Improvements of Motor Performances in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Effects of Dialyzed Leucocyte Extracts from Human Serum
Abstract
1. Introduction
2. Materials and Methods
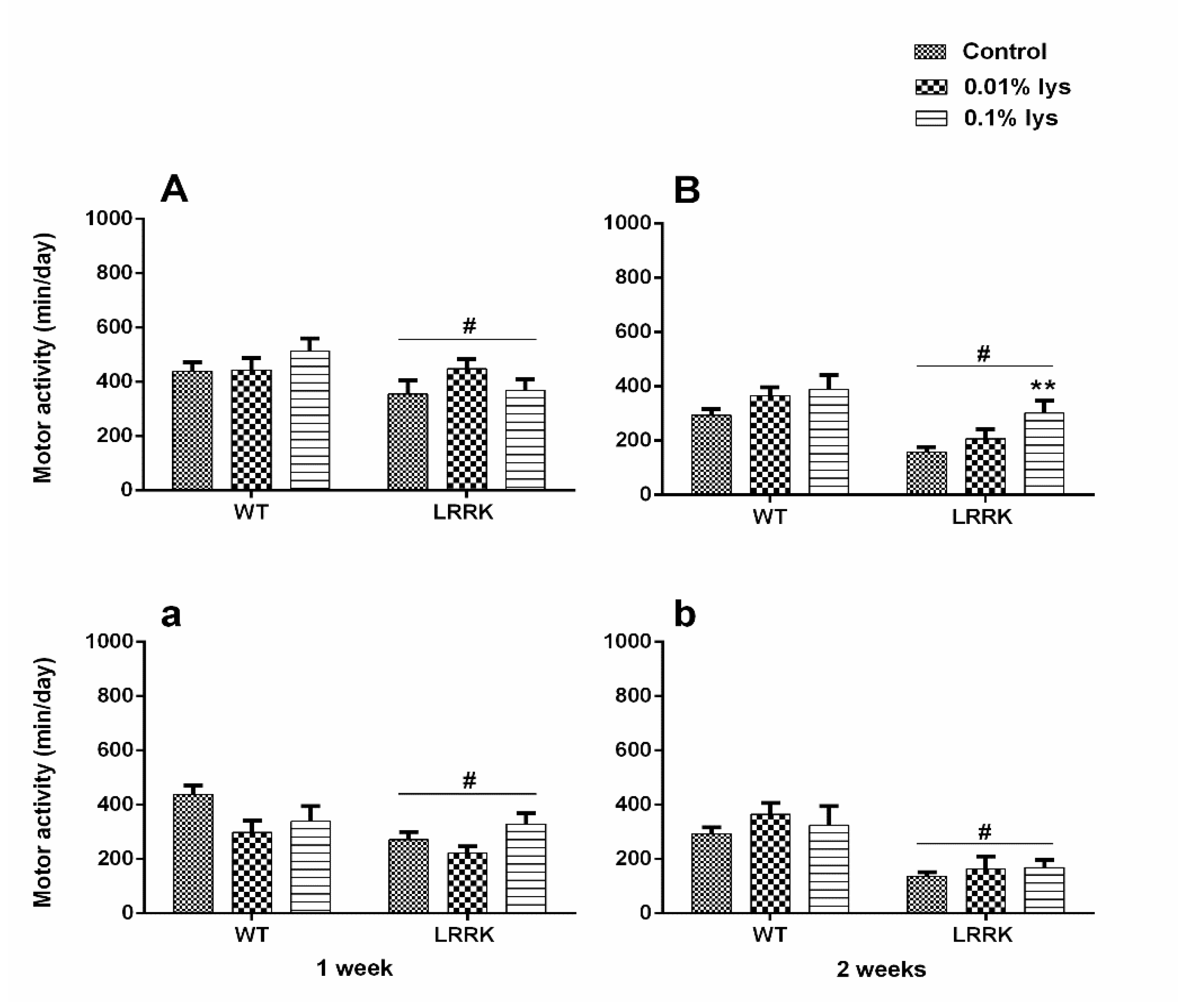
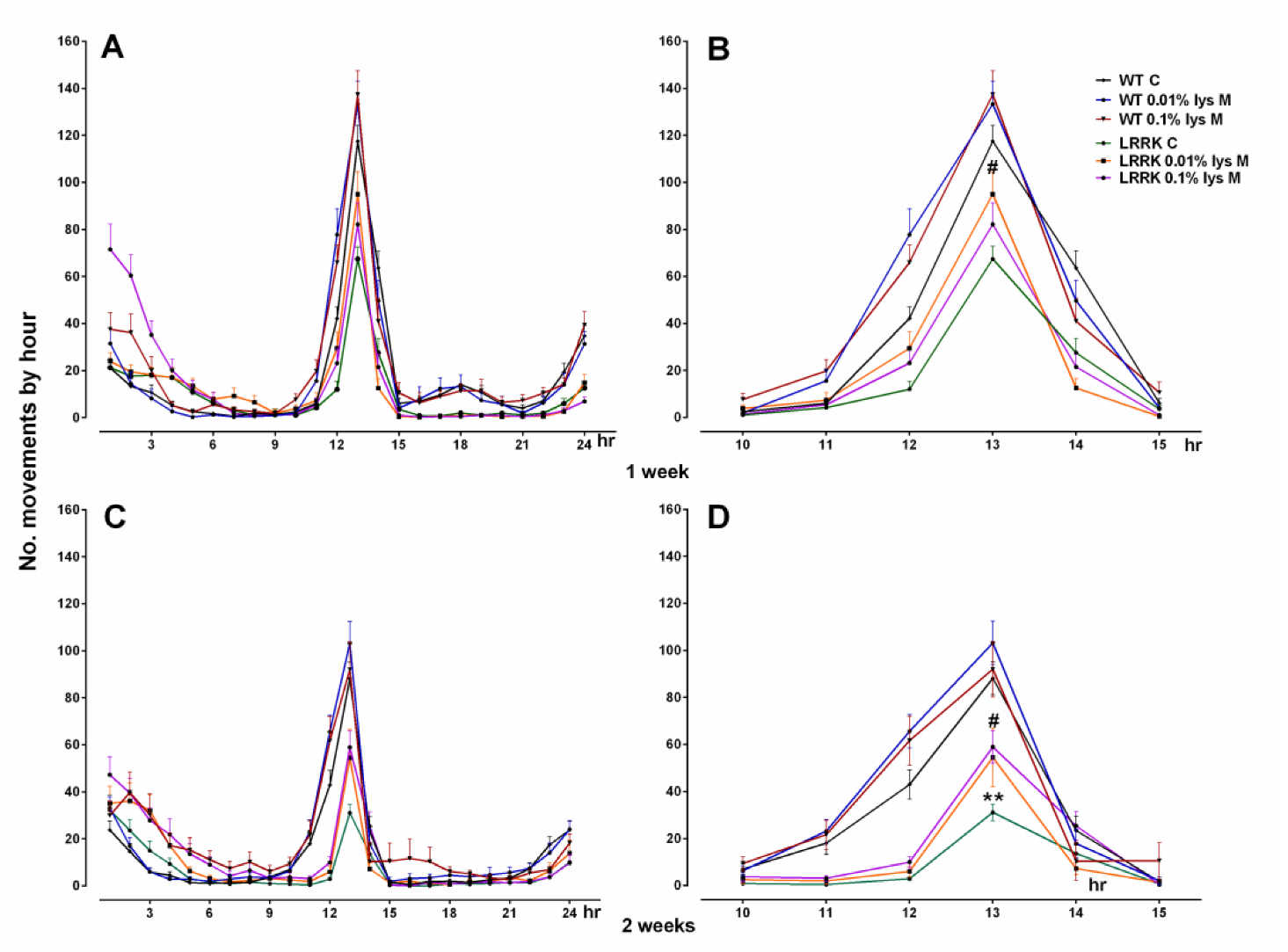
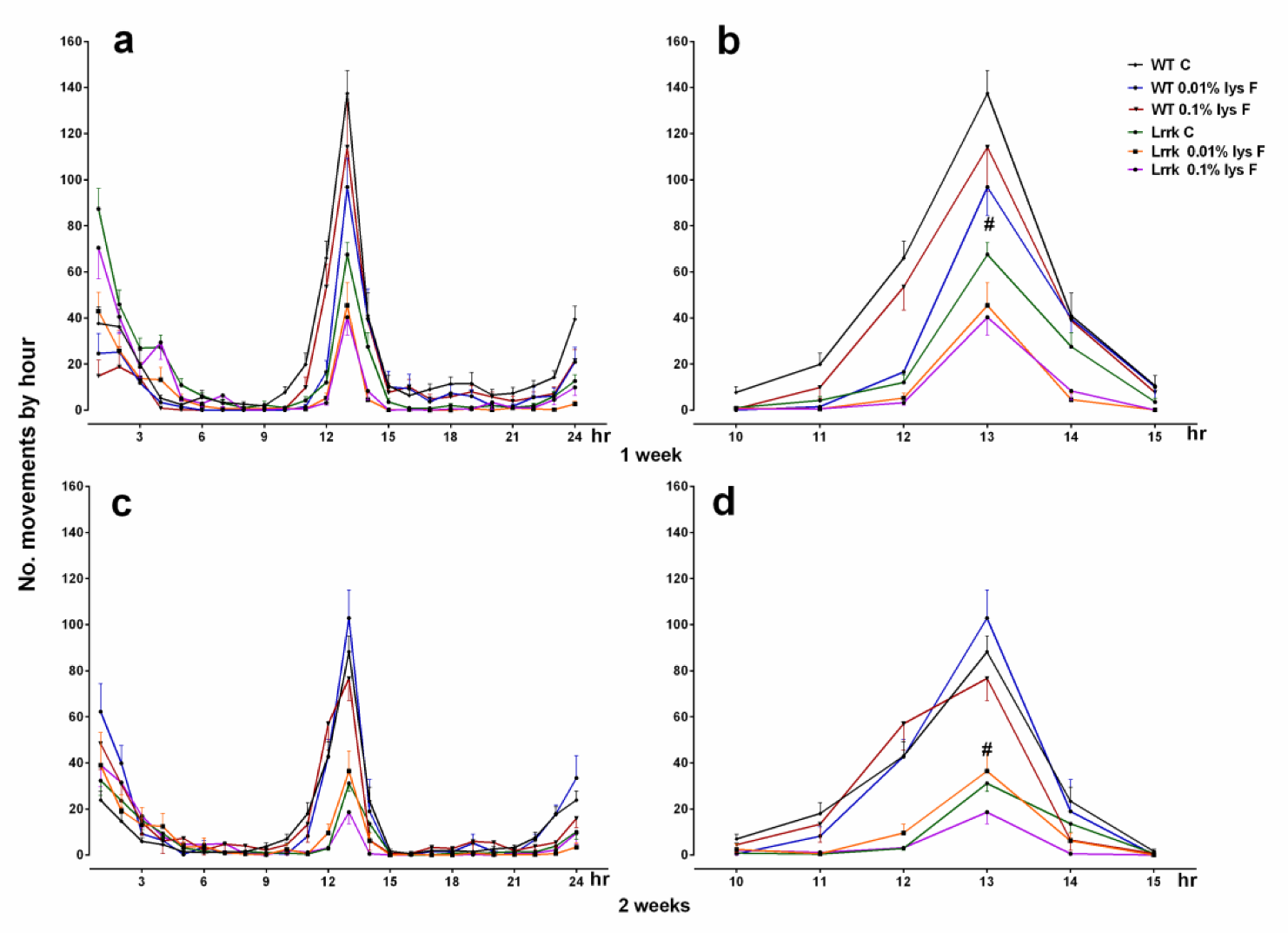
2.1. Motor Activity
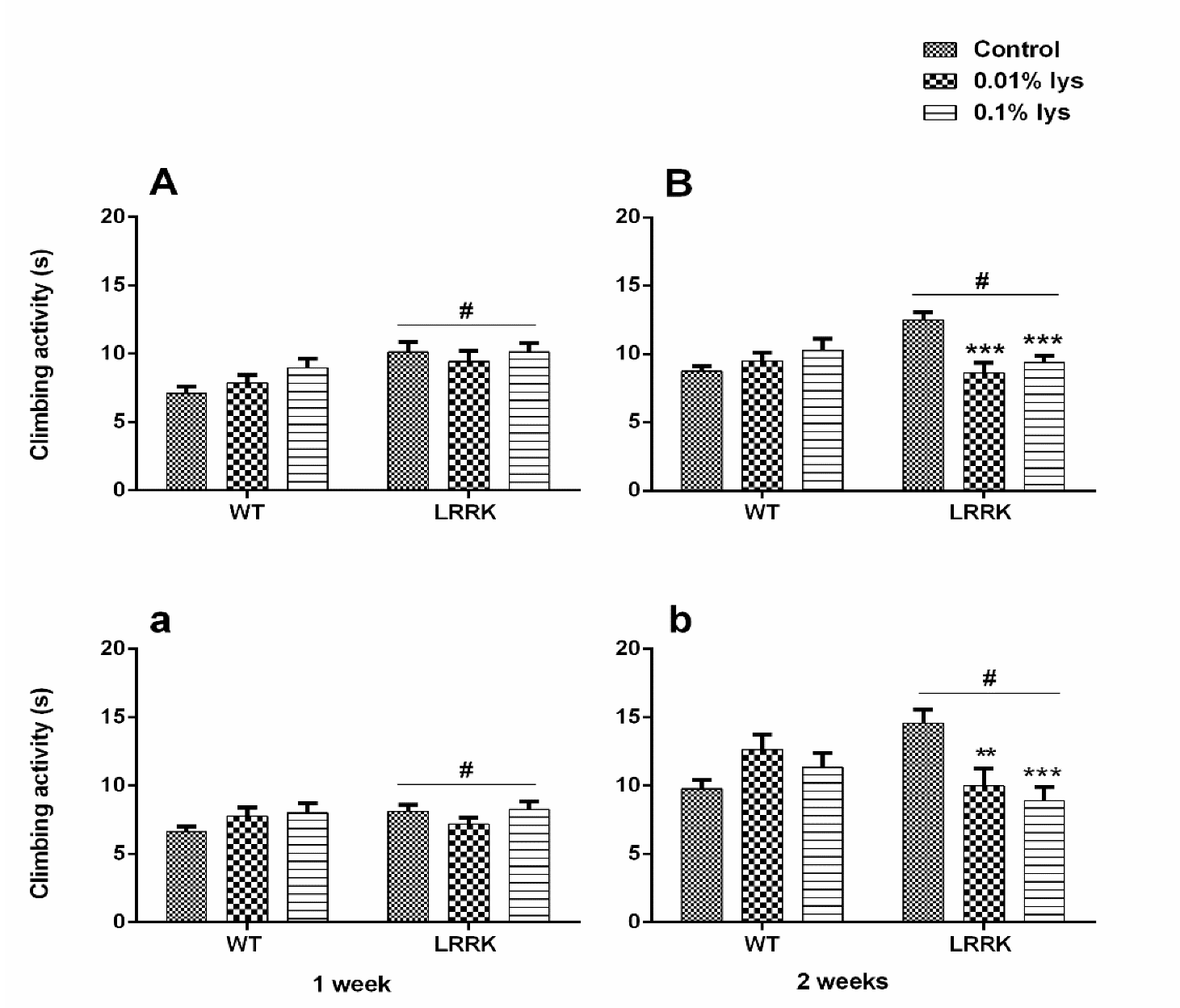
2.2. Climbing Assay
2.3. DLE Preparation
2.4. Immunohistochemistry
2.5. Statistics
3. Results
3.1. Motor Activity
3.2. Climbing Activity
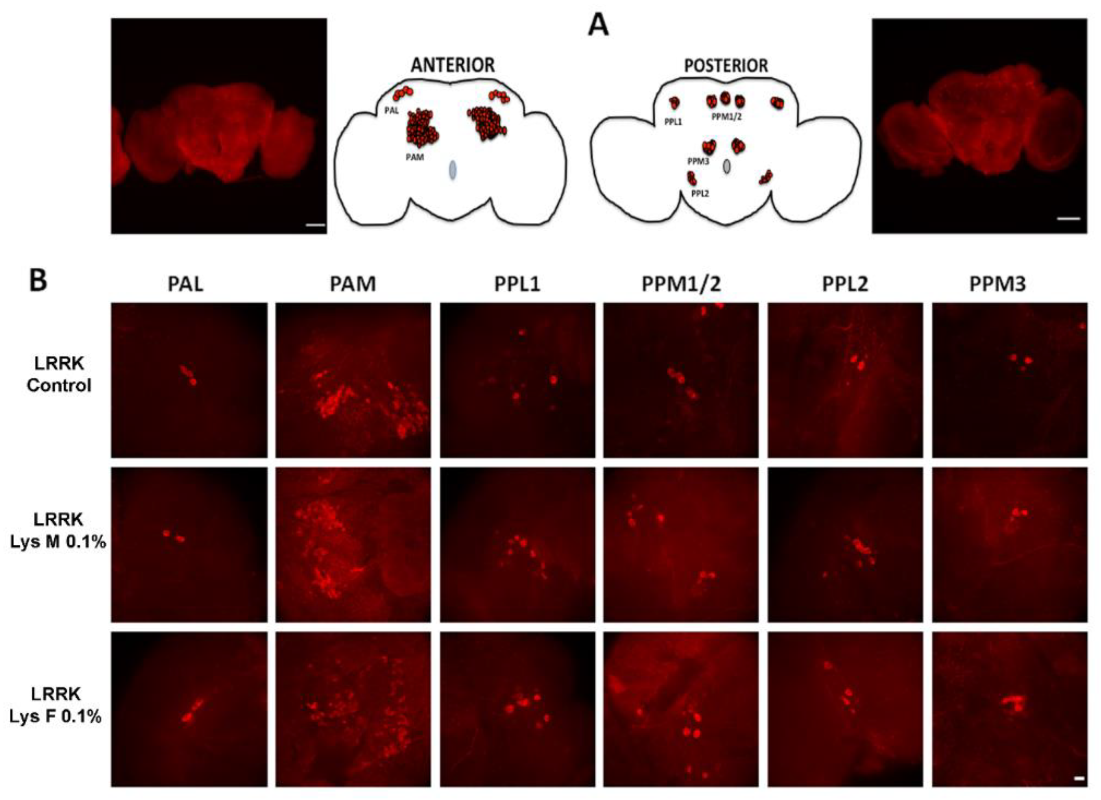
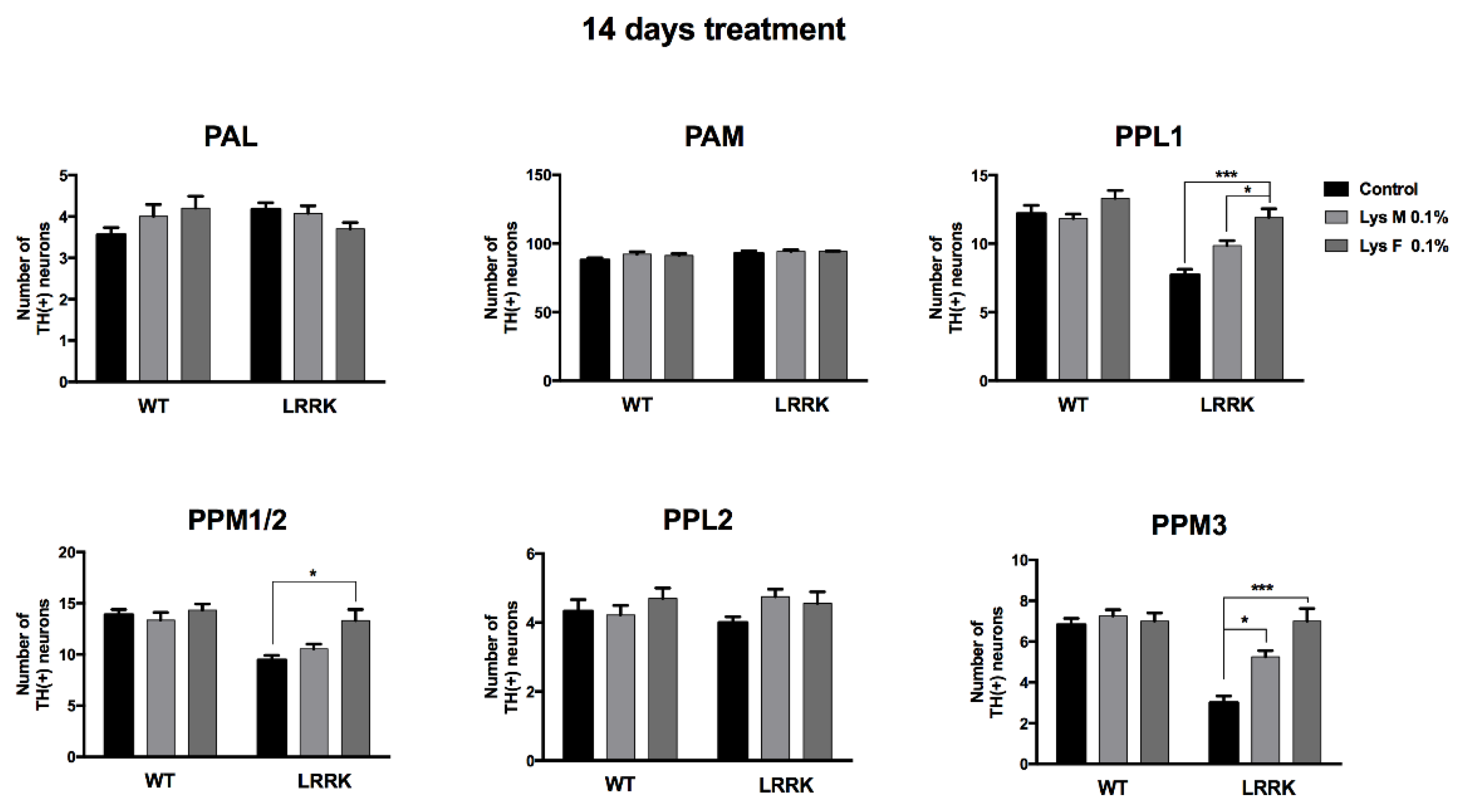
3.3. Immunohistochemistry
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Rijk, M.C.; Tzourio, C.; Breteler, M.M.; Dartigues, J.F.; Amaducci, L.; Lopez-Pousa, S.; Manubens-Bertran, J.M.; Alpérovitch, A.; Rocca, W.A. Prevalence of parkinsonism and Parkinson’s disease in Europe: The EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Twelves, D.; Perkins, K.S.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Disord. 2003, 18, 19–31. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.L.; Breteler, M.M.B. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef]
- Racette, B.A.; Willis, A.W. Time to change the blind men and the elephant approach to Parkinson disease? Neurology 2015, 85, 190–196. [Google Scholar] [CrossRef]
- Covy, J.P.; Giasson, B.I. α-Synuclein, leucine-rich repeat kinase-2, and manganese in the pathogenesis of Parkinson disease. Neurotoxicology 2011, 32, 622–629. [Google Scholar] [CrossRef]
- Wright Willis, A.; Evanoff, B.A.; Lian, M.; Criswell, S.R.; Racette, B.A. Geographic and ethnic variation in Parkinson disease: A population-based study of US Medicare beneficiaries. Neuroepidemiology 2010, 34, 143–151. [Google Scholar] [CrossRef]
- Willis, A.W.; Evanoff, B.A.; Lian, M.; Galarza, A.; Wegrzyn, A.; Schootman, M.; Racette, B.A. Metal emissions and urban incident Parkinson disease: A community health study of Medicare beneficiaries by using geographic information systems. Am. J. Epidemiol. 2010, 172, 1357–1363. [Google Scholar] [CrossRef]
- Plotegher, N.; Duchen, M.R. Crosstalk between Lysosomes and Mitochondria in Parkinson’s Disease. Front. Cell Dev. Biol. 2017, 5, 110. [Google Scholar] [CrossRef]
- Verstraeten, A.; Theuns, J.; Van Broeckhoven, C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet. 2015, 31, 140–149. [Google Scholar] [CrossRef]
- Biskup, S.; Moore, D.J. Detrimental deletions: Mitochondria, aging and Parkinson’s disease. Bioessays 2006, 28, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Higashi, S.; Biskup, S.; West, A.B.; Trinkaus, D.; Dawson, V.L.; Faull, R.L.; Waldvogel, H.J.; Arai, H.; Dawson, T.M.; Moore, D.J.; et al. Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007, 1155, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Galter, D.; Westerlund, M.; Carmine, A.; Lindqvist, E.; Sydow, O.; Olson, L. LRRK2 expression linked to dopamine-innervated areas. Ann. Neurol. 2006, 59, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Taymans, J.M.; Van den Haute, C.; Baekelandt, V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J. Neurochem. 2006, 98, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V. Parkinson’s disease: The LRRK2-G2019S mutation: Opening a novel era in Parkinson’s disease genetics. Eur. J. Hum. Genet. 2006, 14, 1061–1062. [Google Scholar] [CrossRef]
- Xie, C.L.; Pan, J.L.; Wang, W.W.; Zhang, Y.; Zhang, S.F.; Gan, J.; Liu, Z.G. The association between the LRRK2 G2385R variant and the risk of Parkinson’s disease: A meta-analysis based on 23 case-control studies. Neurol. Sci. 2014, 35, 1495–1504. [Google Scholar] [CrossRef]
- Mata, I.F.; Kachergus, J.M.; Taylor, J.P.; Lincoln, S.; Aasly, J.; Lynch, T.; Hulihan, M.M.; Cobb, S.A.; Wu, R.M.; Lu, C.S.; et al. Lrrk2 pathogenic substitutions in Parkinson’s disease. Neurogenetics 2005, 6, 171–177. [Google Scholar] [CrossRef]
- Berg, D.; Schweitzer, K.J.; Leitner, P.; Zimprich, A.; Lichtner, P.; Belcredi, P.; Brüssel, T.; Schulte, C.; Maass, S.; Nägele, T.; et al. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson’s disease *. Brain 2005, 128, 3000–3011. [Google Scholar] [CrossRef]
- Schrag, A.; Schott, J.M. Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. Lancet Neurol. 2006, 5, 355–363. [Google Scholar] [CrossRef]
- Wallings, R.; Manzoni, C.; Bandopadhyay, R. Cellular processes associated with LRRK2 function and dysfunction. FEBS J. 2015, 282, 2806–2826. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Manzoni, C.; Cookson, M.R.; Lewis, P.A. The LRRK2 signalling system. Cell Tissue Res. 2018, 373, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Lee, Y. Disease model organism for Parkinson disease: Drosophila melanogaster. BMP Rep. 2019, 52, 250–258. [Google Scholar] [CrossRef]
- Celotto, A.M.; Palladino, M.J. Drosophila: A “model” model system to study neurodegeneration. Mol. Interv. 2005, 5, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, S.; Bhat, K.M.; Setzu, M.D.; Solla, P.; Angioy, A.M.; Marotta, R.; Ruffilli, R.; Marrosu, F.; Liscia, A. Impaired sense of smell in a Drosophila Parkinson’s model. PLoS ONE 2013, 8, e73156. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, S.; De Rose, F.; Marotta, R.; Ruffilli, R.; Fanti, M.; Secci, P.P.; Mostallino, M.C.; Setzu, M.D.; Zuncheddu, M.A.; Collu, I.; et al. Mucuna pruriens (Velvet bean) rescues motor, olfactory, mitochondrial and synaptic impairment in PINK1B9 Drosophila melanogaster genetic model of Parkinson’s disease. PLoS ONE 2014, 9, e110802. [Google Scholar] [CrossRef] [PubMed]
- Nikonova, E.V.; Xiong, Y.; Tanis, K.Q.; Dawson, V.L.; Vogel, R.L.; Finney, E.M.; Stone, D.J.; Reynolds, I.J.; Kern, J.T.; Dawson, T.M. Transcriptional responses to loss or gain of function of the leucine-rich repeat kinase 2 (LRRK2) gene uncover biological processes modulated by LRRK2 activity. Hum. Mol. Genet. 2012, 21, 163–174. [Google Scholar] [CrossRef]
- Li, X.; Patel, J.C.; Wang, J.; Avshalumov, M.V.; Nicholson, C.; Buxbaum, J.D.; Elder, G.A.; Rice, M.E.; Yue, Z. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J. Neurosci. 2010, 30, 1788–1797. [Google Scholar] [CrossRef]
- Matikainen-Ankney, B.A.; Kezunovic, N.; Mesias, R.E.; Tian, Y.; Williams, F.M.; Huntley, G.W.; Benson, D.L. Altered Development of Synapse Structure and Function in Striatum Caused by Parkinson’s Disease-Linked LRRK2-G2019S Mutation. J. Neurosci. 2016, 36, 7128–7141. [Google Scholar] [CrossRef]
- Roosen, D.A.; Cookson, M.R. LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol. Neurodegener. 2016, 11, 73. [Google Scholar] [CrossRef]
- Carrion, M.D.P.; Marsicano, S.; Daniele, F.; Marte, A.; Pischedda, F.; Di Cairano, E.; Piovesana, E.; von Zweydorf, F.; Kremmer, E.; Gloeckner, C.J.; et al. The LRRK2 G2385R variant is a partial loss-of-function mutation that affects synaptic vesicle trafficking through altered protein interactions. Sci. Rep. 2017, 7, 5377. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Peng, R.; Wu, Y.R.; Wu, R.M.; Wu-Chou, Y.H.; Tan, L.C.; An, X.K.; Chen, C.M.; Fook-Chong, S.; Lu, C.S. LRRK2 G2385R modulates age at onset in Parkinson’s disease: A multi-center pooled analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, C.; Crosio, C.; Vitale, C.; Sanna, G.; Carrì, M.T.; Barone, P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum. Mol. Genet. 2007, 16, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- De Rose, F.; Marotta, R.; Poddighe, S.; Talani, G.; Catelani, T.; Setzu, M.D.; Solla, P.; Marrosu, F.; Sanna, E.; Kasture, S.; et al. Functional and Morphological Correlates in the Drosophila LRRK2 loss-of-function Model of Parkinson’s Disease: Drug Effects of Withania somnifera (Dunal) Administration. PLoS ONE 2016, 11, e0146140. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, W.; Lee, S.; Chung, J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem. Biophys. Res. Commun. 2007, 358, 534–539. [Google Scholar] [CrossRef]
- Joshi, N.; Singh, S. Updates on immunity and inflammation in Parkinson disease pathology. J. Neurosci. Res. 2018, 96, 379–390. [Google Scholar] [CrossRef]
- Kortekaas, R.; Leenders, K.L.; van Oostrom, J.C.; Vaalburg, W.; Bart, J.; Willemsen, A.T.; Hendrikse, N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Lawrence, H.S. The transfer in humans of delayed skin sensitivity to streptococcal m substance and to tuberculin with disrupted leucocytes. J. Clin. Investig. 1955, 34, 219–230. [Google Scholar] [CrossRef]
- Viza, D.; Fudenberg, H.H.; Palareti, A.; Ablashi, D.; De Vinci, C.; Pizza, G. Transfer factor: An overlooked potential for the prevention and treatment of infectious diseases. Folia Biol. (Praha) 2013, 59, 53–67. [Google Scholar]
- Singleton, A.B.; Farrer, M.J.; Bonifati, V. The genetics of Parkinson’s disease: Progress and therapeutic implications. Mov. Disord. 2013, 28, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Yu, Y.; Li, X.; Wang, T.; Jiang, H.; Ren, Q.; Jiao, Y.; Sawa, A.; Moran, T.; et al. A Drosophila model for LRRK2-linked parkinsonism. Proc. Natl. Acad. Sci. USA 2008, 105, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; James, W.S.; Cowley, S.A. LRRK2 in peripheral and central nervous system innate immunity: Its link to Parkinson’s disease. Biochem. Soc. Trans. 2017, 45, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 338, 394–397. [Google Scholar] [CrossRef]
- Kouli, A.; Horne, C.B.; Williams-Gray, C.H. Toll-like receptors and their therapeutic potential in Parkinson’s disease and α-synucleinopathies. Brain Behav. Immun. 2019, 81, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Davis, R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits 2009, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, K. Current perspective on the link between neuroinflammation and neurogenesis. Metab. Brain Dis. 2015, 30, 355–365. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diana, A.; Collu, M.; Casu, M.A.; Mocci, I.; Aguilar-Santelises, M.; Setzu, M.D. Improvements of Motor Performances in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Effects of Dialyzed Leucocyte Extracts from Human Serum. Brain Sci. 2020, 10, 45. https://doi.org/10.3390/brainsci10010045
Diana A, Collu M, Casu MA, Mocci I, Aguilar-Santelises M, Setzu MD. Improvements of Motor Performances in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Effects of Dialyzed Leucocyte Extracts from Human Serum. Brain Sciences. 2020; 10(1):45. https://doi.org/10.3390/brainsci10010045
Chicago/Turabian StyleDiana, Andrea, Maria Collu, Maria Antonietta Casu, Ignazia Mocci, Miguel Aguilar-Santelises, and Maria Dolores Setzu. 2020. "Improvements of Motor Performances in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Effects of Dialyzed Leucocyte Extracts from Human Serum" Brain Sciences 10, no. 1: 45. https://doi.org/10.3390/brainsci10010045
APA StyleDiana, A., Collu, M., Casu, M. A., Mocci, I., Aguilar-Santelises, M., & Setzu, M. D. (2020). Improvements of Motor Performances in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Effects of Dialyzed Leucocyte Extracts from Human Serum. Brain Sciences, 10(1), 45. https://doi.org/10.3390/brainsci10010045





