys, J.G.R.; Menendez de la Prida, L.; Wendling, F.; Bragin, A.; Avoli, M.; Timofeev, I.; Lopes da Silva, F.H. Mechanisms of physiological and epileptic HFO generation. Prog. Neurobiol. 2012, 98, 250–264. [Google Scholar]
- Dzhala, V.I.; Staley, K.J. Mechanisms of fast ripples in the hippocampus. J. Neurosci. 2004, 24, 8896–8906. [Google Scholar] [CrossRef]
- Sotero, R.C. Modeling the Generation of Phase-Amplitude Coupling in Cortical Circuits: From Detailed Networks to Neural Mass Models. BioMed Res. Int. 2015, 2015, 915606. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Frauscher, B.; Gotman, J. Phase-amplitude coupling is elevated in deep sleep and in the onset zone of focal epileptic seizures. Front. Hum. Neurosci. 2016, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Samiee, S.; Lévesque, M.; Avoli, M.; Baillet, S. Phase-amplitude coupling and epileptogenesis in an animal model of mesial temporal lobe epilepsy. Neurobiol. Dis. 2018, 114, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Vendramin Pasquetti, M.; Meier, L.; Righes Marafiga, J.; Barbieri Caus, L.; Bretanha Lopes Tort, A.; Elisa Calcagnotto, M. Hippocampal CA1 and cortical interictal oscillations in the pilocarpine model of epilepsy. Brain Res. 2019, 1722, 146351. [Google Scholar] [CrossRef]
- Buzsáki, G.; Wang, X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar]
- Amorim, B.O.; Covolan, L.; Ferreira, E.; Brito, J.G.; Nunes, D.P.; de Morais, D.G.; Nobrega, J.N.; Rodrigues, A.M.; de Almeida, A.C.G.; Hamani, C. Deep brain stimulation induces antiapoptotic and anti-inflammatory effects in epileptic rats. J. Neuroinflamm. 2015, 12, 1–5. [Google Scholar]
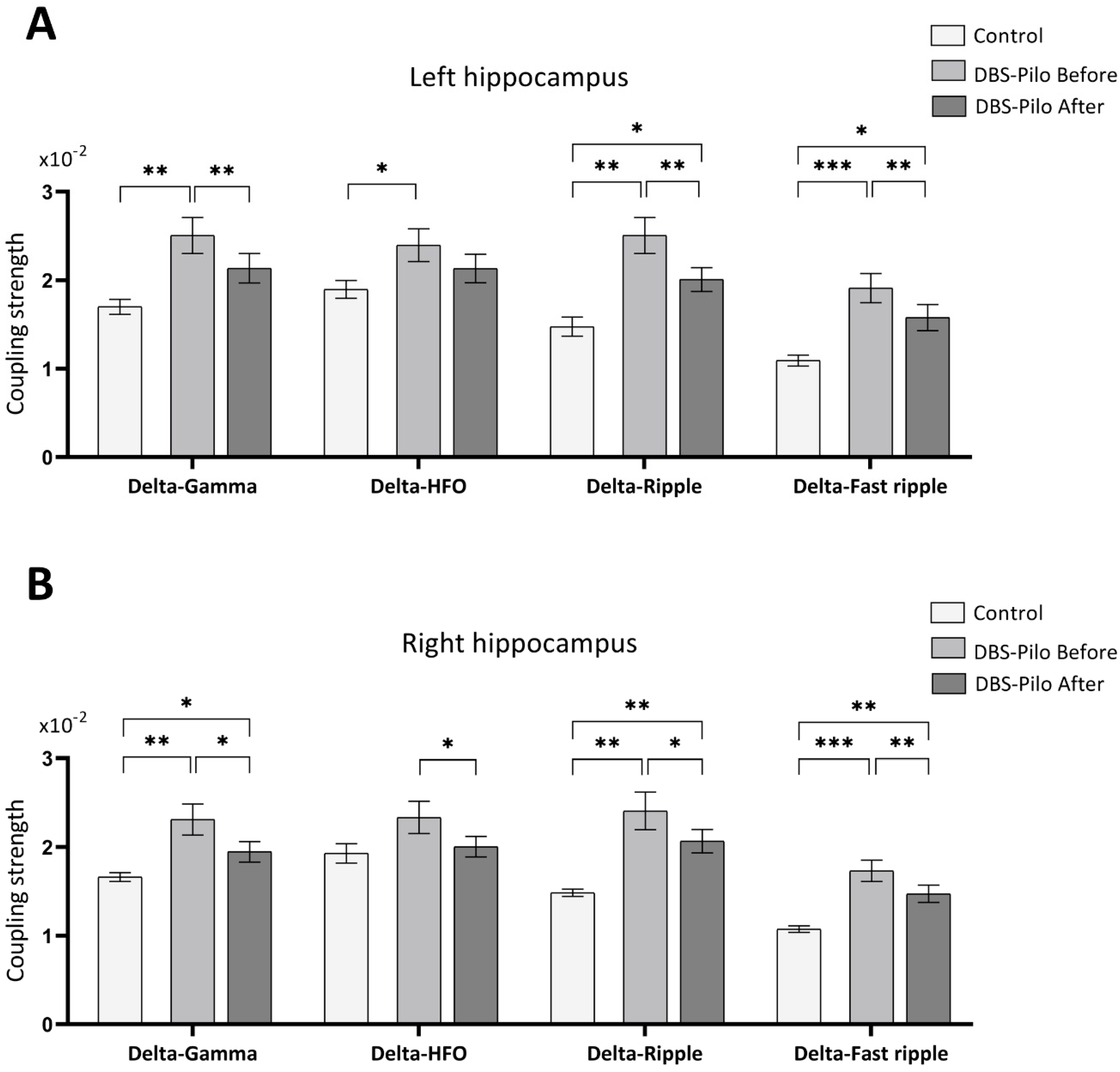
| Electrode Position | Group | Delta-Gamma | Delta-HFO | Delta-Ripple | Delta-Fast Ripple |
|---|---|---|---|---|---|
| L.H. | Control | 1.70 × 10−2 ± 9 × 10−4 | 1.9 × 10−2 ± 1 × 10−3 | 1.47 × 10−2 ± 1.1 × 10−3 | 1.09 × 10−2 ± 6 × 10−4 |
| DBS-Pilo Before | 2.51 × 10−2 ± 2 × 10−3 | 2.4 × 10−2 ± 1.8 × 10−3 | 2.51 × 10−2 ± 2 × 10−3 | 1.91 × 10−2 ± 1.7 × 10−3 | |
| DBS-Pilo After | 2.14 × 10−2 ± 1.7 × 10−3 | 2.13 × 10−2 ± 1.6 × 10−3 | 2.01 × 10−2 ± 1.4 × 10−3 | 1.58 × 10−2 ± 1.5 × 10−3 | |
| R.H. | Control | 1.66 × 10−2 ± 5 × 10−4 | 1.93 × 10−2 ± 1.1 × 10−3 | 1.48 × 10−2 ± 4 × 10−4 | 1.12 × 10−2 ± 5 × 10−4 |
| DBS-Pilo Before | 2.31 × 10−2 ± 1.8 × 10−3 | 2.33 × 10−2 ± 1.8 × 10−3 | 2.41 × 10−2 ± 2.1 × 10−3 | 1.73 × 10−2 ± 1.2 × 10−3 | |
| DBS-Pilo After | 1.95 × 10−2 ± 1.2 × 10−3 | 2 × 10−2 ± 1.2 × 10−3 | 2.07 × 10−2 ± 1.3 × 10−3 | 1.47 × 10−2 ± 1 × 10−3 |
| Time Interval | Electrode Position | Pearson Test | Delta-Gamma | Delta-HFO | Delta-Ripple | Delta-Fast Ripple |
|---|---|---|---|---|---|---|
| Before DBS | Left hippocampus | r | −0.86 | −0.63 | −0.86 | −0.75 |
| p | 0.0027 ** | 0.0713 | 0.0031 ** | 0.0201 * | ||
| Right hippocampus | r | 0.003 | −0.48 | −0.65 | −0.62 | |
| p | 0.9940 | 0.1872 | 0.0570 | 0.0761 | ||
| After DBS | Left hippocampus | r | −0.77 | −0.67 | −0.84 | −0.88 |
| p | 0.0143 ** | 0.0487 * | 0.0048 ** | 0.0017 ** | ||
| Right hippocampus | r | −0.03 | −0.36 | −0.45 | −0.73 | |
| p | 0.9431 | 0.3448 | 0.2226 | 0.0251 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihály, I.; Orbán-Kis, K.; Gáll, Z.; Berki, Á.-J.; Bod, R.-B.; Szilágyi, T. Amygdala Low-Frequency Stimulation Reduces Pathological Phase-Amplitude Coupling in the Pilocarpine Model of Epilepsy. Brain Sci. 2020, 10, 856. https://doi.org/10.3390/brainsci10110856
Mihály I, Orbán-Kis K, Gáll Z, Berki Á-J, Bod R-B, Szilágyi T. Amygdala Low-Frequency Stimulation Reduces Pathological Phase-Amplitude Coupling in the Pilocarpine Model of Epilepsy. Brain Sciences. 2020; 10(11):856. https://doi.org/10.3390/brainsci10110856
Chicago/Turabian StyleMihály, István, Károly Orbán-Kis, Zsolt Gáll, Ádám-József Berki, Réka-Barbara Bod, and Tibor Szilágyi. 2020. "Amygdala Low-Frequency Stimulation Reduces Pathological Phase-Amplitude Coupling in the Pilocarpine Model of Epilepsy" Brain Sciences 10, no. 11: 856. https://doi.org/10.3390/brainsci10110856
APA StyleMihály, I., Orbán-Kis, K., Gáll, Z., Berki, Á.-J., Bod, R.-B., & Szilágyi, T. (2020). Amygdala Low-Frequency Stimulation Reduces Pathological Phase-Amplitude Coupling in the Pilocarpine Model of Epilepsy. Brain Sciences, 10(11), 856. https://doi.org/10.3390/brainsci10110856






