A Disruption in the Balance of Attentional Systems Plays a Role in Trait Anxiety
Abstract
:1. Introduction
1.1. Attentional Functioning in Trait Anxiety
1.2. Representative Evidence of Imbalanced Attentional Functioning
1.3. Current Study
1.4. Hypotheses
- (1)
- Higher levels of trait anxiety will be associated with enhanced early visual information processing, as indicated by a shorter change detection response time without an accompanying cost to accuracy. This pattern is in line with the ACT model’s prediction that accuracy is typically preserved among anxious individual completing a moderately difficult task. However, to do so, individuals must slow down or expend great energy—both of which can be considered a marker of poor efficiency.
- (2)
- Consistent with prior behavioral findings (see [1]), we did not expect trait anxiety to directly predict either efficiency or effectiveness of working memory updating performance on the O-Span task. Trait anxiety will moderate the relation between early visual information processing and effectiveness of updating functioning for individuals higher in trait anxiety. The relation between the change detection response time and performance effectiveness on the O-Span task would be weaker.
- (3)
- We expected that trait anxiety would be positively related to SCL reactivity (i.e., change in SCL from baseline to trial) during the O-Span, reflecting increased effort. We also predicted that SCL reactivity would similarly moderate the relation between the change detection response time and O-Span recall, while controlling for the influence of trait anxiety level.
- (4)
- Finally, to test the importance of perceived traits, we examined the relation of perceived attention control and temperament (i.e., negative affect and extraversion/surgency [43,44]) with trait anxiety levels. We expected that trait anxiety would be associated with greater negative affect, lower extraversion/surgency, lower attentional control, and greater neutral perceptual sensitivity.
2. Materials and Methods
2.1. Participants
2.2. Apparatus
2.2.1. Audiovisual
2.2.2. Physiology
2.3. Measures
2.3.1. Trait Anxiety Level
2.3.2. Dispositional Attentional and Socioemotional Functioning
2.3.3. Visual Change Detection Task
2.3.4. Operation Span Task
2.3.5. SCL Reactivity
2.4. Procedure
2.5. Data Reduction and Assumption Checks
3. Results
3.1. Early Visual Information Processing
3.2. Balance between Early Visual Information Processing and Effectiveness of Working Memory Updating
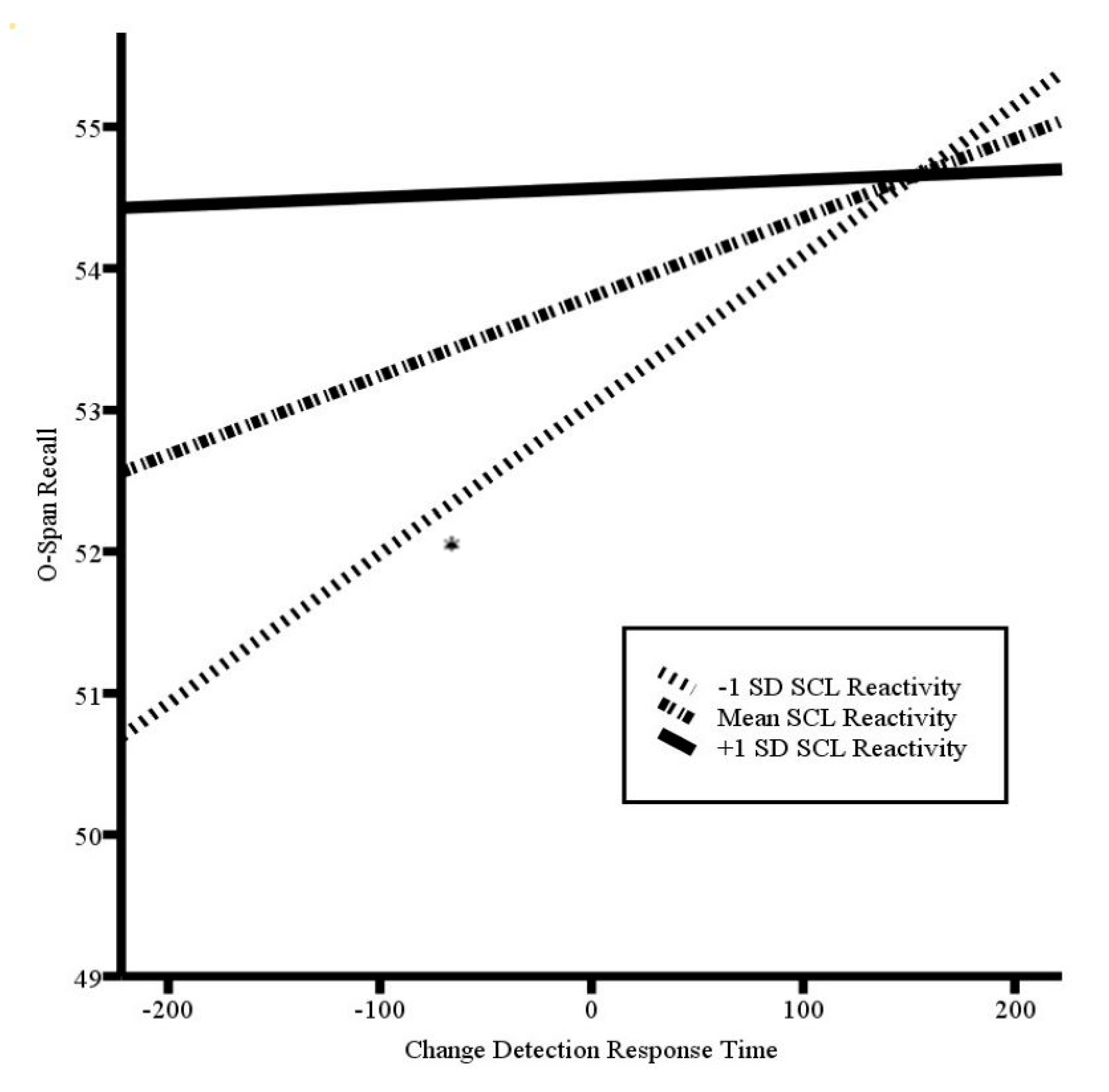
3.3. Effortful Central Executive Recruitment as Indicated by SCL Reactivity
3.4. Perceived Attentional Functioning
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berggren, N.; Derakshan, N. Attentional control deficits in trait anxiety: Why you see them and why you don’t. Biol. Psychol. 2013, 92, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.J. Trait anxiety and impoverished prefrontal control of attention. Nat. Neurosci. 2009, 12, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Unguetti, A.P.; Acosta, A.; Callejas, A.; Lupiáñez, J. Attention and Anxiety: Different Attentional Functioning Under State and Trait Anxiety. Psychol. Sci. 2010, 21, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef]
- Cisler, J.M.; Koster, E.H.W. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin. Psychol. Rev. 2010, 30, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Bar-Haim, Y.; Lamy, D.; Pergamin, L.; Bakermans-Kranenburg, M.J.; van Ijzendoorn, M.H. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007, 133, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Mathews, A. Why worry? The cognitive function of anxiety. Behav. Res. Ther. 1990, 28, 455–468. [Google Scholar] [CrossRef]
- Pineles, S.L.; Mineka, S. Attentional Biases to Internal and External Sources of Potential Threat in Social Anxiety. J. Abnorm. Psychol. 2005, 114, 314–318. [Google Scholar] [CrossRef]
- MacLeod, C.; Mathews, A. Cognitive Bias Modification Approaches to Anxiety. Annu. Rev. Clin. Psychol. 2012, 8, 189–217. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Edgar, K.; Taber-Thomas, B.; Auday, E.; Morales, S. Temperament and Attention as Core Mechanisms in the Early Emergence of Anxiety. Child. Emot. 2014, 26, 42–56. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, M.W.; Mogg, K.; May, J.; Richards, A.; Mathews, A. Bias in interpretation of ambiguous sentences related to threat in anxiety. J. Abnorm. Psychol. 1991, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Exploring the Central Executive. Q. J. Exp. Psychol. Sect. A 1996, 49, 5–28. [Google Scholar] [CrossRef]
- McRae, K.; Hughes, B.; Chopra, S.; Gabrieli, J.D.E.; Gross, J.J.; Ochsner, K.N. The Neural Bases of Distraction and Reappraisal. J. Cogn. Neurosci. 2009, 22, 248–262. [Google Scholar] [CrossRef]
- Koster, E.H.W.; De Lissnyder, E.; Derakshan, N.; De Raedt, R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clin. Psychol. Rev. 2011, 31, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Reinholdt-Dunne, M.L.; Mogg, K.; Bradley, B.P. Attention control: Relationships between self-report and behavioral measures, and symptoms of anxiety and depression. Cogn. Emot. 2013, 27, 430–440. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working Memory. In Psychology of Learning and Motivation; Bower, G.H., Ed.; Academic Press: New York, PA, USA, 1974; Volume 8, pp. 47–89. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [Green Version]
- Derakshan, N.; Eysenck, M.W. Anxiety, Processing Efficiency, and Cognitive Performance: New developments from attentional control theory. Eur. Psychol. 2009, 14, 168–176. [Google Scholar] [CrossRef]
- Egloff, B.; Hock, M. Interactive effects of state anxiety and trait anxiety on emotional Stroop interference. Personal. Individ. Differ. 2001, 31, 875–882. [Google Scholar] [CrossRef]
- Mogg, K.; Bradley, B.P.; Williams, R.; Mathews, A. Subliminal processing of emotional information in anxiety and depression. J. Abnorm. Psychol. 1993, 102, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Kalanthroff, E.; Henik, A.; Derakshan, N.; Usher, M. Anxiety, emotional distraction, and attentional control in the Stroop task. Emotion 2016, 16, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derakshan, N.; Ansari, T.L.; Hansard, M.; Shoker, L.; Eysenck, M.W. Anxiety, inhibition, efficiency, and effectiveness: An investigation using the antisaccade task. Exp. Psychol. 2009, 56, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ansari, T.L.; Derakshan, N. The neural correlates of cognitive effort in anxiety: Effects on processing efficiency. Biol. Psychol. 2011, 86, 337–348. [Google Scholar] [CrossRef]
- Basten, U.; Stelzel, C.; Fiebach, C.J. Trait Anxiety Modulates the Neural Efficiency of Inhibitory Control. J. Cogn. Neurosci. 2011, 23, 3132–3145. [Google Scholar] [CrossRef] [Green Version]
- Mathews, A.; Fox, E.; Yiend, J.; Calder, A. The face of fear: Effects of eye gaze and emotion on visual attention. Vis. Cogn. 2003, 10, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Eysenck, M.W.; Calvo, M.G. Anxiety and Performance: The Processing Efficiency Theory. Cogn. Emot. 1992, 6, 409–434. [Google Scholar] [CrossRef]
- Berggren, N.; Blonievsky, T.; Derakshan, N. Enhanced visual detection in trait anxiety. Emotion 2015, 15, 477–483. [Google Scholar] [CrossRef]
- Moriya, J.; Tanno, Y. Attentional resources in social anxiety and the effects of perceptual load. Cogn. Emot. 2010, 24, 1329–1348. [Google Scholar] [CrossRef]
- Weymar, M.; Keil, A.; Hamm, A.O. Timing the fearful brain: Unspecific hypervigilance and spatial attention in early visual perception. Soc. Cogn. Affect. Neurosci. 2014, 9, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Duff, S.C.; Logie, R.H. Processing and storage in working memory span. Q. J. Exp. Psychol. Sect. A 2001, 54, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, N.; Spillers, G.J. Working memory capacity: Attention control, secondary memory, or both? A direct test of the dual-component model. J. Mem. Lang. 2010, 62, 392–406. [Google Scholar] [CrossRef]
- Shipstead, Z.; Harrison, T.L.; Engle, R.W. Working memory capacity and the scope and control of attention. Atten. Percept. Psychophys. 2015, 77, 1863–1880. [Google Scholar] [CrossRef] [Green Version]
- Aarts, K.; Pourtois, G. Anxiety not only increases, but also alters early error-monitoring functions. Cogn. Affect. Behav. Neurosci. 2010, 10, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Miu, A.C.; Heilman, R.M.; Houser, D. Anxiety impairs decision-making: Psychophysiological evidence from an Iowa Gambling Task. Biol. Psychol. 2008, 77, 353–358. [Google Scholar] [CrossRef]
- Mehler, B.; Reimer, B.; Coughlin, J.F. Sensitivity of Physiological Measures for Detecting Systematic Variations in Cognitive Demand from a Working Memory Task: An On-Road Study across Three Age Groups. Hum. Factors 2012, 54, 396–412. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Berntson, G.G.; Larsen, J.T.; Poehlmann, K.M.; Ito, T.A. The psychophysiology of emotion. Handb. Emot. 2000, 2, 173–191. [Google Scholar]
- Luck, S.J.; Vogel, E.K. The capacity of visual working memory for features and conjunctions. Nature 1997, 390, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.L.; Engle, R.W. Is working memory capacity task dependent? J. Mem. Lang. 1989, 28, 127–154. [Google Scholar] [CrossRef]
- Robert, J.; Hockey, G. Compensatory control in the regulation of human performance under stress and high workload: A cognitive-energetical framework. Biol. Psychol. 1997, 45, 73–93. [Google Scholar] [CrossRef]
- Derryberry, D.; Reed, M.A. Anxiety-related attentional biases and their regulation by attentional control. J. Abnorm. Psychol. 2002, 111, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rothbart, M.K. Becoming Who We Are: Temperament and Personality in Development; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Jylhä, P.; Isometsä, E. The relationship of neuroticism and extraversion to symptoms of anxiety and depression in the general population. Depress. Anxiety 2006, 23, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D. Manual for the State-Trait Anxiety Inventory STAI (Form Y); Mind Garden: Palo Alto, CA, USA, 1983. [Google Scholar]
- Evans, D.E.; Rothbart, M.K. Developing a model for adult temperament. J. Res. Personal. 2007, 41, 868–888. [Google Scholar] [CrossRef]
- Pailian, H.; Halberda, J. The reliability and internal consistency of one-shot and flicker change detection for measuring individual differences in visual working memory capacity. Mem. Cognit. 2015, 43, 397–420. [Google Scholar] [CrossRef] [Green Version]
- Tokowicz, N.; Michael, E.B.; Kroll, J.F. The roles of study-abroad experience and working-memory capacity in the types of errors made during translation. Biling. Lang. Cogn. 2004, 7, 255–272. [Google Scholar] [CrossRef]
- Conway, A.R.A.; Cowan, N.; Bunting, M.F.; Therriault, D.J.; Minkoff, S.R.B. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence 2002, 30, 163–183. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Iglewicz, B. Fine-Tuning Some Resistant Rules for Outlier Labeling. J. Am. Stat. Assoc. 1987, 82, 1147–1149. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis a Regression–Based Approach; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Atkinson, A.C. Fast Very Robust Methods for the Detection of Multiple Outliers. J. Am. Stat. Assoc. 1994, 89, 1329–1339. [Google Scholar] [CrossRef]
- Tortella-Feliu, M.; Morillas-Romero, A.; Balle, M.; Bornas, X.; Llabrés, J.; Pacheco-Unguetti, A.P. Attentional control, attentional network functioning, and emotion regulation styles. Cogn. Emot. 2014, 28, 769–780. [Google Scholar] [CrossRef]

| Accuracy | Response Latency | |||||
|---|---|---|---|---|---|---|
| Variable | n | Mean a | SD | n | Mean | SD |
| Change Detection—Total | 55 | 0.72 | 0.07 | 55 | 760.85 | 201.79 |
| Small Array—Total | 55 | 0.84 | 0.10 | 53 | 693.35 | 159.22 |
| Change | 55 | 0.73 | 0.18 | 55 | 746.92 | 213.61 |
| No Change | 55 | 0.94 | 0.07 | 55 | 691.96 | 216.83 |
| Medium Array—Total | 55 | 0.70 | 0.09 | 55 | 768.85 | 202.72 |
| Change | 55 | 0.51 | 0.17 | 55 | 797.50 | 208.85 |
| No Change | 55 | 0.89 | 0.08 | 55 | 740.20 | 210.24 |
| Large Array—Total | 55 | 0.62 | 0.08 | 55 | 794.26 | 220.12 |
| Change | 55 | 0.39 | 0.15 | 55 | 806.96 | 236.54 |
| No Change | 55 | 0.85 | 0.10 | 55 | 781.57 | 220.10 |
| Operation Span | ||||||
| Recall | 52 | 53.35 | 4.47 | -- | -- | -- |
| Operation Decision | 52 | 0.88 | 0.09 | 53 | 2063.57 | 350.01 |
| Variable | B | SE(B) | t |
|---|---|---|---|
| Constant | 53.000 | 0.609 | 87.03 ** |
| Trait Anxiety | 0.078 | 0.048 | 1.63 |
| CD RT | 0.008 | 0.004 | 2.14 * |
| Trait Anxiety × CD RT | −0.001 | 0.000 | −3.28 ** |
| −1 SD Trait Anxiety | 0.014 | 0.004 | 3.54 ** |
| Mean Trait Anxiety | 0.008 | 0.004 | 2.13 * |
| +1 SD Trait Anxiety | 0.002 | 0.004 | 0.43 |
| Variable | B | SE(B) | t |
|---|---|---|---|
| Constant | 52.66 | 3.207 | 16.42 ** |
| SCL-R | 0.015 | 0.017 | 0.38 |
| CD RT | 0.006 | 0.017 | 0.89 |
| SCL-R × CD RT | −0.000 | 0.000 | −2.11 * |
| −1 SD SCL-R | 0.010 | 0.005 | 2.22 * |
| Mean SCL-R | 0.006 | 0.006 | 1.00 |
| +1 SD SCL-R | 0.000 | 0.007 | 0.09 |
| Trait Anxiety | 0.030 | 0.075 | 0.40 |
| Variable | Mean | SD | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|---|---|
| 1 | Trait Anxiety | 37.3 | 9.59 | -- | |||
| 2 | Negative Affect | 3.89 | 0.62 | 0.441 ** | -- | ||
| 3 | Extraversion/Surgency | 4.94 | 0.84 | −0.425 ** | −0.209 | -- | |
| 4 | Attentional Control | 3.76 | 1.04 | −0.338 * | −0.375 ** | −0.121 | -- |
| 5 | Neutral Perceptual Sensitivity | 4.75 | 0.82 | −0.226 | −0.207 | −0.029 | −0.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnick, M.R.; Pérez-Edgar, K.E.; Soto, J.A. A Disruption in the Balance of Attentional Systems Plays a Role in Trait Anxiety. Brain Sci. 2020, 10, 761. https://doi.org/10.3390/brainsci10100761
Minnick MR, Pérez-Edgar KE, Soto JA. A Disruption in the Balance of Attentional Systems Plays a Role in Trait Anxiety. Brain Sciences. 2020; 10(10):761. https://doi.org/10.3390/brainsci10100761
Chicago/Turabian StyleMinnick, Mark R., Koraly E. Pérez-Edgar, and José A. Soto. 2020. "A Disruption in the Balance of Attentional Systems Plays a Role in Trait Anxiety" Brain Sciences 10, no. 10: 761. https://doi.org/10.3390/brainsci10100761
APA StyleMinnick, M. R., Pérez-Edgar, K. E., & Soto, J. A. (2020). A Disruption in the Balance of Attentional Systems Plays a Role in Trait Anxiety. Brain Sciences, 10(10), 761. https://doi.org/10.3390/brainsci10100761




