Air Purification Performance of Photocatalytic Concrete Paving Blocks after Seven Years of Service
Abstract
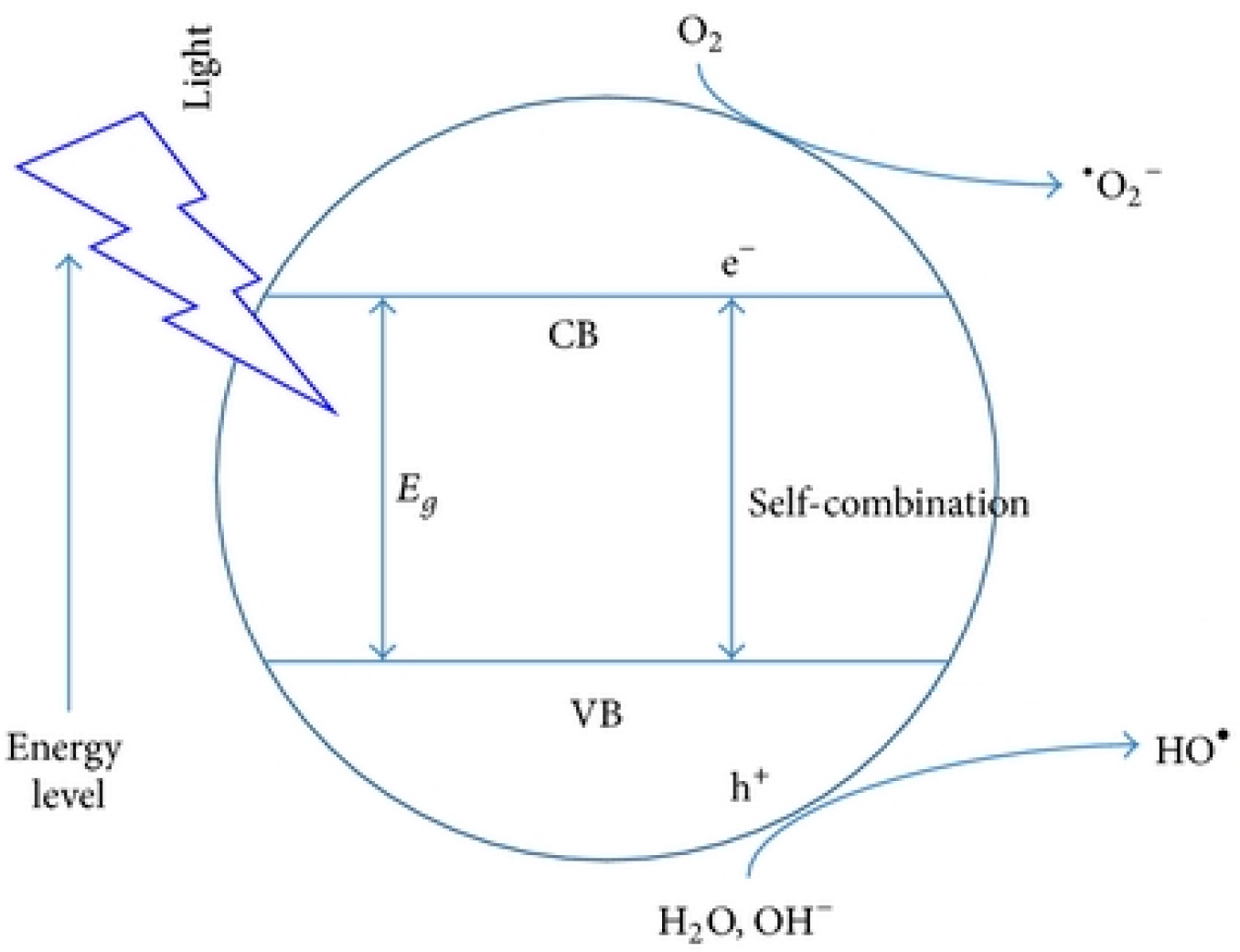
:1. Introduction
2. Experimental Procedure
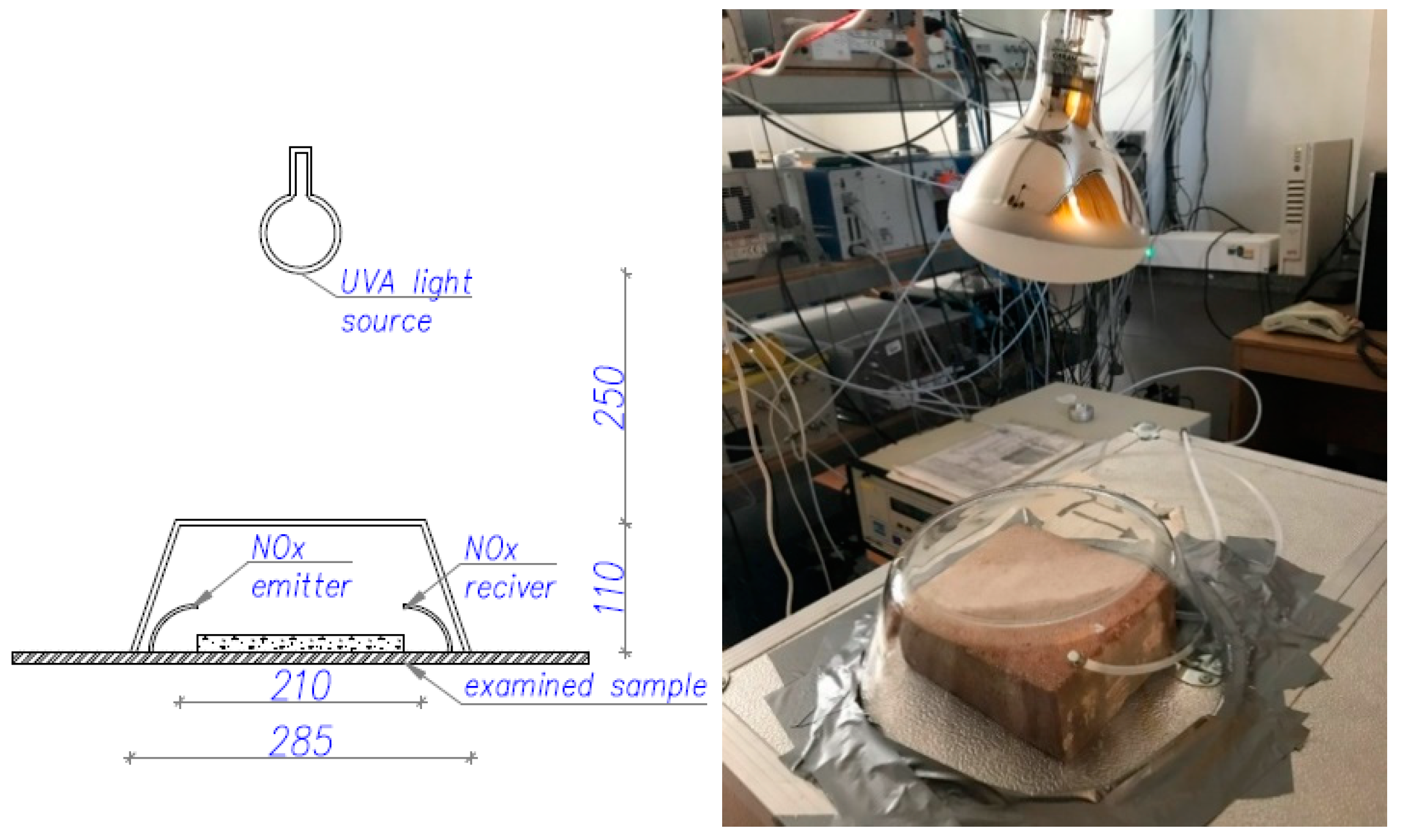
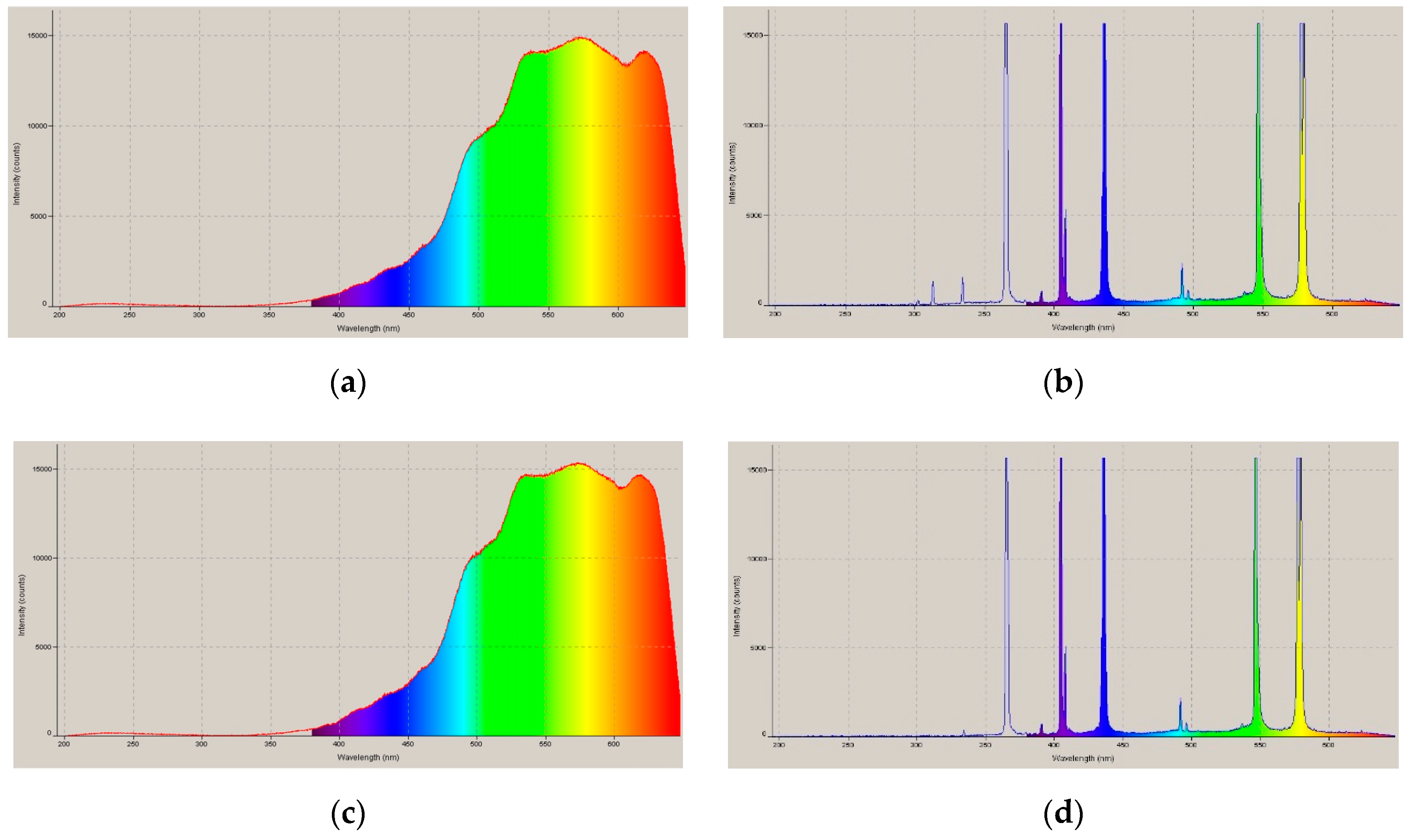
2.1. Test for AirPurification Performance
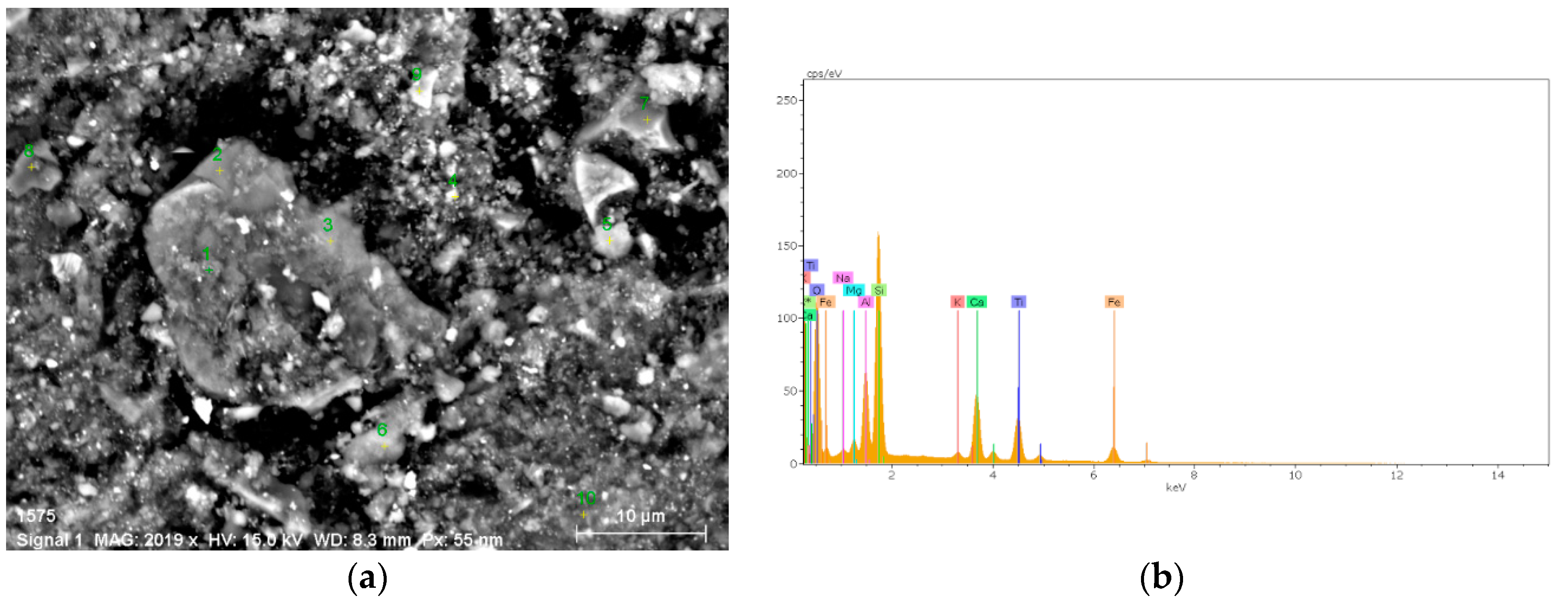
2.2. SEM Analysis
3. Results
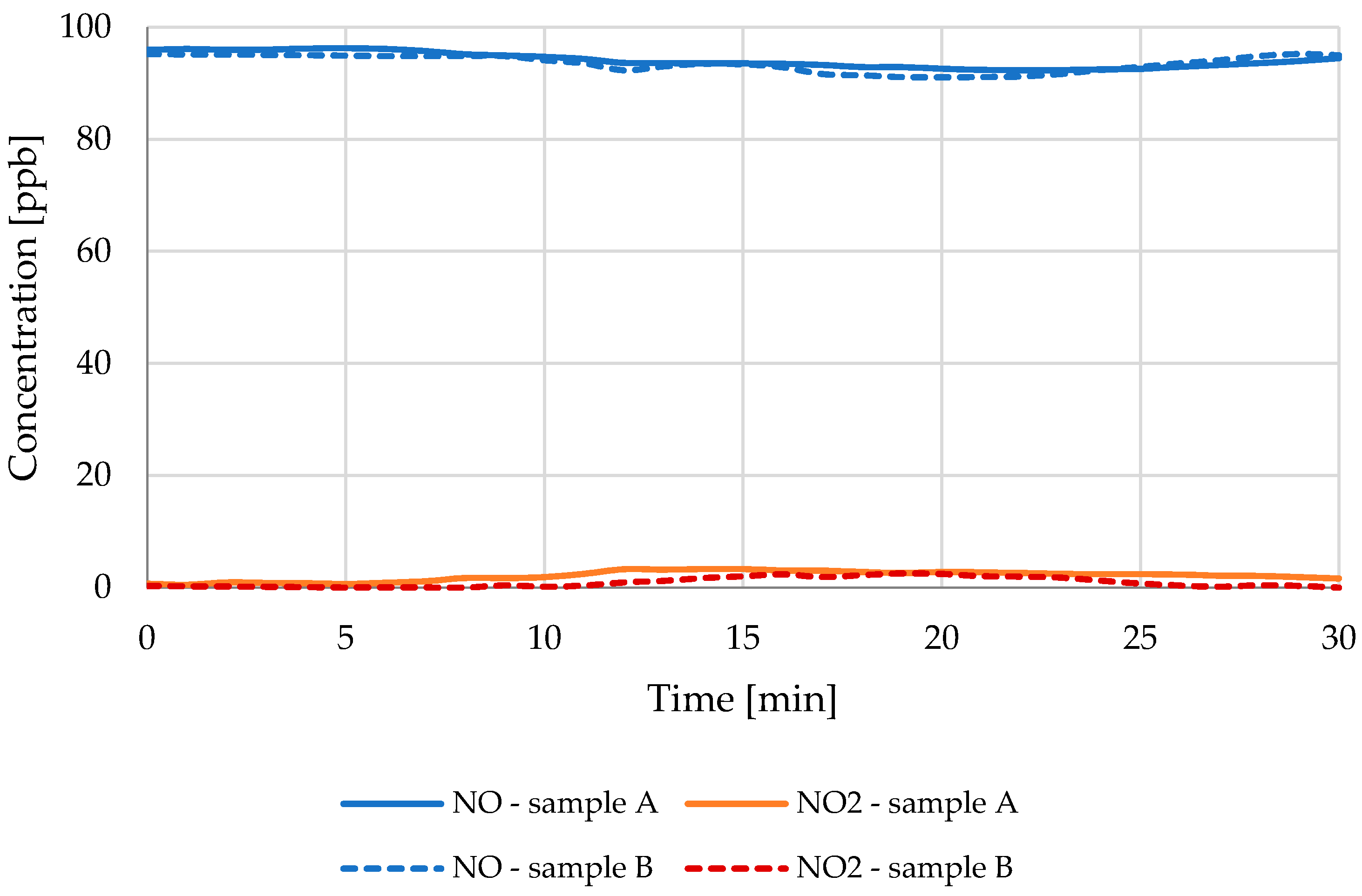
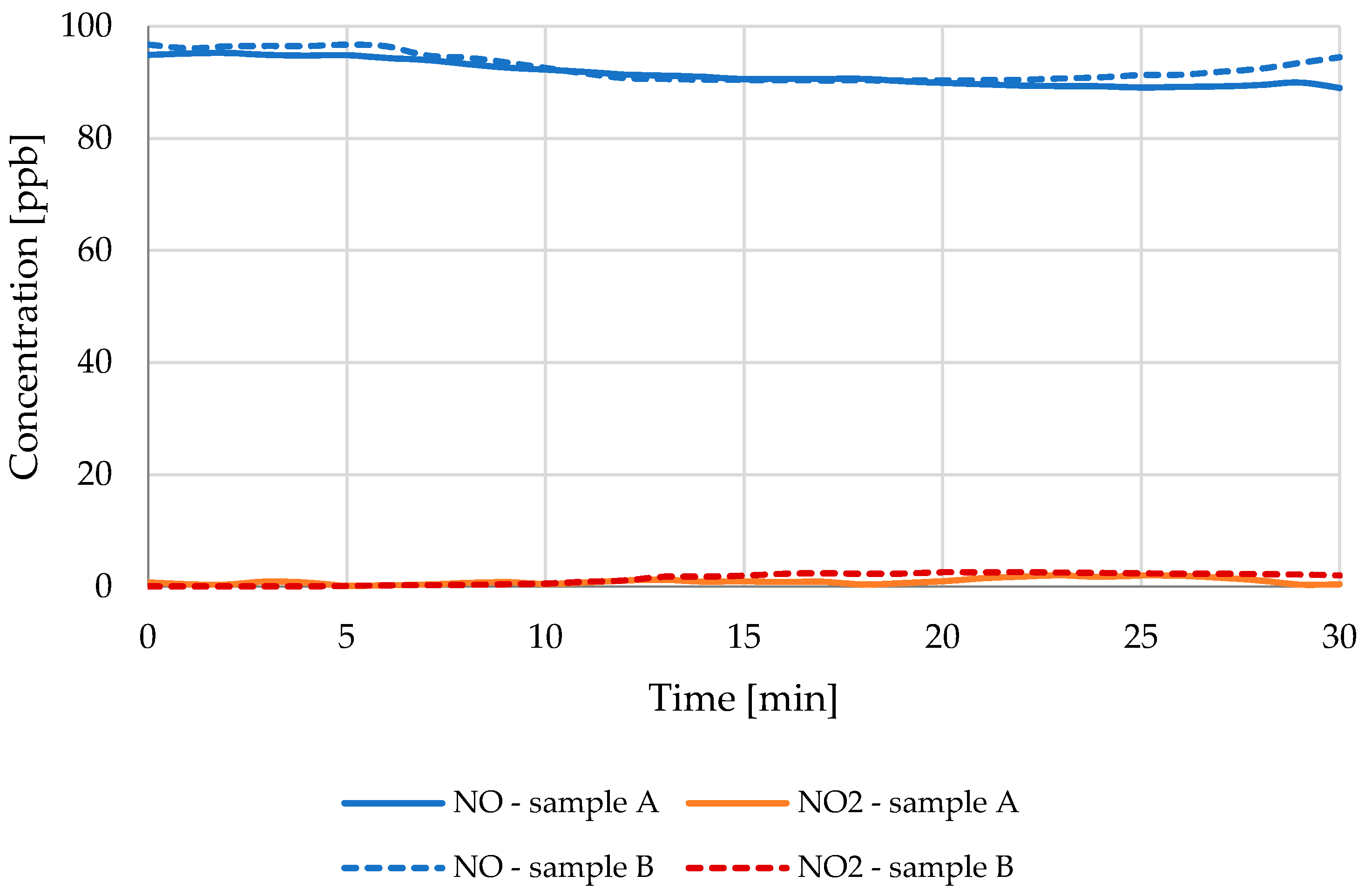
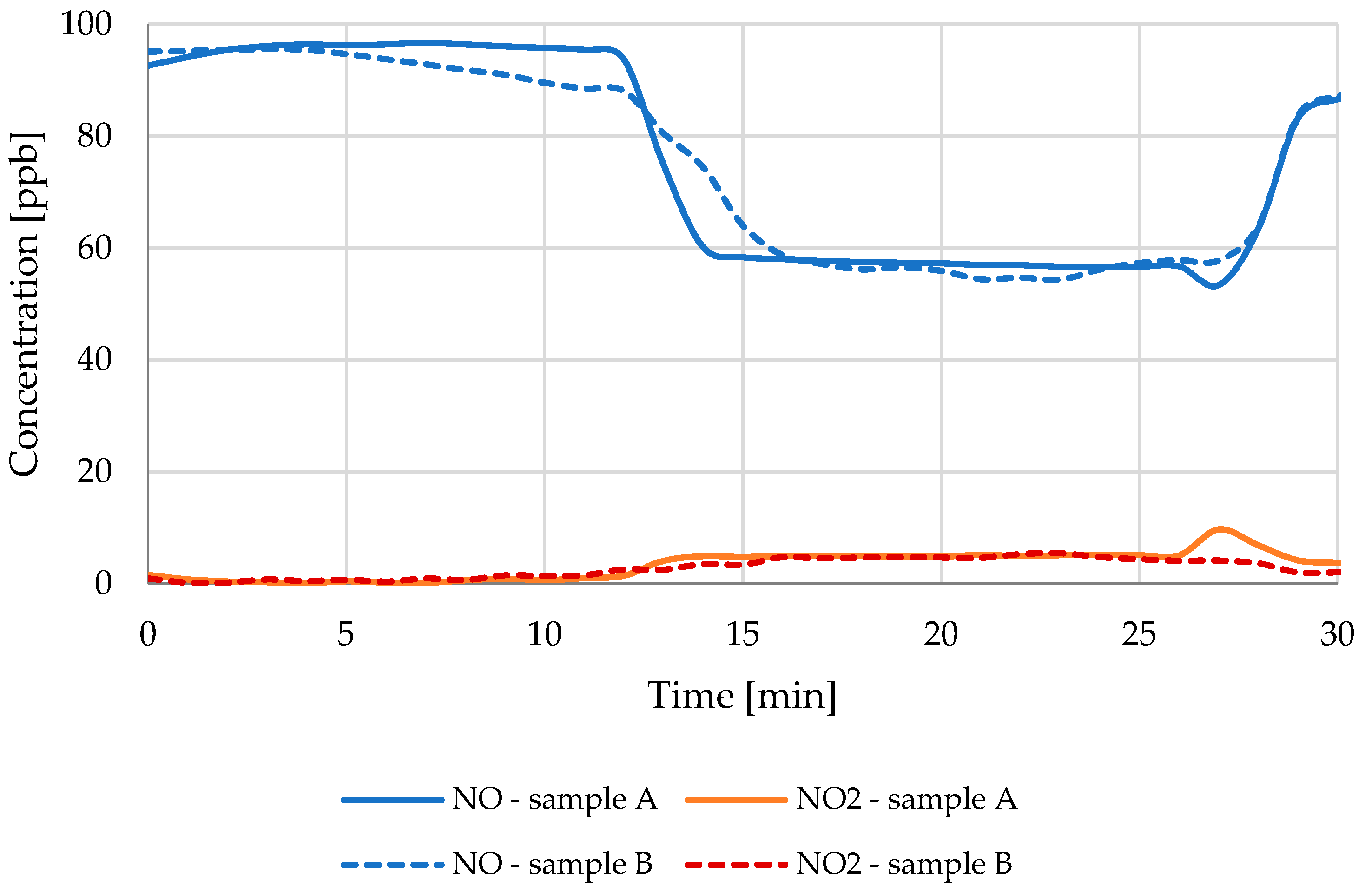
3.1. NO Reduction
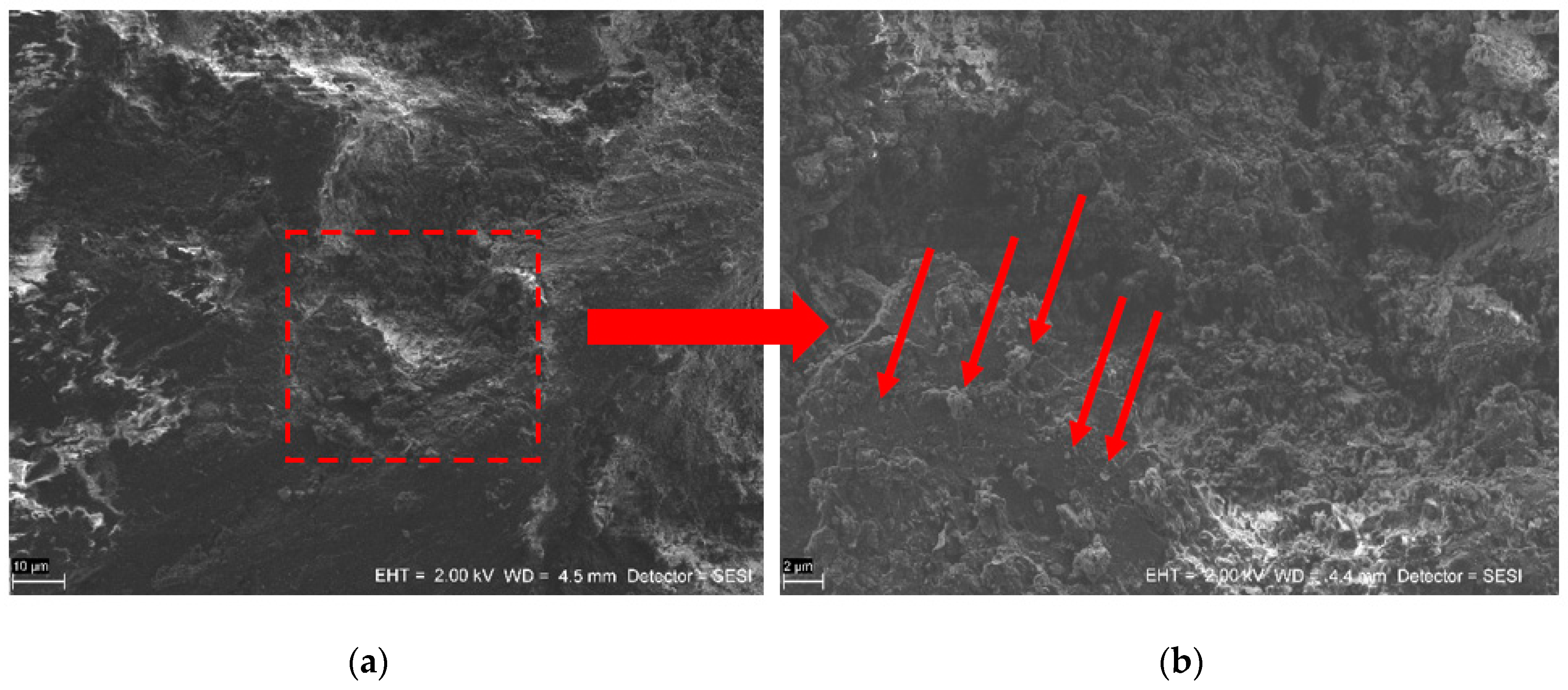
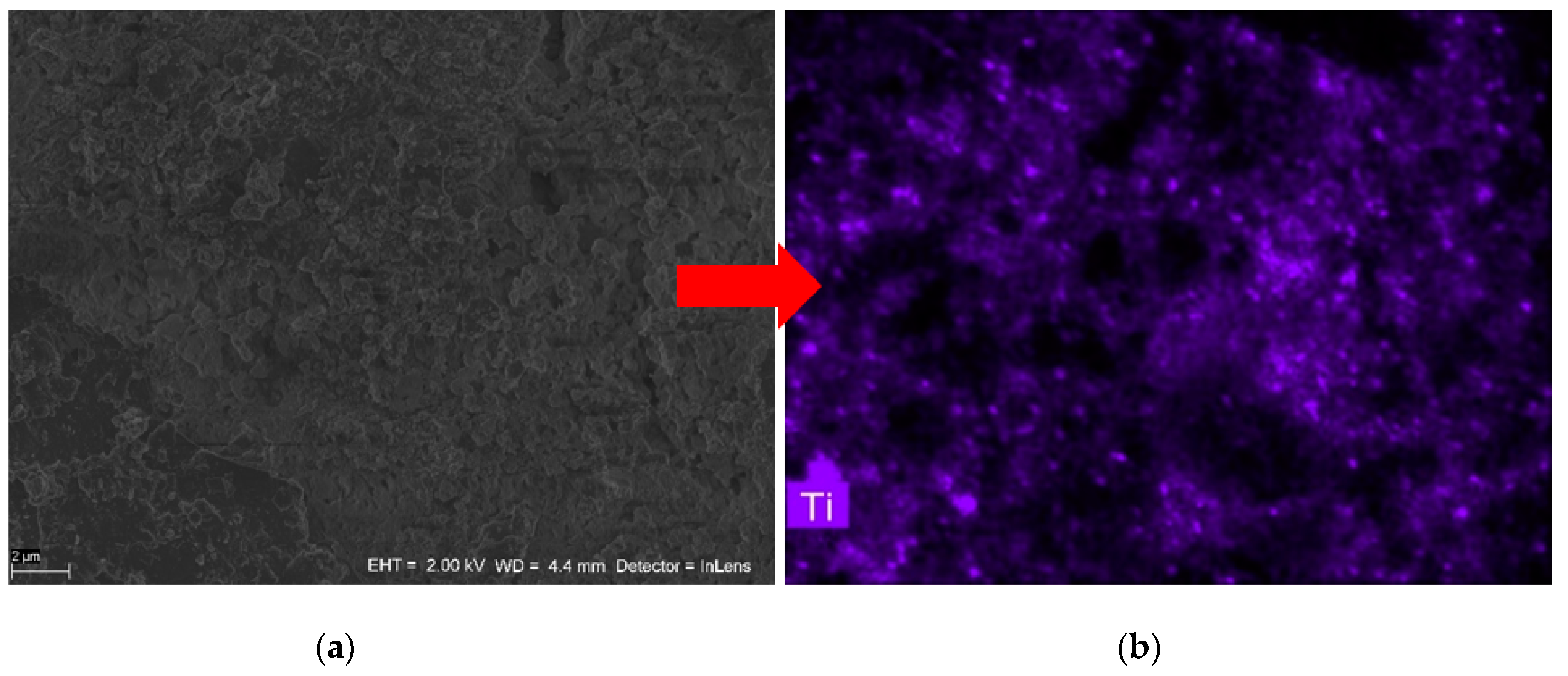
3.2. SEM Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muilwijk, C.; Schrijvers, P.J.C.; Werz, S.; Kenjeres, S. Simulations of photochemical smog formation in complex urban areas. Atmos. Environ. 2016, 147, 470–484. [Google Scholar] [CrossRef]
- Guerrini, G.L.; Beldens, A.; Crispino, M.; D’Ambarosio, G.; Vismaro, S. Environmental benefits of innovative photocatalytic cementitous road material. In Proceedings of the 10th International Conference on Concrete Pavement, Quebec City, QC, Canada, 8–12 July 2012. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, G.L. Photocatalytic performance in a city tunnel in Rome: NOx monitoring results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- Boonen, E.; Akylas, V.; Barmpas, F.; Boreave, A.; Bottalico, L.; Cazaunau, M.; Chen, H.; Daele, V.; De Marco, T.; Doussin, J.F.; et al. Construction of a photocatalytic de–polluting field site in the Leopold II tunnel in Brussels. J. Environ. Manag. 2015, 155, 136–144. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Beeldens, A.; Barmpas, F.; Doussin, J.F.; Manganelli, G.; Herrmann, H.; Kleffmann, J.; Mellouki, A. Impact of photocatalytic remediation of pollutnats on urban air quality. Front. Environ. Sci. Eng. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Qu, Y. TiO2—Based Photocatalytic Process for Purification of Polluted Water: Bridging Fundamentals to Applications. J. Nanomater. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Shen, W.; Zang, C.; Li, Q.; Zhang, W.; Cao, L.; Ye, P. Preparation of titanium dioxide nano particle modified photocatalytic self–cleaning concrete. J. Clean. Prod. 2015, 87, 762–765. [Google Scholar] [CrossRef]
- Husken, G.; Hunger, M.; Bruwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Macphee, D.E.; Folli, A. Photocatalytic concretes–The interface between photocatalysis and cement chemistry. Cem. Concr. Res. 2016, 85, 48–54. [Google Scholar] [CrossRef]
- Poon, C.S.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Cassar, L.; Beeldens, A.; Pimpinelli, N.; Guerrini, G.L. Photocatalysis of cementitous materials. In Proceedings of the International RILEM Symposium on Photocatalysis, Environment and Construction Materials, Florence, Italy, 8–9 October 2012. [Google Scholar]
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Beeldens, A. An environmental friendly solution for air purification and self-cleaning effect: The application of TiO2 as photocatalyst in concrete. In Proceedings of the 8th International Conference on Concrete Blocks Paving, San Francisco, CA, USA, 6–8 November 2006. [Google Scholar]
- Guerrini, G.L. Some observations regarding in–service performance Photocatalytic paving block surfaces. Betonwerk Fertigteil-Technik BFT 2009, 5, 16–25. [Google Scholar]
- ISO 22197-1:2016, Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide; ISO: Geneva, Switzerland, 2016.
- UNI-11247:2010, Determination of the Degradation of Nitrogen Oxides in the Air by Inorganic Photocatalytic Materials: Continuous Flow Test Method; Ente Nazionale Italiano di Unificazione: Milano, Italy, 2010; (Italian Standard).
- JIS TR Z 0018, Photocatalytic Materials—Air Purification Test Procedure; Japanese Standards Association: Tokyo, Japan, 2002; (Japan Standard).
- Folli, A.; Macphee, D.E. Future challenges for photocatalytic concrete technology. In Proceedings of the 34th Cement and Concrete Science Conference, University of Sheffield, Sheffield, UK, 14–17 September 2014. [Google Scholar]
- Bloh, J.; Folli, A.; Macphee, D. Photocatalytic NOx abatement: Why the selectivity matters. RSC Adv. 2014, 4, 45726–45734. [Google Scholar] [CrossRef]

| Light Source | 70 W | 70 W | 300 W |
|---|---|---|---|
| Surface Cleanliness | Dusty | Cleaned | Cleaned |
| ΔNO * | −4 ppb ± 1.3 | −5 ppb ± 0.3 | −43 ppb ± 1.0 |
| ΔNO2 * | +3 ppb ± 0.6 | +2 ppb ± 0.5 | +9 ppb ± 3.1 |
| ΔNOx * (ΔNO + ΔNO2) | −1 ppb | −4 ppb | −34 ppb |
| NO reduction * % | 4% | 5% | 45% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowski, H.; Jackiewicz-Rek, W.; Chilmon, K.; Jarosławski, J.; Tryfon-Bojarska, A.; Gąsiński, A. Air Purification Performance of Photocatalytic Concrete Paving Blocks after Seven Years of Service. Appl. Sci. 2019, 9, 1735. https://doi.org/10.3390/app9091735
Witkowski H, Jackiewicz-Rek W, Chilmon K, Jarosławski J, Tryfon-Bojarska A, Gąsiński A. Air Purification Performance of Photocatalytic Concrete Paving Blocks after Seven Years of Service. Applied Sciences. 2019; 9(9):1735. https://doi.org/10.3390/app9091735
Chicago/Turabian StyleWitkowski, Hubert, Wioletta Jackiewicz-Rek, Karol Chilmon, Janusz Jarosławski, Anna Tryfon-Bojarska, and Arkadiusz Gąsiński. 2019. "Air Purification Performance of Photocatalytic Concrete Paving Blocks after Seven Years of Service" Applied Sciences 9, no. 9: 1735. https://doi.org/10.3390/app9091735




