The Effect of Blindness on Spatial Asymmetries
Abstract
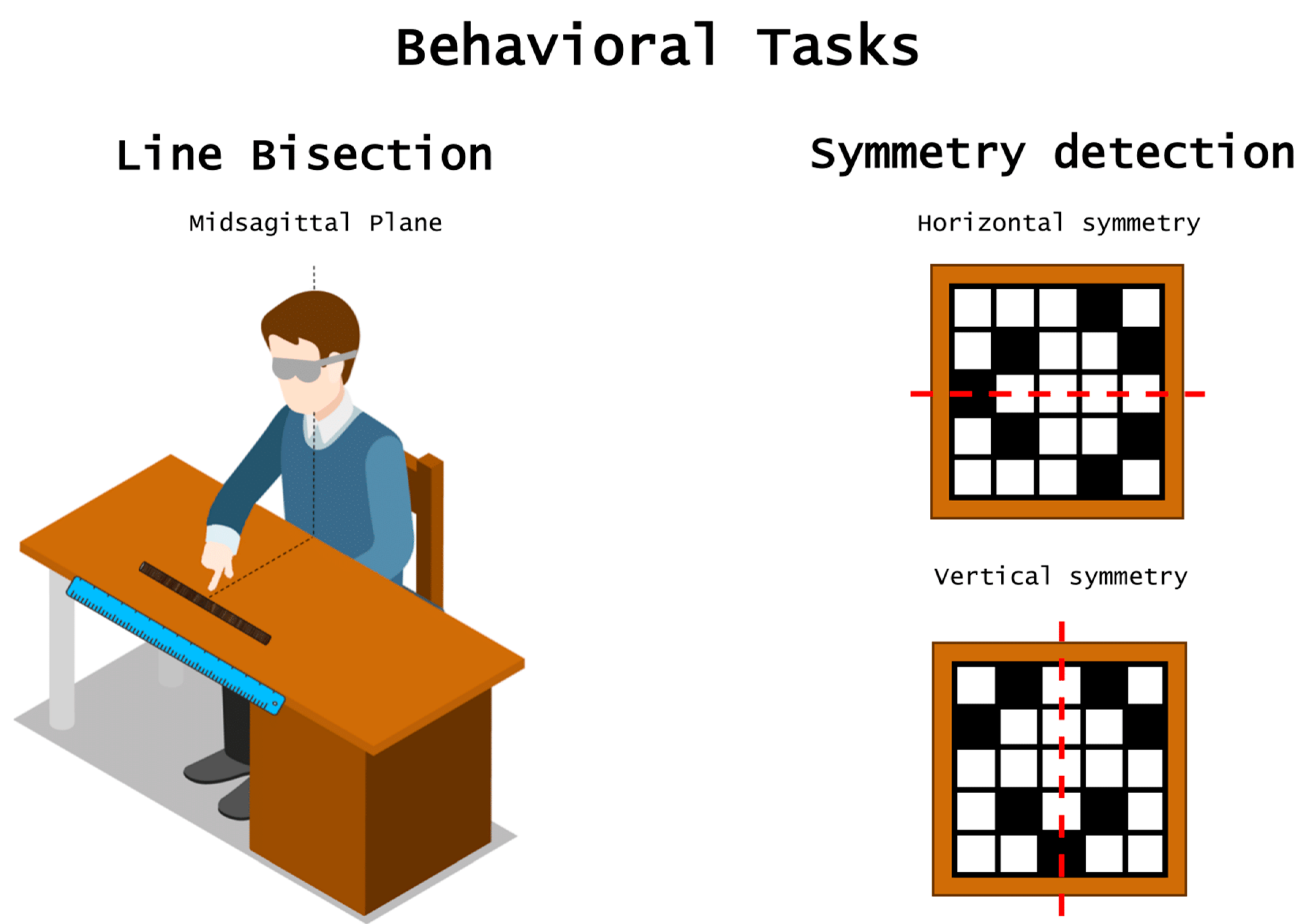
:1. Introduction
2. Pseudoneglect
3. Mirror Symmetry
4. Localization Tasks
5. The Role of Sensorimotor Experience
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Concha, M.L.; Bianco, I.H.; Wilson, S.W. Encoding asymmetry within neural circuits. Nat. Rev. Neurosci. 2012, 13, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O.; Ocklenburg, S. Ontogenesis of Lateralization. Neuron 2017, 94, 249–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zozulinsky, P.; Greenbaum, L.; Brande-Eilat, N.; Braun, Y.; Shalev, I.; Tomer, R. Dopamine system genes are associated with orienting bias among healthy individuals. Neuropsychologia 2014, 62, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Robertson, I.H.; Gill, M.; Bellgrove, M.A. Dopaminergic genotype influences spatial bias in healthy adults. Neuropsychologia 2010, 48, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Packheiser, J.; Schmitz, J.; Rook, N.; Güntürkün, O.; Peterburs, J.; Grimshaw, G.M. Hugs and kisses—The role of motor preferences and emotional lateralization for hemispheric asymmetries in human social touch. Neurosci. Biobehav. Rev. 2018, 95, 353–360. [Google Scholar] [CrossRef]
- Latham, A.J.; Patston, L.L.M.; Tippett, L.J. The precision of experienced action video-game players: Line bisection reveals reduced leftward response bias. Atten. Percept. Psychophys. 2014, 76, 2193–2198. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.; Kumsta, R.; Moser, D.; Güntürkün, O.; Ocklenburg, S. DNA methylation of dopamine-related gene promoters is associated with line bisection deviation in healthy adults. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Beste, C.; Arning, L.; Peterburs, J.; Güntürkün, O. The ontogenesis of language lateralization and its relation to handedness. Neurosci. Biobehav. Rev. 2014, 43, 191–198. [Google Scholar] [CrossRef]
- Kosslyn, S.M. Image And Brain; MIT Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Cattaneo, Z.; Vecchi, T.; Cornoldi, C.; Mammarella, I.; Bonino, D.; Ricciardi, E.; Pietrini, P. Imagery and spatial processes in blindness and visual impairment. Neurosci. Biobehav. Rev. 2008, 32, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Merabet, L.B.; Rizzo, J.F.; Amedi, A.; Somers, D.C.; Pascual-Leone, A. What blindness can tell us about seeing again: Merging neuroplasticity and neuroprostheses. Nat. Rev. Neurosci. 2005, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, E.; Bonino, D.; Pellegrini, S.; Pietrini, P. Mind the blind brain to understand the sighted one! Is there a supramodal cortical functional architecture? Neurosci. Biobehav. Rev. 2014, 41, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.L.; Nettleton, N.C.; Nathan, G.; Wilson, L. Tactual-kinesthetic matching of horizontal extents by the long-term blind: Absence or reversal of normal left-side underestimation. Neuropsychologia 1986, 24, 261–264. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Rinaldi, L.; Geraci, C.; Cecchetto, C.; Papagno, C. Spatial biases in deaf, blind, and deafblind individuals as revealed by a haptic line bisection task. Q. J. Exp. Psychol. 2018, 7, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, Z.; Fantino, M.; Tinti, C.; Pascual-Leone, A.; Silvanto, J.; Vecchi, T. Spatial Biases in Peripersonal Space in Sighted and Blind Individuals Revealed by a Haptic Line Bisection Paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2011, 37, 1110–1121. [Google Scholar] [CrossRef]
- Sampaio, E.; Gouarir, C.; Mvondo, D.M. Tactile and Visual Bisection Tasks by Sighted and Blind Children. Dev. Neuropsychol. 1995, 11, 109–127. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Fantino, M.; Silvanto, J.; Vallar, G.; Vecchi, T. Tapping effects on numerical bisection. Exp. Brain Res. 2011, 208, 21–28. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Fantino, M.; Tinti, C.; Silvanto, J.; Vecchi, T. Crossmodal interaction between the mental number line and peripersonal haptic space representation in sighted and blind individuals. Atten. Percept. Psychophys. 2010, 72, 885–890. [Google Scholar] [CrossRef]
- Rinaldi, L.; Vecchi, T.; Fantino, M.; Merabet, L.B.; Cattaneo, Z. The effect of hand movements on numerical bisection judgments in early blind and sighted individuals. Cortex 2015, 71, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Bauer, C.; Yazzolino, L.; Hirsch, G.; Cattaneo, Z.; Vecchi, T.; Merabet, L.B. Neural correlates associated with superior tactile symmetry perception in the early blind. Cortex 2015, 63, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, Z.; Fantino, M.; Silvanto, J.; Tinti, C.; Pascual-Leone, A.; Vecchi, T. Symmetry perception in the blind. Acta Psychol. (Amsterdam) 2010, 134, 398–402. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Vecchi, T.; Fantino, M.; Herbert, A.M.; Merabet, L.B. The effect of vertical and horizontal symmetry on memory for tactile patterns in late blind individuals. Atten. Percept. Psychophys. 2013, 75, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collignon, O.; Davare, M.; Olivier, E.; De Volder, A.G. Reorganisation of the right occipito-parietal stream for auditory spatial processing in early blind humans. a transcranial magnetic stimulation study. Brain Topogr. 2009, 21, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Vercillo, T.; Tonelli, A.; Gori, M. Intercepting a sound without vision. PLoS ONE 2017, 5, e0177407. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Kincade, M.J.; Lewis, C.; Snyder, A.Z.; Sapir, A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat. Neurosci. 2005, 8, 1603–1610. [Google Scholar] [CrossRef]
- Shulman, G.L.; Pope, D.L.W.; Astafiev, S.V.; McAvoy, M.P.; Snyder, A.Z.; Corbetta, M. Right Hemisphere Dominance during Spatial Selective Attention and Target Detection Occurs Outside the Dorsal Frontoparietal Network. J. Neurosci. 2010, 30, 3640–3651. [Google Scholar] [CrossRef] [Green Version]
- Jewell, G.; McCourt, M.E. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000, 38, 93–110. [Google Scholar] [CrossRef]
- Benwell, C.S.Y.; Harvey, M.; Thut, G. On the neural origin of pseudoneglect: EEG-correlates of shifts in line bisection performance with manipulation of line length. Neuroimage 2014, 86, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Fink, G.R.; Marshall, J.C.; Shah, N.J.; Weiss, P.H.; Halligan, P.W.; Grosse-Ruyken, M.; Ziemons, K.; Zilles, K.; Freund, H.J. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology 2000, 54, 1324–1331. [Google Scholar] [CrossRef]
- Fink, G.R.; Marshall, J.C.; Weiss, P.H.; Zilles, K. The neural basis of vertical and horizontal line bisection judgments: An fMRI study of normal volunteers. Neuroimage 2001, 14, S59–S67. [Google Scholar] [CrossRef]
- Zago, L.; Petit, L.; Jobard, G.; Hay, J.; Mazoyer, B.; Tzourio-Mazoyer, N.; Karnath, H.O.; Mellet, E. Pseudoneglect in line bisection judgement is associated with a modulation of right hemispheric spatial attention dominance in right-handers. Neuropsychologia 2017, 94, 75–83. [Google Scholar] [CrossRef]
- Brooks, J.L.; Darling, S.; Malvaso, C.; Della Sala, S. Adult developmental trajectories of pseudoneglect in the tactile, visual and auditory modalities and the influence of starting position and stimulus length. Brain Cogn. 2016, 103, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, J.L.; Della Sala, S.; Logie, R.H. Tactile rod bisection in the absence of visuo-spatial processing in children, mid-age and older adults. Neuropsychologia 2011, 49, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Coudereau, J.P.; Gueguen, N.; Pratte, M.; Sampaio, E. Tactile precision in right-handed archery experts with visual disabilities: A pseudoneglect effect? Laterality 2006, 11, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ciricugno, A.; Rinaldi, L.; Vecchi, T.; Merabet, L.B.; Cattaneo, Z. The role of binocular vision in driving pseudoneglect in visual and haptic bisection: Evidence from strabismic and monocular blind individuals. Multisens. Res. 2020, 33, 549–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, H.L.; Lora, A.N.; Heilman, K.M. Effects of monocular viewing and eye dominance on spatial attention. Brain 2002, 125, 2023–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girelli, L.; Marinelli, C.V.; Grossi, G.; Arduino, L.S. Cultural and biological factors modulate spatial biases over development. Laterality 2017, 22, 725–739. [Google Scholar] [CrossRef]
- Rinaldi, L.; Di Luca, S.; Toneatto, C.; Girelli, L. The effects of hemispheric dominance, literacy acquisition, and handedness on the development of visuospatial attention: A study in preschoolers and second graders. J. Exp. Child Psychol. 2020, 195, 104830. [Google Scholar] [CrossRef]
- Fierro, B.; Brighina, F.; Piazza, A.; Oliveri, M.; Bisiach, E. Timing of right parietal and frontal cortex activity in visuo-spatial perception: A TMS study in normal individuals. Neuroreport 2001, 12, 2605–2607. [Google Scholar] [CrossRef] [Green Version]
- Fink, G.R.; Marshall, J.C.; Weiss, P.H.; Toni, I.; Zilles, K. Task instructions influence the cognitive strategies involved in line bisection judgements: Evidence from modulated neural mechanisms revealed by fMRI. Neuropsychologia 2002, 40, 119–130. [Google Scholar] [CrossRef]
- Foxe, J.J.; McCourt, M.E.; Javitt, D.C. Right hemisphere control of visuospatial attention: Line-bisection judgments evaluated with high-density electrical mapping and source analysis. Neuroimage 2003, 19, 710–726. [Google Scholar] [CrossRef]
- Collignon, O.; Lassonde, M.; Voss, P.; Vandewalle, G.; Charbonneau, G.; Lepore, F.; Albouy, G. Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proc. Natl. Acad. Sci. USA 2011, 108, 4435–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, A.; Schwartz, D.; Stevens, A.A. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia 2007, 45, 2307–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gougoux, F.; Zatorre, R.J.; Lassonde, M.; Voss, P.; Lepore, F. A functional neuroimaging study of sound localization: Visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005, 3, e27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlierde, A.; De Volder, A.G.; Wanet-Defalque, M.C.; Veraart, C. Occipito-parietal cortex activation during visuo-spatial imagery in early blind humans. Neuroimage 2003, 19, 698–709. [Google Scholar] [CrossRef]
- Weeks, R.; Horwitz, B.; Aziz-Sultan, A.; Tian, B.; Wessinger, C.M.; Cohen, L.G.; Hallett, M.; Rauschecker, J.P. A positron emission tomographic study of auditory localization in the congenitally blind. J. Neurosci. 2000, 20, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.M.; Crosson, J.B.; Crucian, G.P.; Heilman, K.M. Far bias on the radial line bisection task: Measuring perceptual-attentional and motor-intentional bias in normal subjects. Cortex 2002, 38, 769–778. [Google Scholar] [CrossRef]
- Chieffi, S.; Iavarone, A.; Carlomagno, S. Effects of spatiotopic factors on bisection of radial lines. Exp. Brain Res. 2008, 189, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Szpak, A.; Thomas, N.A.; Nicholls, M.E.R. Hemispheric asymmetries in perceived depth revealed through a radial line bisection task. Exp. Brain Res. 2016, 234, 807–813. [Google Scholar] [CrossRef]
- Suavansri, K.; Falchook, A.D.; Williamson, J.B.; Heilman, K.M. Right up there: Hemispatial and hand asymmetries of altitudinal pseudoneglect. Brain Cogn. 2012, 79, 216–220. [Google Scholar] [CrossRef]
- Drain, M.; Reuter-Lorenz, P.A. Vertical Orienting Control: Evidence for Attentional Bias and “Neglect” in the Intact Brain. J. Exp. Psychol. Gen. 1996, 125, 139–158. [Google Scholar] [CrossRef]
- Brooks, J.L.; Logie, R.H.; McIntosh, R.; Sala, S. Della Representational pseudoneglect in an auditory-driven spatial working memory task. Q. J. Exp. Psychol. 2011, 64, 2168–2180. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.L.; Della Sala, S.; Darling, S. Representational pseudoneglect: A review. Neuropsychol. Rev. 2014, 24, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Darling, S.; Logie, R.H.; Della Sala, S. Representational pseudoneglect in line bisection. Psychon. Bull. Rev. 2012, 19, 879–883. [Google Scholar] [CrossRef] [PubMed]
- McGeorge, P.; Beschin, N.; Colnaghi, A.; Rusconi, M.L.; Della Sala, S. A lateralized bias in mental imagery: Evidence for representational pseudoneglect. Neurosci. Lett. 2007, 421, 259–263. [Google Scholar] [CrossRef]
- Dehaene, S.; Bossini, S.; Giraux, P. The Mental Representation of Parity and Number Magnitude. J. Exp. Psychol. Gen. 1993, 122, 371–396. [Google Scholar] [CrossRef]
- Longo, M.R.; Lourenco, S.F. Spatial attention and the mental number line: Evidence for characteristic biases and compression. Neuropsychologia 2007. [Google Scholar] [CrossRef]
- Loftus, A.M.; Nicholls, M.E.R.; Mattingley, J.B.; Chapman, H.L.; Bradshaw, J.L. Pseudoneglect for the bisection of mental number lines. Q. J. Exp. Psychol. 2009, 62, 925–945. [Google Scholar] [CrossRef]
- Göbel, S.M.; Calabria, M.; Farnè, A.; Rossetti, Y. Parietal rTMS distorts the mental number line: Simulating “spatial” neglect in healthy subjects. Neuropsychologia 2006, 44, 860–868. [Google Scholar] [CrossRef]
- Castronovo, J.; Seron, X. Numerical Estimation in Blind Subjects: Evidence of the Impact of Blindness and Its Following Experience. J. Exp. Psychol. Hum. Percept. Perform. 2007. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Fantino, M.; Silvanto, J.; Tinti, C.; Vecchi, T. Blind individuals show pseudoneglect in bisecting numerical intervals. Atten. Percept. Psychophys. 2011, 73, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Szücs, D.; Csépe, V. The parietal distance effect appears in both the congenitally blind and matched sighted controls in an acoustic number comparison task. Neurosci. Lett. 2005, 384, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Doricchi, F.; Guariglia, P.; Gasparini, M.; Tomaiuolo, F. Dissociation between physical and mental number line bisection in right hemisphere brain damage. Nat. Neurosci. 2005. [Google Scholar] [CrossRef] [PubMed]
- Treder, M.S. Behind the looking-glass: A review on human symmetry perception. Symmetry 2010, 2, 1510–1543. [Google Scholar] [CrossRef]
- Ballesteros, S.; Manga, D.; Reales, J.M. Haptic discrimination of bilateral symmetry in 2-dimensional and 3-dimensional unfamiliar displays. Percept. Psychophys. 1997, 59, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, Z.; Bona, S.; Bauer, C.; Silvanto, J.; Herbert, A.M.; Vecchi, T.; Merabet, L.B. Symmetry detection in visual impairment: Behavioral evidence and neural correlates. Symmetry 2014, 6, 427–443. [Google Scholar] [CrossRef]
- Tyler, C.W.; Baseler, H.A.; Kontsevich, L.L.; Likova, L.T.; Wade, A.R.; Wandell, B.A. Predominantly extra-retinotopic cortical response to pattern symmetry. Neuroimage 2005, 24, 306–314. [Google Scholar] [CrossRef]
- Sasaki, Y.; Vanduffel, W.; Knutsen, T.; Tyler, C.; Tootell, R. Symmetry activates extrastriate visual cortex in human and nonhuman primates. Proc. Natl. Acad. Sci. USA 2005, 102, 3159–3163. [Google Scholar] [CrossRef] [Green Version]
- Bona, S.; Herbert, A.; Toneatto, C.; Silvanto, J.; Cattaneo, Z. The causal role of the lateral occipital complex in visual mirror symmetry detection and grouping: An fMRI-guided TMS study. Cortex 2014, 51, 46–55. [Google Scholar] [CrossRef]
- Cattaneo, Z. The neural basis of mirror symmetry detection: A review. J. Cogn. Psychol. 2017, 29, 259–268. [Google Scholar] [CrossRef]
- Bertamini, M.; Silvanto, J.; Norcia, A.M.; Makin, A.D.J.; Wagemans, J. The neural basis of visual symmetry and its role in mid-and high-level visual processing. Ann. N. Y. Acad. Sci. 2018, 1426, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Hillger, L.A.; Koenig, O. Separable mechanisms in face processing: Evidence from hemispheric specialization. J. Cogn. Neurosci. 1991, 3, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.A.; Patai, E.Z.; Julian, J.B.; Spiers, H.J. The cognitive map in humans: Spatial navigation and beyond. Nat. Neurosci. 2017, 20, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Weeks, R.A.; Aziz-Sultan, A.; Bushara, K.O.; Tian, B.; Wessinger, C.M.; Dang, N.; Rauschecker, J.P.; Hallett, M. A PET study of human auditory spatial processing. Neurosci. Lett. 1999, 262, 155–158. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Bouffard, M.; Ahad, P.; Belin, P. Where is “where” in the human auditory cortex? Nat. Neurosci. 2002, 5, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Van Boven, R.W.; Ingeholm, J.E.; Beauchamp, M.S.; Bikle, P.C.; Ungerleider, L.G. Tactile form and location processing in the human brain. Proc. Natl. Acad. Sci. USA 2005, 102, 12601–12605. [Google Scholar] [CrossRef] [Green Version]
- Röder, B.; Rösler, F.; Spence, C. Early Vision Impairs Tactile Perception in the Blind. Curr. Biol. 2004, 14, 121–124. [Google Scholar] [CrossRef]
- Lessard, N.; Paré, M.; Lepore, F.; Lassonde, M. Early-blind human subjects localize sound sources better than sighted subjects. Nature 1998, 395, 278–280. [Google Scholar] [CrossRef]
- Voss, P.; Gougoux, F.; Lassonde, M.; Zatorre, R.J.; Lepore, F. A positron emission tomography study during auditory localization by late-onset blind individuals. Neuroreport 2006, 17, 383–388. [Google Scholar] [CrossRef]
- Röder, B.; Teder-Sälejärvi, W.; Sterr, A.; Rösler, F.; Hillyard, S.A.; Neville, H.J. Improved auditory spatial tuning in blind humans. Nature 1999, 400, 162–166. [Google Scholar] [CrossRef]
- Poirier, C.; Collignon, O.; Scheiber, C.; Renier, L.; Vanlierde, A.; Tranduy, D.; Veraart, C.; De Volder, A.G. Auditory motion perception activates visual motion areas in early blind subjects. Neuroimage 2006, 31, 279–285. [Google Scholar] [CrossRef]
- Arno, P.; De Volder, A.G.; Vanlierde, A.; Wanet-Defalque, M.C.; Streel, E.; Robert, A.; Sanabria-Bohórquez, S.; Veraart, C. Occipital activation by pattern recognition in the early blind using auditory substitution for vision. Neuroimage 2001, 13, 632–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collignon, O.; Davare, M.; De Volder, A.G.; Poirier, C.; Olivier, E.; Veraart, C. Time-course of posterior parietal and occipital cortex contribution to sound localization. J. Cogn. Neurosci. 2008, 20, 1454–1463. [Google Scholar] [CrossRef]
- Bowers, D.; Heilman, K.M. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia 1980, 18, 491–498. [Google Scholar] [CrossRef]
- Bradshaw, J.L.; Nathan, G.; Nettleton, N.C.; Wilson, L.; Pierson, J. Why is there a left side underestimation in rod bisection? Neuropsychologia 1987. [Google Scholar] [CrossRef]
- Kinsbourne, M. The cerebral basis of lateral asymmetries in attention. Acta Psychol. 1970, 33, 193–201. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Kinsbourne, M.; Moscovitch, M. Hemispheric control of spatial attention. Brain Cogn. 1990, 12, 240–266. [Google Scholar] [CrossRef]
- Chokron, S.; Imbert, M. Influence of reading habits on line bisection. Cogn. Brain Res. 1993, 1, 219–222. [Google Scholar] [CrossRef]
- Kazandjian, S.; Cavézian, C.; Zivotofsky, A.Z.; Chokron, S. Bisections in two languages: When number processing, spatial representation, and habitual reading direction interact. Neuropsychologia 2010, 48, 4031–4037. [Google Scholar] [CrossRef]
- Rinaldi, L.; Di Luca, S.; Henik, A.; Girelli, L. Reading direction shifts visuospatial attention: An Interactive Account of attentional biases. Acta Psychol. (Amsterdam) 2014, 151, 98–105. [Google Scholar] [CrossRef]
- Leo, A.; Bernardi, G.; Handjaras, G.; Bonino, D.; Ricciardi, E.; Pietrini, P. Increased BOLD variability in the parietal cortex and enhanced parieto-occipital connectivity during tactile perception in congenitally blind individuals. Neural Plast. 2012. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, R.H.; Pascual-Leone, A. Cortical plasticity associated with Braille learning. Trends Cogn. Sci. 1998, 2, 168–174. [Google Scholar] [CrossRef]
- Burton, H.; Snyder, A.Z.; Diamond, J.B.; Raichle, M.E. Adaptive Changes in Early and Late Blind: A fMRI Study of Verb Generation to Heard Nouns. J. Neurophysiol. 2002, 88, 3359–3371. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.G.; Celnik, P.; Pascual-Leone, A.; Corwell, B.; Faiz, L.; Dambrosia, J.; Honda, M.; Sadato, N.; Gerloff, C.; Dolores Catalá, M.; et al. Functional relevance of cross-modal plasticity in blind humans. Nature 1997, 389, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Siuda-Krzywicka, K.; Bola, Ł.; Paplińska, M.; Sumera, E.; Jednoróg, K.; Marchewka, A.; Śliwińska, M.W.; Amedi, A.; Szwed, M. Massive cortical reorganization in sighted braille readers. eLife 2016. [Google Scholar] [CrossRef] [Green Version]
- Bonino, D.; Ricciardi, E.; Bernardi, G.; Sani, L.; Gentili, C.; Vecchi, T.; Pietrini, P. Spatial imagery relies on a sensory independent, though sensory sensitive, functional organization within the parietal cortex: A fMRI study of angle discrimination in sighted and congenitally blind individuals. Neuropsychologia 2015, 68, 59–70. [Google Scholar] [CrossRef]
- Merabet, L.B.; Pascual-Leone, A. Neural reorganization following sensory loss: The opportunity of change. Nat. Rev. Neurosci. 2010, 11, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The plastic human brain cortex. Annu. Rev. Neurosci. 2005. [Google Scholar] [CrossRef] [Green Version]
- Bedny, M.; Richardson, H.; Saxe, R. “Visual” Cortex Responds to Spoken Language in Blind Children. J. Neurosci. 2015, 35, 11674–11681. [Google Scholar] [CrossRef] [Green Version]
- Merabet, L.B.; Hamilton, R.; Schlaug, G.; Swisher, J.D.; Kiriakopoulos, E.T.; Pitskel, N.B.; Kauffman, T.; Pascual-Leone, A. Rapid and reversible recruitment of early visual cortex for touch. PLoS ONE 2008, 3, e3046. [Google Scholar] [CrossRef] [Green Version]
- Chiao, J.; Li, S.-C.; Seligman, R.; Turner, R. The Oxford Handbook of Cultural Neuroscience; Oxford Univ. Press: Oxford, UK, 2016. [Google Scholar]

| Author | Year | Blind Participants | Control Participants |
|---|---|---|---|
| Pseudoneglect (line bisection) | |||
| Bradshaw et al. [13] | 1986 | 10 | 24 |
| Cattaneo et al. [14] | 2018 | 11 | 25 |
| Cattaneo, Fantino, Tinti et al. [15] | 2011 | 17 | 18 |
| Sampaio et al. [16] | 1995 | 20 | 20 |
| Pseudoneglect (numerical bisection) | |||
| Cattaneo, Fantino, Silvanto et al. [17] | 2011 | 18 | 10 |
| Cattaneo, Fantino, Tinti et al. [18] | 2010 | 17 | 23 |
| Rinaldi et al. [19] | 2015 | 16 | 16 |
| Mirror symmetry | |||
| Bauer et al. [20] | 2015 | 8 | 7 |
| Cattaneo, Fantino, Silvanto et al. [21] | 2010 | 16 | 26 |
| Cattaneo et al. [22] | 2013 | 12 | 12 |
| Localization tasks | |||
| Collignon et al. [23] | 2009 | 6 | 0 |
| Vercillo et al. [24] | 2017 | 8 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, L.; Ciricugno, A.; Merabet, L.B.; Vecchi, T.; Cattaneo, Z. The Effect of Blindness on Spatial Asymmetries. Brain Sci. 2020, 10, 662. https://doi.org/10.3390/brainsci10100662
Rinaldi L, Ciricugno A, Merabet LB, Vecchi T, Cattaneo Z. The Effect of Blindness on Spatial Asymmetries. Brain Sciences. 2020; 10(10):662. https://doi.org/10.3390/brainsci10100662
Chicago/Turabian StyleRinaldi, Luca, Andrea Ciricugno, Lotfi B. Merabet, Tomaso Vecchi, and Zaira Cattaneo. 2020. "The Effect of Blindness on Spatial Asymmetries" Brain Sciences 10, no. 10: 662. https://doi.org/10.3390/brainsci10100662
APA StyleRinaldi, L., Ciricugno, A., Merabet, L. B., Vecchi, T., & Cattaneo, Z. (2020). The Effect of Blindness on Spatial Asymmetries. Brain Sciences, 10(10), 662. https://doi.org/10.3390/brainsci10100662





