Combined Supra- and Sub-Lesional Epidural Electrical Stimulation for Restoration of the Motor Functions after Spinal Cord Injury in Mini Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedures
2.2. Implantation of Stimulating and Reference Electrodes
2.3. Contusion Injury
2.4. Postoperative Care
2.5. Training on the Treadmill
2.6. Epidural Stimulation
2.7. Electrophysiological Assessment
2.8. Joint Kinematics
2.9. Behavioral Test
2.10. Data Analysis
3. Results
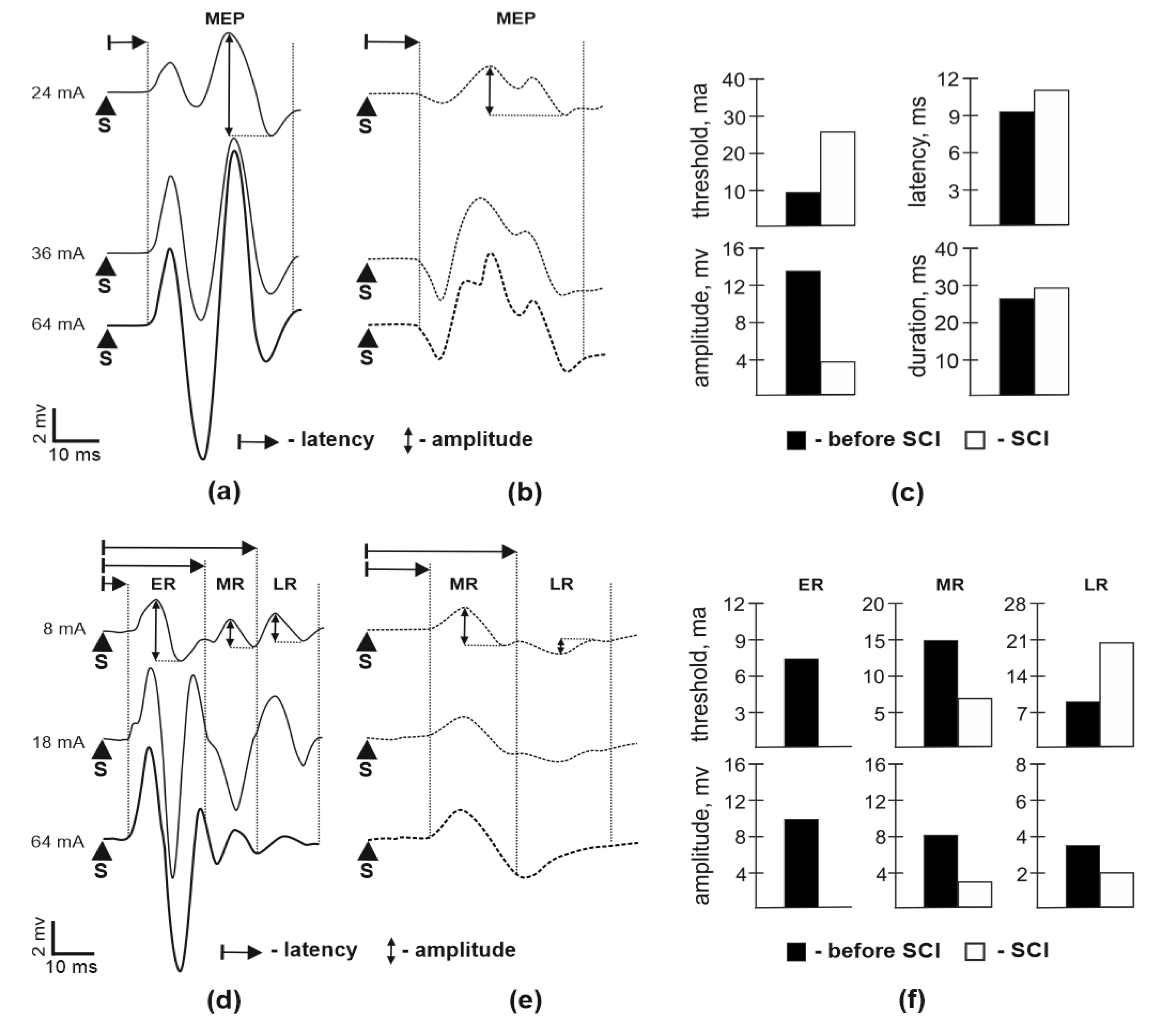
3.1. Motor Potentials Evoked by EES
3.2. M-Response and H-Reflex Evaluation
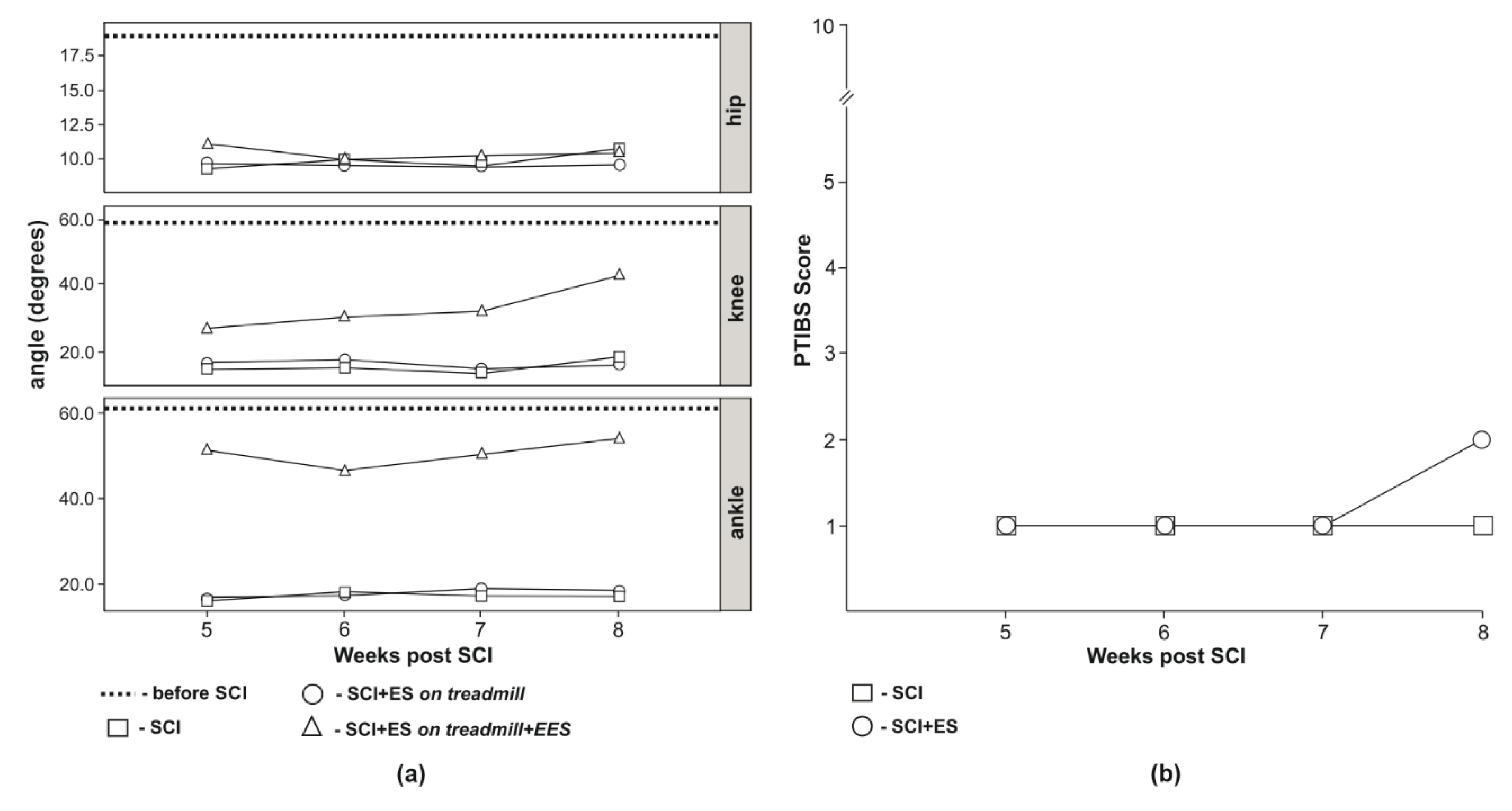
3.3. Video Analysis of Hind Limb Joints Kinematics
3.4. Behavioral Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Biering-Sorensen, F.; Noonan, V.K. Standardization of Data for Clinical Use and Research in Spinal Cord Injury. Brain Sci. 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Curt, A.; Van Hedel, H.J.A.; Klaus, D.; Dietz, V. Recovery from a spinal cord injury: Significance of compensation, neural plasticity, and repair. J. Neurotrauma 2008, 25, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Granger, N.; Olby, N.J.; Spitzbarth, I.; Jeffery, N.D.; Tipold, A.; Nout-Lomas, Y.S.; da Costa, R.C.; Stein, V.M.; Noble-Haeusslein, L.J.; et al. Targeting Translational Successes through CANSORT-SCI: Using Pet Dogs To Identify Effective Treatments for Spinal Cord Injury. J. Neurotrauma 2017, 34, 2007–2018. [Google Scholar] [CrossRef]
- Donovan, J.; Kirshblum, S. Clinical Trials in Traumatic Spinal Cord Injury. Neurotherapeutics 2018, 15, 654–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrijevic, M.R.; Gerasimenko, Y.; Pinter, M.M. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 1998, 860, 360–376. [Google Scholar] [CrossRef]
- Sheffler, L.R.; Chae, J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 2007, 35, 562–590. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef] [Green Version]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seanez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Grahn, P.J.; Lavrov, I.A.; Sayenko, D.G.; Van Straaten, M.G.; Gill, M.L.; Strommen, J.A.; Calvert, J.S.; Drubach, D.I.; Beck, L.A.; Linde, M.B.; et al. Enabling Task-Specific Volitional Motor Functions via Spinal Cord Neuromodulation in a Human With Paraplegia. Mayo Clin. Proc. 2017, 92, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Davis, J.; Bersch, I.; Goldberg, G.; Gorgey, A.S. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen. Res. 2020, 15, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, I.; Musienko, P.E.; Selionov, V.A.; Zdunowski, S.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Activation of spinal locomotor circuits in the decerebrated cat by spinal epidural and/or intraspinal electrical stimulation. Brain Res. 2015, 1600, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Lavrov, I. Spinal Epidural Stimulation Strategies: Clinical Implications of Locomotor Studies in Spinal Rats. Neuroscientist 2017, 23, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Hachmann, J.T.; Calvert, J.S.; Grahn, P.J.; Drubach, D.I.; Lee, K.H.; Lavrov, I.A. Review of Epidural Spinal Cord Stimulation for Augmenting Cough after Spinal Cord Injury. Front. Hum. Neurosci. 2017, 11, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formento, E.; Minassian, K.; Wagner, F.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Rowald, A.; Bloch, J.; Micera, S.; Capogrosso, M.; Courtine, G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018, 21, 1728–1741. [Google Scholar] [CrossRef]
- Toossi, A.; Everaert, D.G.; Perlmutter, S.I.; Mushahwar, V.K. Functional organization of motor networks in the lumbosacral spinal cord of non-human primates. Sci. Rep. 2019, 9, 13539. [Google Scholar] [CrossRef] [Green Version]
- Gerasimenko, I.P.; Avelev, V.D.; Nikitin, O.A.; Lavrov, I.A. Initiation of locomotor activity in spinalized cats by epidural stimulation of the spinal cord. Rossiiskii Fiziologicheskii Zhurnal Imeni IM Sechenova 2001, 87, 1161–1170. [Google Scholar]
- Lavrov, I.; Gerasimenko, Y.P.; Ichiyama, R.M.; Courtine, G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J. Neurophysiol. 2006, 96, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, I.; Dy, C.J.; Fong, A.J.; Gerasimenko, Y.; Courtine, G.G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J. Neurosci. 2008, 28, 6022–6029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavrov, I.; Fox, L.; Shen, J.; Han, Y.; Cheng, J. Gap Junctions Contribute to the Regulation of Walking-Like Activity in the Adult Mudpuppy (Necturus Maculatus). PLoS ONE 2016, 11, e0152650. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, M.; Wagner, F.B.; Gandar, J.; Moraud, E.M.; Wenger, N.; Milekovic, T.; Shkorbatova, P.; Pavlova, N.; Musienko, P.; Bezard, E.; et al. Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat. Protoc. 2018, 13, 2031–2061. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Fouad, K.; Bastiaanse, C.M. Neuronal coordination of arm and leg movements during human locomotion. Eur. J. Neurosci. 2001, 14, 1906–1914. [Google Scholar] [CrossRef]
- Roy, R.R.; Edgerton, V.R. Neurobiological perspective of spasticity as occurs after a spinal cord injury. Exp. Neurol. 2012, 235, 116–122. [Google Scholar] [CrossRef]
- Song, W.; Amer, A.; Ryan, D.; Martin, J.H. Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury. Exp. Neurol. 2016, 277, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Gerasimenko, Y.P.; Avelev, V.D.; Nikitin, O.A.; Lavrov, I.A. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2003, 33, 247–254. [Google Scholar] [CrossRef]
- Lavrov, I.; Cheng, J. Activation of NMDA receptors is required for the initiation and maintenance of walking-like activity in the mudpuppy (Necturus Maculatus). Can. J. Physiol. Pharmacol. 2004, 82, 637–644. [Google Scholar] [CrossRef]
- Lavrov, I.; Cheng, J. Methodological optimization of applying neuroactive agents for the study of locomotor-like activity in the mudpuppies (Necturus maculatus). J. Neurosci. Methods 2008, 174, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Cuellar, C.A.; Mendez, A.A.; Islam, R.; Calvert, J.S.; Grahn, P.J.; Knudsen, B.; Pham, T.; Lee, K.H.; Lavrov, I.A. The role of functional neuroanatomy of the lumbar spinal cord in effect of epidural stimulation. Front. Neuroanat. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Gerasimenko, Y.; Gorodnichev, R.; Puhov, A.; Moshonkina, T.; Savochin, A.; Selionov, V.; Roy, R.R.; Lu, D.C.; Edgerton, V.R. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J. Neurophysiol. 2015, 113, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Madigan, N.N.; Hakim, J.S.; Dadsetan, M.; McMahon, S.S.; Yaszemski, M.J.; Windebank, A.J. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J. Tissue Eng. Regen. Med. 2018, 12, e398–e407. [Google Scholar] [CrossRef] [Green Version]
- Hakim, J.S.; Rodysill, B.R.; Chen, B.K.; Schmeichel, A.M.; Yaszemski, M.J.; Windebank, A.J.; Madigan, N.N. Combinatorial tissue engineering partially restores function after spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Inman, D.M.; Steward, O. Ascending sensory, but not other long-tract axons, regenerate into the connective tissue matrix that forms at the site of a spinal cord injury in mice. J. Comp. Neurol. 2003, 462, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Godfrey, S.; Thomas, C.K. Interlimb reflexes induced by electrical stimulation of cutaneous nerves after spinal cord injury. PLoS ONE 2016, 11, e0153063. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kitaura, M.; Wu, S.; Kataoka, K.; Suzuki, K.; Endo, K.; Nishimura, Y.; Ide, C. Electrophysiological and horseradish peroxidase-tracing studies of nerve regeneration through alginate-filled gap in adult rat spinal cord. Neurosci. Lett. 2002, 318, 121–124. [Google Scholar] [CrossRef]
- Lee, J.H.T.; Jones, C.F.; Okon, E.B.; Anderson, L.; Tigchelaar, S.; Kooner, P.; Godbey, T.; Chua, B.; Gray, G.; Hildebrandt, R.; et al. A Novel Porcine Model of Traumatic Thoracic Spinal Cord Injury. J. Neurotrauma 2013, 30, 142–159. [Google Scholar] [CrossRef]
- Shik, M.L.; Severin, F.V.; Orlovsky, G.N. Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr. Clin. Neurophysiol. 1969, 26, 549. [Google Scholar]
- Cazalets, J.R.; Borde, M.; Clarac, F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J. Neurosci. 1995, 15, 4943–4951. [Google Scholar] [CrossRef] [Green Version]
- Cote, M.-P.; Murray, M.; Lemay, M.A. Rehabilitation Strategies after Spinal Cord Injury: Inquiry into the Mechanisms of Success and Failure. J. Neurotrauma 2017, 34, 1841–1857. [Google Scholar] [CrossRef] [Green Version]
- Boekhoff, T.M.; Flieshardt, C.; Ensinger, E.-M.; Fork, M.; Kramer, S.; Tipold, A. Quantitative magnetic resonance imaging characteristics: Evaluation of prognostic value in the dog as a translational model for spinal cord injury. J. Spinal Disord. Tech. 2012, 25, E81–E87. [Google Scholar] [CrossRef]
- Zurita, M.; Aguayo, C.; Bonilla, C.; Otero, L.; Rico, M.; Rodríguez, A.; Vaquero, J. The pig model of chronic paraplegia: A challenge for experimental studies in spinal cord injury. Prog. Neurobiol. 2012, 97, 288–303. [Google Scholar] [CrossRef]
- Islamov, R.R.; Izmailov, A.A.; Sokolov, M.E.; Fadeev, F.O.; Bashirov, F.V.; Eremeev, A.A.; Shaymardanova, G.F.; Shmarov, M.M.; Naroditskiy, B.S.; Chelyshev, Y.A.A.; et al. Evaluation of direct and cell-mediated triple-gene therapy in spinal cord injury in rats. Brain Res. Bull. 2017, 132, 44–52. [Google Scholar] [CrossRef]
- Islamov, R.R.; Rizvanov, A.A.; Fedotova, V.Y.; Izmailov, A.A.; Safiullov, Z.Z.; Garanina, E.E.; Salafutdinov, I.I.; Sokolov, M.E.; Mukhamedyarov, M.A.; Palotás, A. Tandem Delivery of Multiple Therapeutic Genes Using Umbilical Cord Blood Cells Improves Symptomatic Outcomes in ALS. Mol. Neurobiol. 2017, 54, 4756–4763. [Google Scholar] [CrossRef]
- Fong, A.J.; Cai, L.L.; Otoshi, C.K.; Reinkensmeyer, D.J.; Burdick, J.W.; Roy, R.R.; Edgerton, V.R. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 2005, 25, 11738–11747. [Google Scholar] [CrossRef]
- Rossignol, S.; Dubuc, R.; Gossard, J.-P. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef]
- Nikitin, O.; Bogacheva, I.N.; Musienko, P.E.; Savohin, A.A.; Gerasimenko, Y.P. Afferent activation of step movements of spinalised cat in the early period after spinalisation. Vestn. Tv.GU Biol. Ecol. Sect. 2007, 6, 22–30. [Google Scholar]
- Pavlova, N.V.; Bogacheva, I.N.; Bazhenova, E.Y.; Gorsky, O.V.; Moshonkina, T.R.; Gerasimenko, Y. Restoration of Motor Functions in Spinal Rats by Electrical Stimulation of the Spinal Cord and Locomotor Training. Russ. J. Physiol. 2019, 105, 565–577. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Bogacheva, I.N.; Shcherbakova, N.A.; Makarovskii, A.N. Bioelectric activity of spinal cord in patients with vertebrospinal pathologies. Bull. Exp. Biol. Med. 2001, 132, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.P.; Makarovskii, A.N.; Nikitin, O.A. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci. Behav. Physiol. 2002, 32, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, R.M.; Gerasimenko, Y.P.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005, 383, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Choe, J.; Nandra, M.S.; Zhong, H.; Roy, R.R.; Tai, Y.-C.; Edgerton, V.R. Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats. J. Neuroeng. Rehabil. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.K.; Sureddi, S.; Alam, M.; Zhong, H.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Unique Spatiotemporal Neuromodulation of the Lumbosacral Circuitry Shapes Locomotor Success after Spinal Cord Injury. J. Neurotrauma 2016, 33, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Shapkov, I.T.; Shapkova, E.I. Spinal locomotor generators in humans: Problems in assessing effectiveness of stimulations. Med. Tekh. 1998, 24–27. [Google Scholar] [PubMed]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef]
- Ichiyama, R.M.; Gerasimenko, Y.; Jindrich, D.L.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neurosci. Lett. 2008, 438, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Cuellar, C.A.; Tang, S.; Islam, R.; Wen, H.; Huang, C.; Manduca, A.; Trzasko, J.D.; Knudsen, B.E.; Lee, K.H.; et al. Functional Ultrasound Imaging of Spinal Cord Hemodynamic Responses to Epidural Electrical Stimulation: A Feasibility Study. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Cuellar, C.A.; Song, P.; Islam, R.; Huang, C.; Wen, H.; Knudsen, B.E.; Gong, P.; Lok, U.W.; Chen, S.; et al. Changes in spinal cord hemodynamics reflect modulation of spinal network with different parameters of epidural stimulation. Neuroimage 2020, 221. [Google Scholar] [CrossRef]
- Hansen, C.N.; Faw, T.D.; White, S.; Buford, J.A.; Grau, J.W.; Basso, D.M. Sparing of Descending Axons Rescues Interneuron Plasticity in the Lumbar Cord to Allow Adaptive Learning After Thoracic Spinal Cord Injury. Front. Neural Circuits 2016, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Cazalets, J.R.; Bertrand, S. Coupling between lumbar and sacral motor networks in the neonatal rat spinal cord. Eur. J. Neurosci. 2000, 12, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Ballion, B.; Morin, D.; Viala, D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur. J. Neurosci. 2001, 14, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Juvin, L.; Simmers, J.; Morin, D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J. Neurosci. 2005, 25, 6025–6035. [Google Scholar] [CrossRef]
- Juvin, L.; Le Gal, J.-P.; Simmers, J.; Morin, D. Cervicolumbar coordination in mammalian quadrupedal locomotion: Role of spinal thoracic circuitry and limb sensory inputs. J. Neurosci. 2012, 32, 953–965. [Google Scholar] [CrossRef] [Green Version]
- Zehr, E.P.; Duysens, J. Regulation of arm and leg movement during human locomotion. Neuroscientist 2004, 10, 347–361. [Google Scholar] [CrossRef]
- Zehr, E.P.; Hundza, S.R.; Vasudevan, E.V. The quadrupedal nature of human bipedal locomotion. Exerc. Sport Sci. Rev. 2009, 37, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Solopova, I.A.; Selionov, V.A.; Zhvansky, D.S.; Grishin, A. The mutual influence of the upper and lower extremities during cyclic movements. Hum. Physiol. 2011, 37, 55–64. [Google Scholar] [CrossRef]
- Sylos-Labini, F.; Ivanenko, Y.P.; Maclellan, M.J.; Cappellini, G.; Poppele, R.E.; Lacquaniti, F. Locomotor-like leg movements evoked by rhythmic arm movements in humans. PLoS ONE 2014, 9, e90775. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T. Descending pathways eliciting forelimb stepping in the lateral funiculus: Experimental studies with stimulation and lesion of the cervical cord in decerebrate cats. Brain Res. 1986, 379, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Shik, M. Recognizing propriospinal and reticulospinal systems of initiation of stepping. Motor Control 1997, 1, 310–313. [Google Scholar] [CrossRef]
- Jordan, L.M.; Schmidt, B.J. Propriospinal neurons involved in the control of locomotion: Potential targets for repair strategies? Prog. Brain Res. 2002, 137, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.R.; Dimitrijevic, M.M.; Faganel, J.; Sherwood, A.M. Suprasegmentally induced motor unit activity in paralyzed muscles of patients with established spinal cord injury. Ann. Neurol. 1984, 16, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.R.; Halter, J.A.; Sharkey, P.C.; Sherwood, A.M. Epidural spinal cord stimulation and carry-over effect in chronic spinal cord injury patients. Appl. Neurophysiol. 1987, 50, 449–450. [Google Scholar] [CrossRef]
- Militskova, A.; Mukhametova, E.; Fatykhova, E.; Sharifullin, S.; Cuellar, C.A.; Calvert, J.S.; Grahn, P.J.; Baltina, T.; Lavrov, I. Supraspinal and Afferent Signaling Facilitate Spinal Sensorimotor Network Excitability After Discomplete Spinal Cord Injury: A Case Report. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Alstermark, B.; Lundberg, A.; Pinter, M.; Sasaki, S. Subpopulations and functions of long C3-C5 propriospinal neurones. Brain Res. 1987, 404, 395–400. [Google Scholar] [CrossRef]
- Flynn, J.R.; Graham, B.A.; Galea, M.P.; Callister, R.J. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 2011, 60, 809–822. [Google Scholar] [CrossRef]
- Engesser-Cesar, C.; Anderson, A.J.; Basso, D.M.; Edgerton, V.R.; Cotman, C.W. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma 2005, 22, 157–171. [Google Scholar] [CrossRef]
- Perreau, V.M.; Adlard, P.A.; Anderson, A.J.; Cotman, C.W. Exercise-induced gene expression changes in the rat spinal cord. Gene Expr. 2005, 12, 107–121. [Google Scholar] [CrossRef]
- Shenkman, B.S. From Slow to Fast: Hypogravity-Induced Remodeling of Muscle Fiber Myosin Phenotype. Acta Nat. 2016, 8, 47–59. [Google Scholar] [CrossRef]
- Knikou, M.; Murray, L.M. Repeated transspinal stimulation decreases soleus H-reflex excitability and restores spinal inhibition in human spinal cord injury. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- ElBasiouny, S.M.; Mushahwar, V.K. Suppressing the excitability of spinal motoneurons by extracellularly applied electrical fields: Insights from computer simulations. J. Appl. Physiol. 2007, 103, 1824–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.; Emch, G.S.; Johnson, C.S.; Wrathall, J.R. Effect of spinal cord injury severity on alterations of the H-reflex. Exp. Neurol. 2005, 196, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Pomerantz, F.R.; Wolpaw, J.R. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J. Neurosci. 2013, 33, 2365–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgerton, V.R.; Roy, R.R. Neuromuscular adaptation to actual and simulated weightlessness. Adv. Space Biol. Med. 1994, 4, 33–67. [Google Scholar] [CrossRef]
- De-Doncker, L.; Kasri, M.; Picquet, F.; Falempin, M. Physiologically adaptive changes of the L5 afferent neurogram and of the rat soleus EMG activity during 14 days of hindlimb unloading and recovery. J. Exp. Biol. 2005, 208, 4585–4592. [Google Scholar] [CrossRef] [Green Version]
- Shenkman, B.S.; Kozlovskaya, I.B. Cellular Responses of Human Postural Muscle to Dry Immersion. Front. Physiol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Grillner, S.; Zangger, P. On the central generation of locomotion in the low spinal cat. Exp. Brain Res. 1979, 34, 241–261. [Google Scholar] [CrossRef]
- Grillner, S. Control of locomotion in bipeds, tetrapods and fish. In Handbook of Physiology; American Physiological Society: Bethesda/Rockville, MD, USA, 1981; pp. 1179–1236. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadeev, F.; Eremeev, A.; Bashirov, F.; Shevchenko, R.; Izmailov, A.; Markosyan, V.; Sokolov, M.; Kalistratova, J.; Khalitova, A.; Garifulin, R.; et al. Combined Supra- and Sub-Lesional Epidural Electrical Stimulation for Restoration of the Motor Functions after Spinal Cord Injury in Mini Pigs. Brain Sci. 2020, 10, 744. https://doi.org/10.3390/brainsci10100744
Fadeev F, Eremeev A, Bashirov F, Shevchenko R, Izmailov A, Markosyan V, Sokolov M, Kalistratova J, Khalitova A, Garifulin R, et al. Combined Supra- and Sub-Lesional Epidural Electrical Stimulation for Restoration of the Motor Functions after Spinal Cord Injury in Mini Pigs. Brain Sciences. 2020; 10(10):744. https://doi.org/10.3390/brainsci10100744
Chicago/Turabian StyleFadeev, Filip, Anton Eremeev, Farid Bashirov, Roman Shevchenko, Andrei Izmailov, Vage Markosyan, Mikhail Sokolov, Julia Kalistratova, Anastasiia Khalitova, Ravil Garifulin, and et al. 2020. "Combined Supra- and Sub-Lesional Epidural Electrical Stimulation for Restoration of the Motor Functions after Spinal Cord Injury in Mini Pigs" Brain Sciences 10, no. 10: 744. https://doi.org/10.3390/brainsci10100744
APA StyleFadeev, F., Eremeev, A., Bashirov, F., Shevchenko, R., Izmailov, A., Markosyan, V., Sokolov, M., Kalistratova, J., Khalitova, A., Garifulin, R., Islamov, R., & Lavrov, I. (2020). Combined Supra- and Sub-Lesional Epidural Electrical Stimulation for Restoration of the Motor Functions after Spinal Cord Injury in Mini Pigs. Brain Sciences, 10(10), 744. https://doi.org/10.3390/brainsci10100744






