The Impact of a Structured Exercise Programme upon Cognitive Function in Chronic Fatigue Syndrome Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Initial Examination of Patients
2.2.1. Cognitive Function Measurement
2.2.2. Structured Exercise Programme
2.2.3. Statistical Methods
3. Results
Influence of SEP on Cognitive Function
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Prins, J.B.; Bleijenberg, G.; van der Meer, J.W.M. Chronic fatigue syndrome–Authors’ reply. Lancet 2006, 367, 1575. [Google Scholar] [CrossRef]
- Jason, L.A.; McManimen, S.; Sunnquist, M.; Brown, A.; Newton, J.L.; Strand, E.B. Examining the Institute of Medicine’s Recommendations Regarding Chronic Fatigue Syndrome: Clinical Versus Research Criteria. J. Neurol. Psychol. 2015, 2015, 441577253. [Google Scholar]
- Institute of Medicine (US). Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. In Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Assessing quality of life in older adults with cognitive impairment. Psychosom. Med. 2002, 64, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L. Clinical presentation of chronic fatigue syndrome. In Chronic Fatigue Syndrome; John Wiley: Chichester, UK, 1993; Volume 173, pp. 43–61. [Google Scholar]
- Jain, S.S.; DeLisa, J.A. Chronic fatigue syndrome: A literature review from a physiatric perspective. Am. J. Phys. Med. Rehabil. 1998, 77, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Natelson, B.H.; Lange, G. A status report on chronic fatigue syndrome. Environ. Health Perspect. 2002, 110, 673–677. [Google Scholar] [CrossRef]
- Afari, N.; Buchwald, D. Chronic fatigue syndrome: A review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef]
- Cockshell, S.J.; Mathias, J.L. Cognitive deficits in chronic fatigue syndrome and their relationship to psychological status, symptomatology, and everyday functioning. Neuropsychology 2013, 27, 230. [Google Scholar] [CrossRef]
- Jorgensen, R. Chronic fatigue: An evolutionary concept analysis. J. Adv. Nurs. 2008, 63, 199–207. [Google Scholar] [CrossRef]
- Robinson, L.J.; Gallagher, P.; Watson, S.; Pearce, R.; Finkelmeyer, A.; Maclachlan, L.; Newton, J.L. Impairments in cognitive performance in chronic fatigue syndrome are common, not related to co-morbid depression but do associate with autonomic dysfunction. PLoS ONE 2019, 14, e0210394. [Google Scholar] [CrossRef]
- DeLuca, J.; Johnson, S.K.; Ellis, S.P.; Natelson, B.H. Cognitive functioning is impaired in patients with chronic fatigue syndrome devoid of psychiatric disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 151–155. [Google Scholar] [CrossRef]
- Joyce, E.; Blumenthal, S.; Wessely, S. Memory, attention, and executive function in chronic fatigue syndrome. J. Neurol. Neurosurg. Psychiatry 1996, 60, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Shaw, E.J. Diagnosis and management of chronic fatigue syndrome or myalgic encephalomyelitis (or encephalopathy): Summary of NICE guidance. BMJ 2007, 335, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Vink, M. PACE trial authors continue to ignore their own null effect. J. Health Psychol. 2017, 22, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Cvejic, E.; Lloyd, A.R.; Vollmer-Conna, U. Neurocognitive improvements after best-practice intervention for chronic fatigue syndrome: Preliminary evidence of divergence between objective indices and subjective perceptions. Compr. Psychiatry 2016, 66, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Castell, B.D.; Kazantzis, N.; Moss-Morris, R.E. Cognitive behavioral therapy and graded exercise for chronic fatigue syndrome: A meta-analysis. Clin. Psychol. Sci. Pract. 2011, 18, 311–324. [Google Scholar] [CrossRef]
- Larun, L.; Brurberg, K.G.; Odgaard-Jensen, J.; Price, J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Knapen, J.; Vancampfort, D.; Moriën, Y.; Marchal, Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil. Rehabil. 2015, 37, 1490–1495. [Google Scholar] [CrossRef]
- Wilshire, C.E.; Kindlon, T.; Courtney, R.; Matthees, A.; Tuller, D.; Geraghty, K.; Levin, B. Rethinking the treatment of chronic fatigue syndrome—A reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychol. 2018, 6, 6. [Google Scholar] [CrossRef]
- McPhee, G. Cognitive behaviour therapy and objective assessments in chronic fatigue syndrome. J. Health Psychol. 2017, 22, 1181–1186. [Google Scholar] [CrossRef]
- Price, J.R.; Mitchell, E.; Tidy, E.; Hunot, V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst. Rev. 2008, 3. [Google Scholar] [CrossRef]
- Ickmans, K.; Meeus, M.; Kos, D.; Clarys, P.; Meersdom, G.; Lambrecht, L.; Nijs, J. Cognitive performance is of clinical importance, but is unrelated to pain severity in women with chronic fatigue syndrome. Clin. Rheumatol. 2013, 32, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Moss-Morris, R.; Sharon, C.; Tobin, R.; Baldi, J.C. A randomized controlled graded exercise trial for chronic fatigue syndrome: Outcomes and mechanisms of change. J. Health Psychol. 2005, 10, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Verburgh, L.; Königs, M.; Scherder, E.J.; Oosterlaan, J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. Br. J. Sports Med. 2014, 48, 973–979. [Google Scholar] [CrossRef] [PubMed]
- de Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, S.; Słomko, J.; Tafil-Klawe, M.; Zawadka-Kunikowska, M.; Szrajda, J.; Newton, J.L.; Zalewski, P.; Klawe, J.J. The impact of total sleep deprivation upon cognitive functioning in firefighters. Neuropsychiatr. Dis. Treat. 2018, 14, 1171. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.V.; White, P.D. The role of deconditioning and therapeutic exercise in chronic fatigue syndrome (CFS). J. Ment. Health 2005, 14, 237–252. [Google Scholar] [CrossRef]
- Bavinton, J. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 2011, 377, 823–836. [Google Scholar]
- Fulcher, K.Y.; White, P.D. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ 1997, 314, 1647. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage: Root, Switzerland, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 9 October 2019).
- Patil, I.; Powell, C. Ggstatsplot: ‘ggplot2’ Based Plots with Statistical Details. 2018. Available online: https://CRAN.R-project.org/package=ggstatsplot (accessed on 9 October 2019).
- Blackwood, S.K.; MacHale, S.M.; Power, M.J.; Goodwin, G.M.; Lawrie, S.M. Effects of exercise on cognitive and motor function in chronic fatigue syndrome and depression. J. Neurol. Neurosurg. Psychiatry 1998, 65, 541–546. [Google Scholar] [CrossRef]
- Hallgren, M.; Herring, M.P.; Owen, N.; Dunstan, D.; Ekblom, Ö.; Helgadottir, B.; Nakitanda, O.A.; Forsell, Y. Exercise, physical activity, and sedentary behavior in the treatment of depression: Broadening the scientific perspectives and clinical opportunities. Front. Psychiatry 2016, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Annesi, J. Effects of a cognitive behavioral treatment package on exercise attendance and drop out in fitness centers. Eur. J. Sport Sci. 2016, 3, 1–16. [Google Scholar] [CrossRef]
- Annesi, J.J. Effects of computer feedback on adherence to exercise. Percept. Mot. Ski. 1998, 87, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Marcus, B.H.; Forsyth, L.H.; Stone, E.J.; Dubbert, P.M.; McKenzie, T.L.; Dunn, A.L.; Blair, S.N. Physical activity behavior change: Issues in adoption and maintenance. Health Psychol. 2000, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS ONE 2018, 13, e0197811. [Google Scholar] [CrossRef] [PubMed]
- Deale, A.; Husain, K.; Chalder, T.; Wessely, S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: A 5-year follow-up study. Am. J. Psychiatry 2001, 158, 2038–2042. [Google Scholar] [CrossRef]
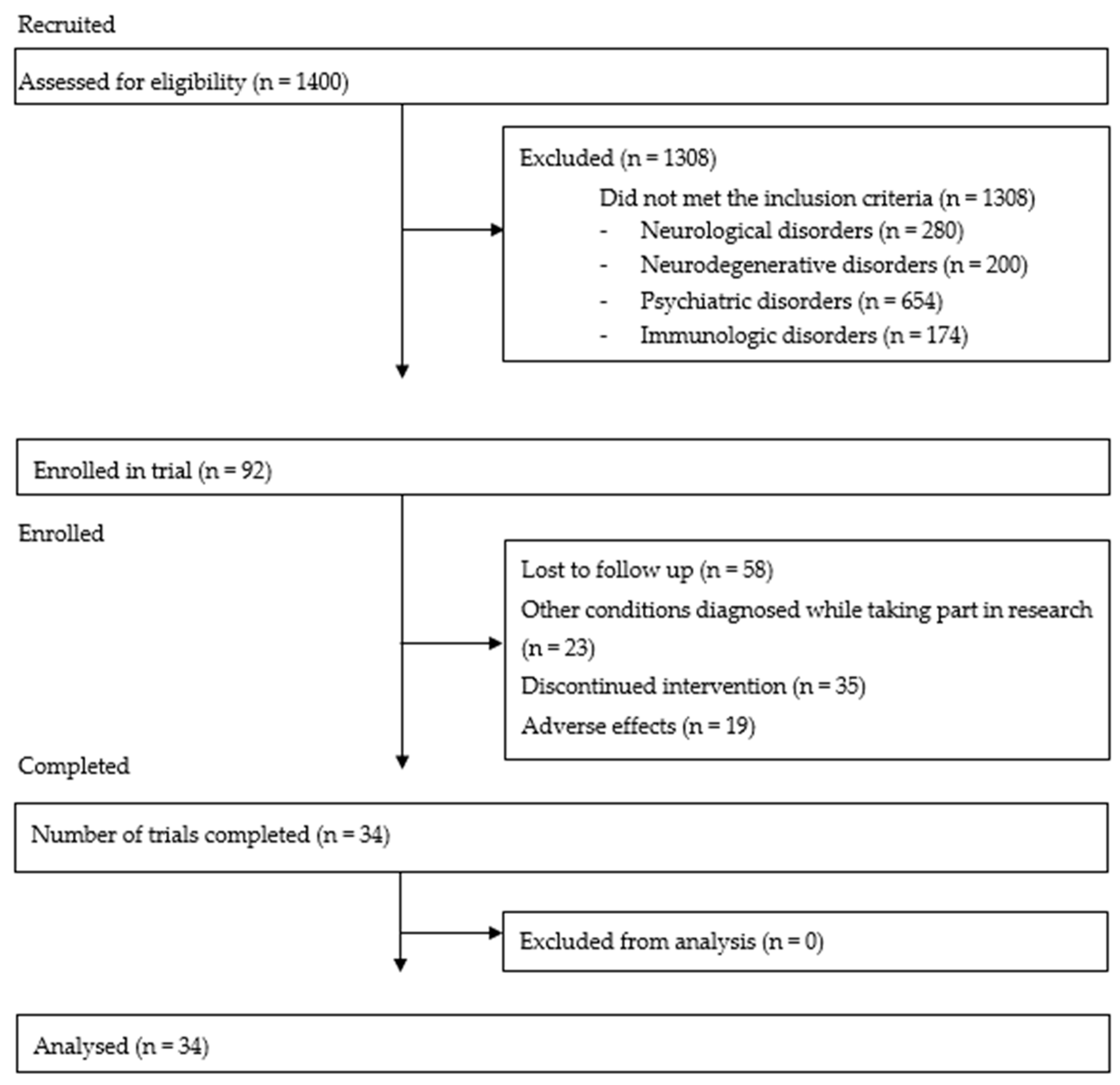
| Variable | Mean (SD) Before | Mean (SD) After | p | FDR |
|---|---|---|---|---|
| SRT.1 correct answers | 19.47 (0.99) | 19.47 (0.83) | 0.89 | 1.07 |
| SRT.1 errors | 0.53 (0.99) | 0.53 (0.83) | 0.89 | 1 |
| SRT.1 correct reaction time | 547.09 (162.59) | 520.56 (129.67) | 0.22 | 0.79 |
| SRT.2 correct answers | 19.18 (2.59) | 19.71 (0.68) | 0.34 | 1.02 |
| SRT.2 errors | 0.82 (2.59) | 0.32 (0.68) | 0.44 | 0.88 |
| SRT.2 correct reaction time | 540.24 (188.58) | 517.32 (106.68) | 0.62 | 0.80 |
| SRT.3 correct answers | 19.62 (0.60) | 19.59 (0.66) | 1.00 | 1.06 |
| SRT.3 errors | 0.38 (0.60) | 1.59 (7.33) | 1.00 | 1 |
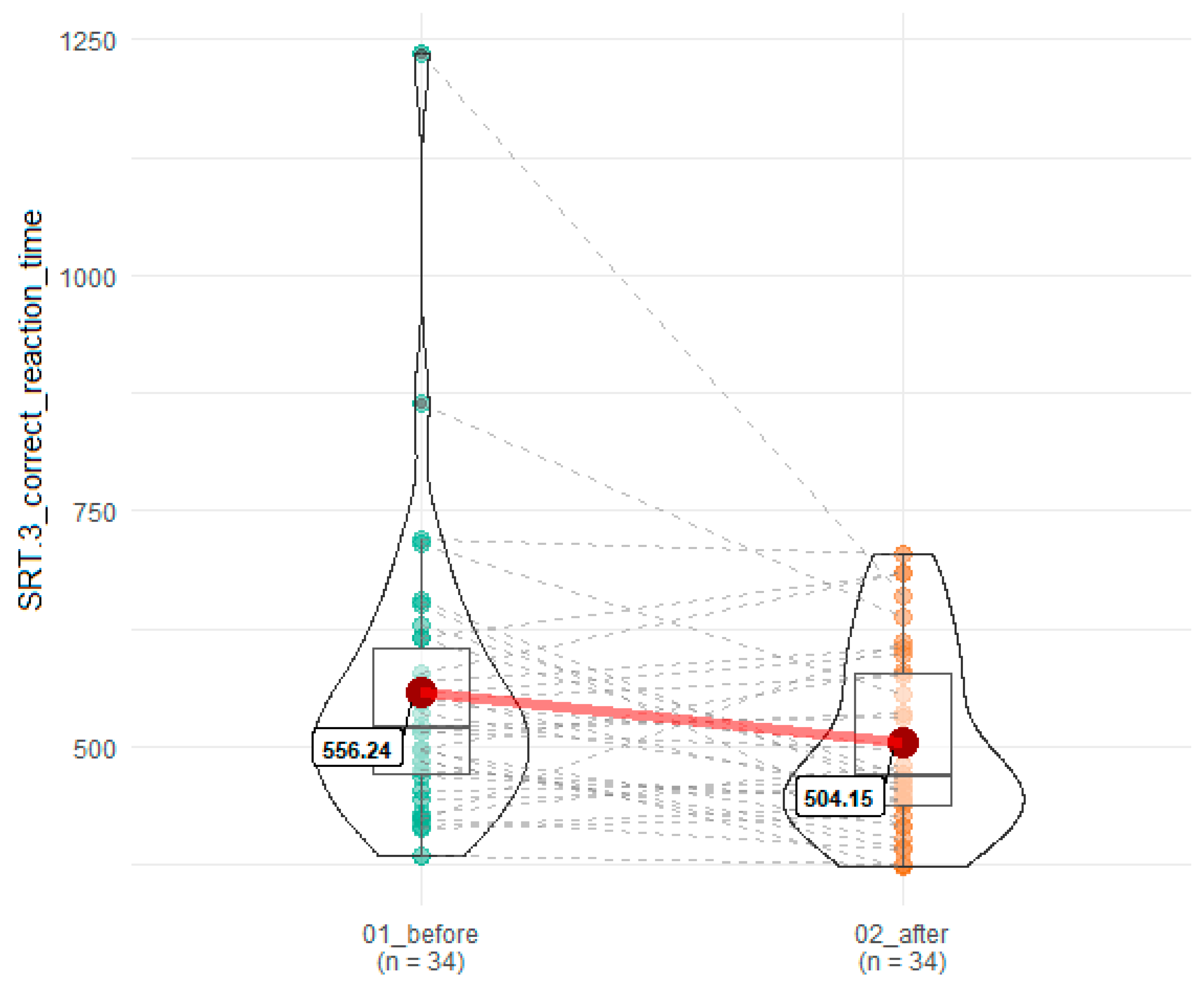
| SRT.3 correct reaction time | 556.24 (157.99) | 504.15 (97.28) | 0.04 | 0.18 |
| CRT correct answers | 29.24 (0.89) | 29.44 (0.75) | 0.41 | 1.05 |
| CRT errors | 0.77 (0.89) | 0.62 (0.82) | 0.53 | 0.80 |
| CRT correct reaction time | 617.97 (175.24) | 596.21 (119.01) | 0.56 | 0.78 |
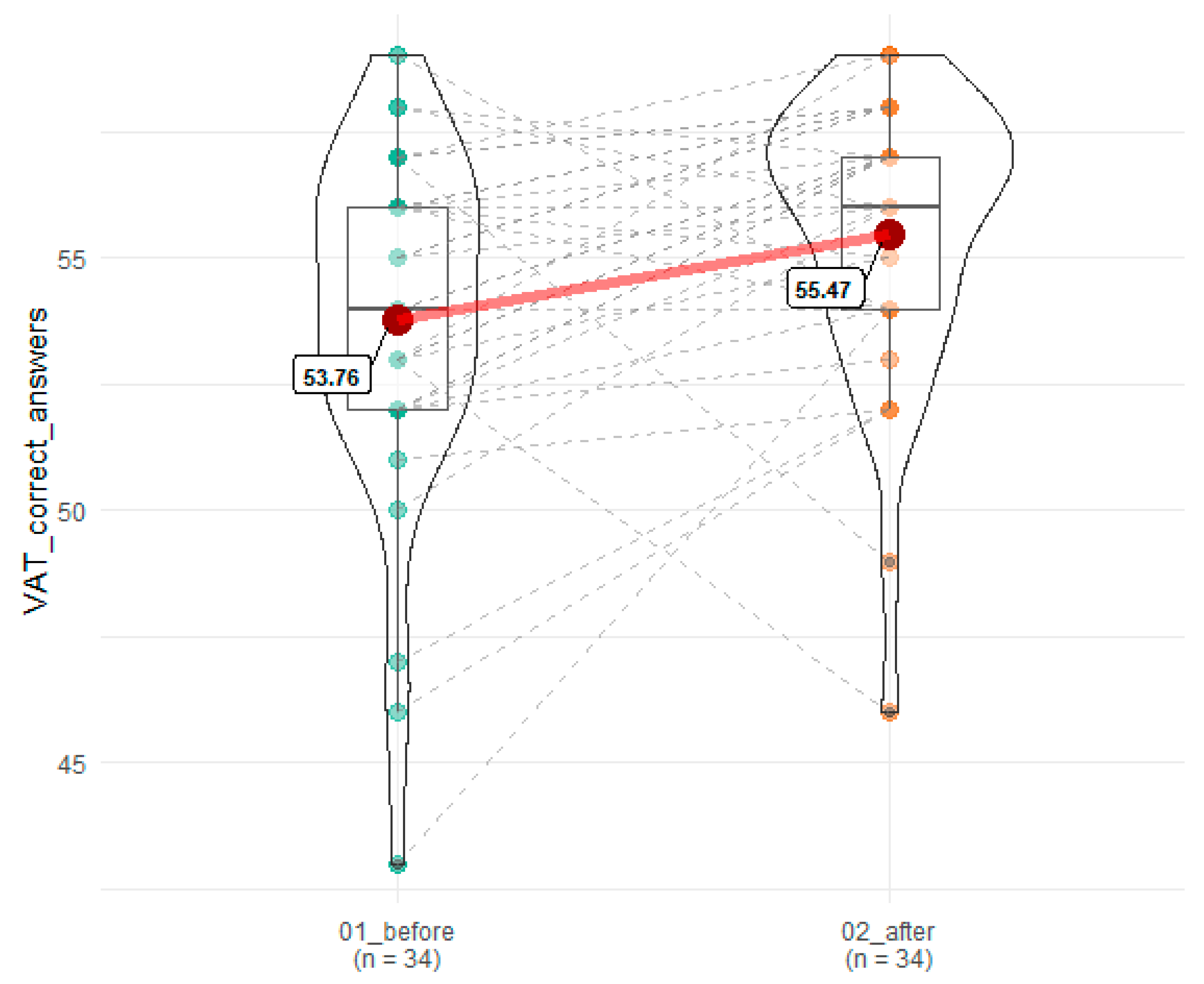
| VAT correct answers | 53.77 (3.49) | 55.47 (2.85) | 0.007 | 0.06 |
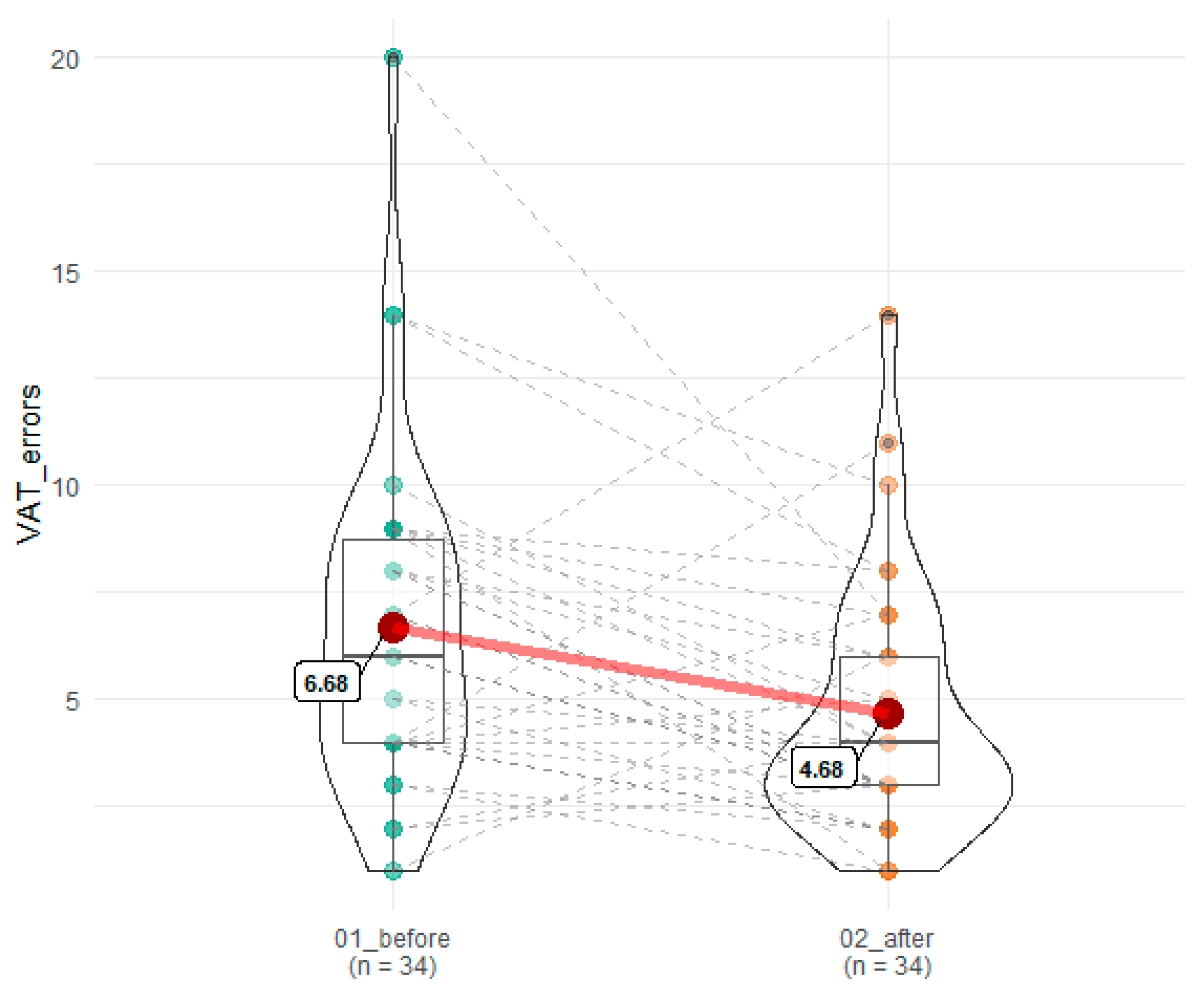
| VAT errors | 6.68 (3.88) | 4.68 (2.96) | 0.004 | 0.07 |
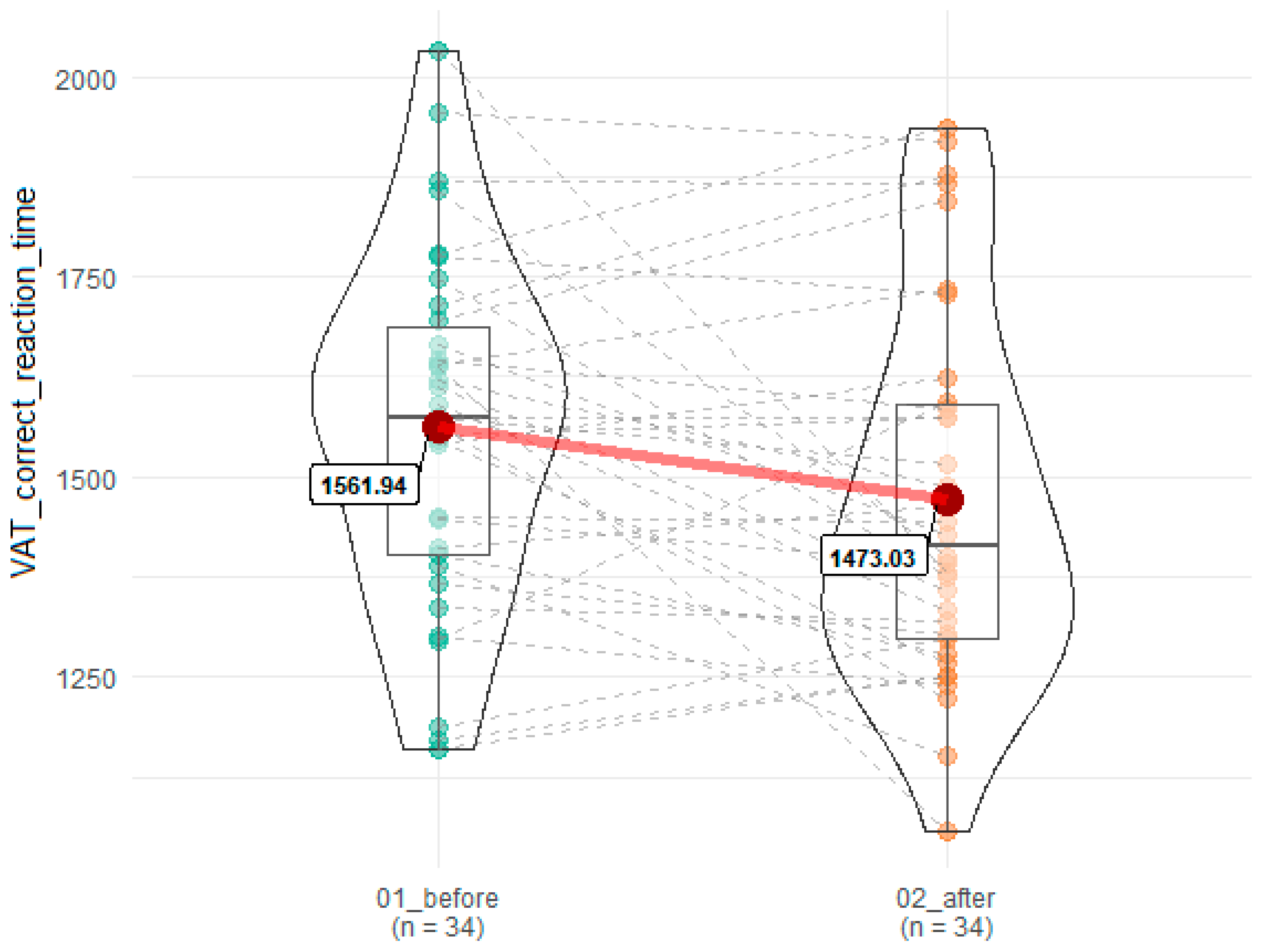
| VAT correct reaction time | 1561.94 (216.66) | 1473.03 (232.90) | 0.02 | 0.12 |
| DMS correct answers | 22.35 (3.54) | 22.94 (3.48) | 0.48 | 0.79 |
| DMS errors | 10.82 (3.73) | 10.71 (5.58) | 0.43 | 0.97 |
| DMS correct reaction time | 1473.59 (265.46) | 1418.79 (360.73) | 0.47 | 0.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewski, P.; Kujawski, S.; Tudorowska, M.; Morten, K.; Tafil-Klawe, M.; Klawe, J.J.; Strong, J.; Estévez-López, F.; Murovska, M.; Newton, J.L.; et al. The Impact of a Structured Exercise Programme upon Cognitive Function in Chronic Fatigue Syndrome Patients. Brain Sci. 2020, 10, 4. https://doi.org/10.3390/brainsci10010004
Zalewski P, Kujawski S, Tudorowska M, Morten K, Tafil-Klawe M, Klawe JJ, Strong J, Estévez-López F, Murovska M, Newton JL, et al. The Impact of a Structured Exercise Programme upon Cognitive Function in Chronic Fatigue Syndrome Patients. Brain Sciences. 2020; 10(1):4. https://doi.org/10.3390/brainsci10010004
Chicago/Turabian StyleZalewski, Paweł, Sławomir Kujawski, Malwina Tudorowska, Karl Morten, Małgorzata Tafil-Klawe, Jacek J. Klawe, James Strong, Fernando Estévez-López, Modra Murovska, Julia L. Newton, and et al. 2020. "The Impact of a Structured Exercise Programme upon Cognitive Function in Chronic Fatigue Syndrome Patients" Brain Sciences 10, no. 1: 4. https://doi.org/10.3390/brainsci10010004
APA StyleZalewski, P., Kujawski, S., Tudorowska, M., Morten, K., Tafil-Klawe, M., Klawe, J. J., Strong, J., Estévez-López, F., Murovska, M., Newton, J. L., & the European Network on ME/CFS (EUROMENE). (2020). The Impact of a Structured Exercise Programme upon Cognitive Function in Chronic Fatigue Syndrome Patients. Brain Sciences, 10(1), 4. https://doi.org/10.3390/brainsci10010004







